Micro total analysis systems (μ-TAS) have been widely used in trace detection in different fields such as biology and medicine[1]. In recent years, many micro fabrication techniques have been developed for application on polymer. So, more and more microfluidic chips employ polymer as materials during fabrication. The development of μ-TAS tends to integrate much more functions in limited size of microchip. Thus, multifunctional integration should be achieved by means of three-dimensional structures fabrication and multilayer bonding[2-4]. Today, many hard polymers such as methyl methacrylate (PMMA) and polycarbonate (PC) have replaced silicon and glass due to their better properties such as optics, biocompatibility and low cost[5-7]. Therefore, we have developed a microwave bonding technique which is suitable for multilayer bonding based on hard polymer such as PMMA. This method will be helpful for multifunctional integration and three dimensional fabrication of μ-TAS.
Many commonly known bonding methods are available based on the polymer.Thermal bonding is a widely used method for bonding a PMMA microfluidic device[8-10].However, the deformation of microstructures can not be ignored due to the pressure involved in the thermal bonding process[11-12]. Furthermore, a temperature approximately equal to glass transition temperature (Tg) is applied and kept under pressure until the bonding process is completed[13]. Thus, the microstructures are easily deformed. Liu[14] proposed a plasma assisted method to achieve surface modification method which decreases the bonding temperature. Hence, the thermal bonding temperature is decreased from 100 ℃ to 85 ℃. However, it is not possible to directly transfer heat and pressure to the bonding interface due to the low thermal conductivity and high deformation rate of the polymer at temperature Tg. Researchers have proposed ultrasonic bonding and microwave bonding techniques to achieve effective bonding[15-20]. These two methods are capable of providing direct interface bonding at low bonding pressure. Furthermore, both of these two methods have achieved short bonding time. Therefore, they can gain high bonding strength with low deformation.
Ultrasonic bonding generally involves energy directors to conduct ultrasonic energy. The ultrasonic energy can melt energy directors in several seconds to seal microstructures. However, fabrication of the energy directors increases the process sequence before bonding. Microwave bonding utilizes the microwaves to heat the microwave absorbing materials at the interface of the PMMA substrates directly. Then, PMMA substrates are melted to seal the microfluidic device. The microstructure deformation is relatively small due to the short heating time and rapid temperature rise. Some researchers apply solvent to assist the microwave bonding based on the PMMA microfluidic devices[21]. However, bubbles are produced if solvent is applied only at the interface of the two substrates. This causes reduction in the bonding strength due to the effective contraction of the bonding area.
In this paper, our work aims to provide a fast, reliable, flexible and low cost microwave bonding method which involves poor solvent to fabricate multilayer microfluidic chip. The proposed method has produced better results comparable to the bonding strength and minimum deformation of the microfluidic chips which are bonded by other heating bonding methods. Furthermore, the achieved bonding rate of the microfluidic chips is close to other microwave bonding techniques. In our study, the developed microwave bonding technique is used successfully to bond multilayer micro-mixer comprising of four layers. The deformation of the micro-channels can be significantly reduced through the microwave power and radiation time adjustment by detecting the results of the samples after each bonding. Moreover, we have achieved both good bonding strength and minimum deformation by analyzing the experimental results without any bubbles. The whole bonding process can be completed in a household microwave oven.
2 Experimental Section 2.1 Equipment and MaterialsThe PMMA substrates used in this work are 3.0 mm in thickness(Stone into Gold Trading Co., Ltd.Dongguan, China).The micro-channels fabrication is carried out using micro-precision engraving machine (VIP3530, Thai Power Electronic Equipment (Beijing)Co., Ltd.Beijing, China).The PMMA substrates are cleaned by using ultrasonic cleaning equipment(KQ-5200DB, Kun Shan Ultrasonic Instruments Co., Ltd, Kun Shan, China).Vacuum oven (DZF-6020, Shanghai Jing Hong Laboratory Instrument Co., Ltd.Shanghai, China)dried PMMA substrates after cleaning. Household microwave oven (MS-1968TW, Tianjin Le Jin Electronic Appliance Co., Ltd. Tianjin, China) can provide the microwave bonding power. The metallurgical microscope (C3203A, Shanghai Precision Instrument Co., Ltd. Shanghai, China) is used for evaluating bonding result and testing micro-mixer. All the reagents used are the analytical reagents (Tianjin Kermel Chemical Reagent Co., Ltd. Tianjin, China). Electronic balance (FA1604, Shanghai Liangping, Instrument Co., Ltd. Shanghai, China) has been used to weigh the reagents.
2.2 Bonding ProcedureThe PMMA plates are cut into square substrates 2 cm×2 cm size. Then, the films are tore on the substrates. The PMMA substrates are cleaned in the ultrasonic cleaning equipment for 5 min and dried in vacuum oven. To decrease bonding area errors and avoid bubbles at the interface, the alignment and fixation of two PMMA substrates should be performed under the metallurgical microscope in alcohol solution before bonding. Alcohol is applied as poor organic solvent and PMMA substrates are fixed by using binder clips. Then, we put the fixed the PMMA substrates are fixed in a 80 mL cubage beaker with 45 mL alcohol poured in. This ensures that the surface of the liquid alcohol is higher than the fixed PMMA substrates. The alcohol is heated in the microwave oven with different power and time. Thus, different results of microwave bonding are obtained. Bubbles cause leakage and reduction in the bonding strength due to the contraction of the bonding interface effective area. To ensure repeatability of each experiment, the heated alcohol is replaced after each experiment. To guarantee no bubble appearance after bonding at the interface of the substrates, the binder clips should press PMMA substrates evenly. Furthermore, the difference in PMMA substrate thickness should not be greater than 3 μm at 1.5 cm×1.5 cm square in size. Fig. 1(a) shows the fixed PMMA substrates in the beaker and Fig. 1(b) is the substrates after bonding. The substrates are bonded under 350 W microwave power for 35 s. The bonding rate of the sample is nearly 100%. The PMMA substrates are employed without micro-channels to avoid errors in detection of the bonding strength due to the size error of bonding area.

|
Figure 1 PMMA substrates before and after microwave bonding |
3 Results and Discussion 3.1 Bonding Strength
Fig. 2 illustrates the heating rate during the bonding process at different microwave powers. It can be seen that the maximum temperature is staying at 81 ℃ compare to 78.4 ℃ which is the theoretical boiling point of ethanol. Therefore, the maximum bonding temperature is still much lower than Tg of PMMA. Low temperature can decrease the deformation of the PMMA substrates during the bonding process. Furthermore, binder clips provided 14.7 N/cm2 bonding pressure which is low as compared to 1 215 N/cm2 maximum bonding strength. Low bonding pressure can also decrease the deformation rate of the PMMA substrates.
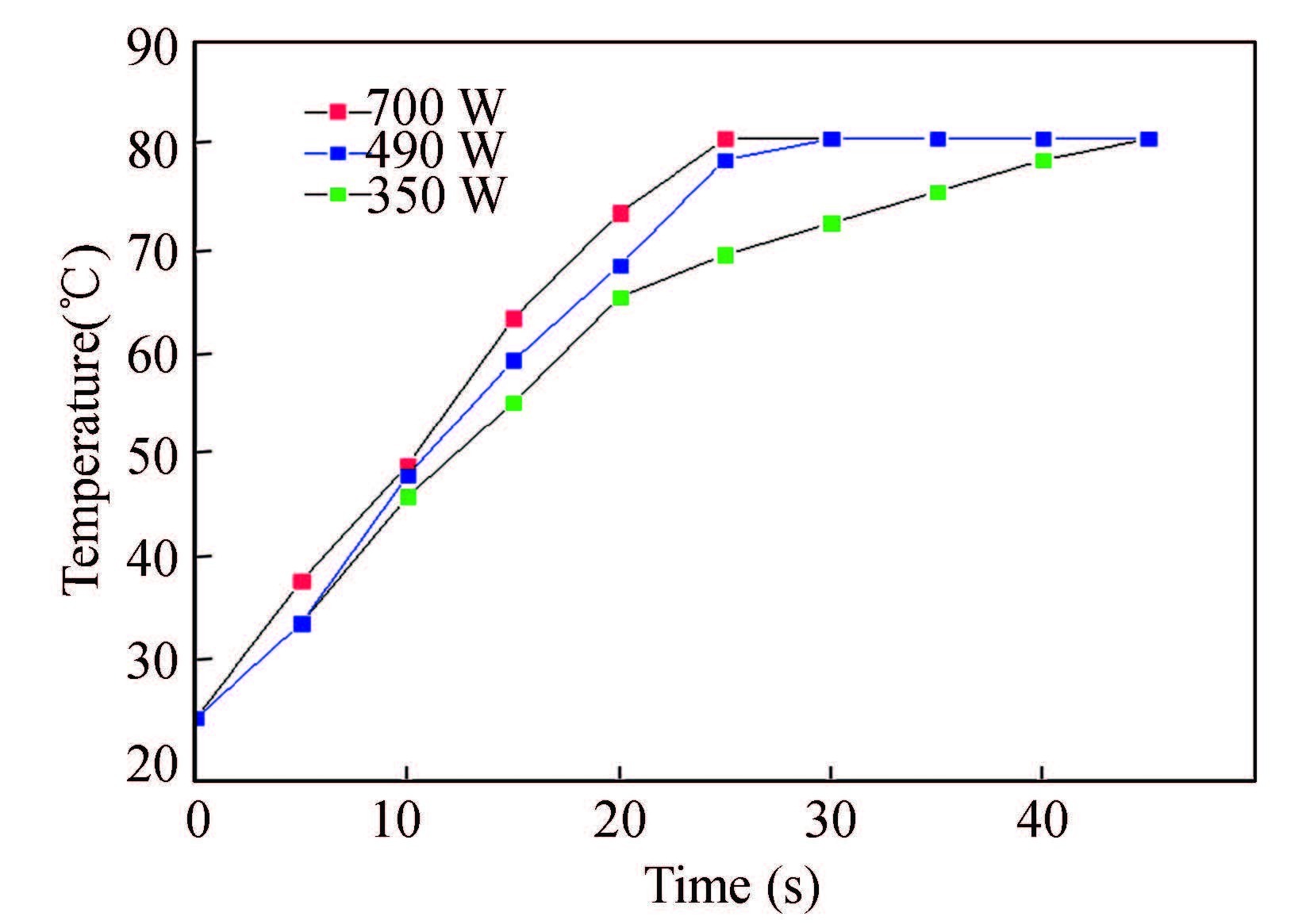
|
Figure 2 Heating rate at different microwave power |
Different microwave power and bonding time result in different bonding strengths and deformation of the micro-channels. The household microwave oven has three power levels: 350 W, 490 W and 700 W. The bonding time was set from several seconds to tens of seconds. The bonding strength is measured using drawing force meter (Wuxi Dajishan Instrument Engineering Equipment Co., Ltd. Wuxi, China) at different bonding conditions. The bonding strength measurements are shown in Fig. 3. It illustrates the drawing force measurement system used in this work and the sketch of the drawing force measurements. The bonded substrate is glued to the connectors by using two components modified acrylate adhesives (GLH-302, Fushun GELIAHAO Chemical Co., Ltd. Fushun, China).The bonded substrate is then fixed to the connectors and dried at room temperature for 24 h. After that, the bonding strength of the bonded substrate is measured by imposing drawing force. Then the bonding strength can be calculated by averaging the drawing forces to an area of 2.25 cm2. Each measurement is repeated five times and the bonding strength is calculated as an average value.
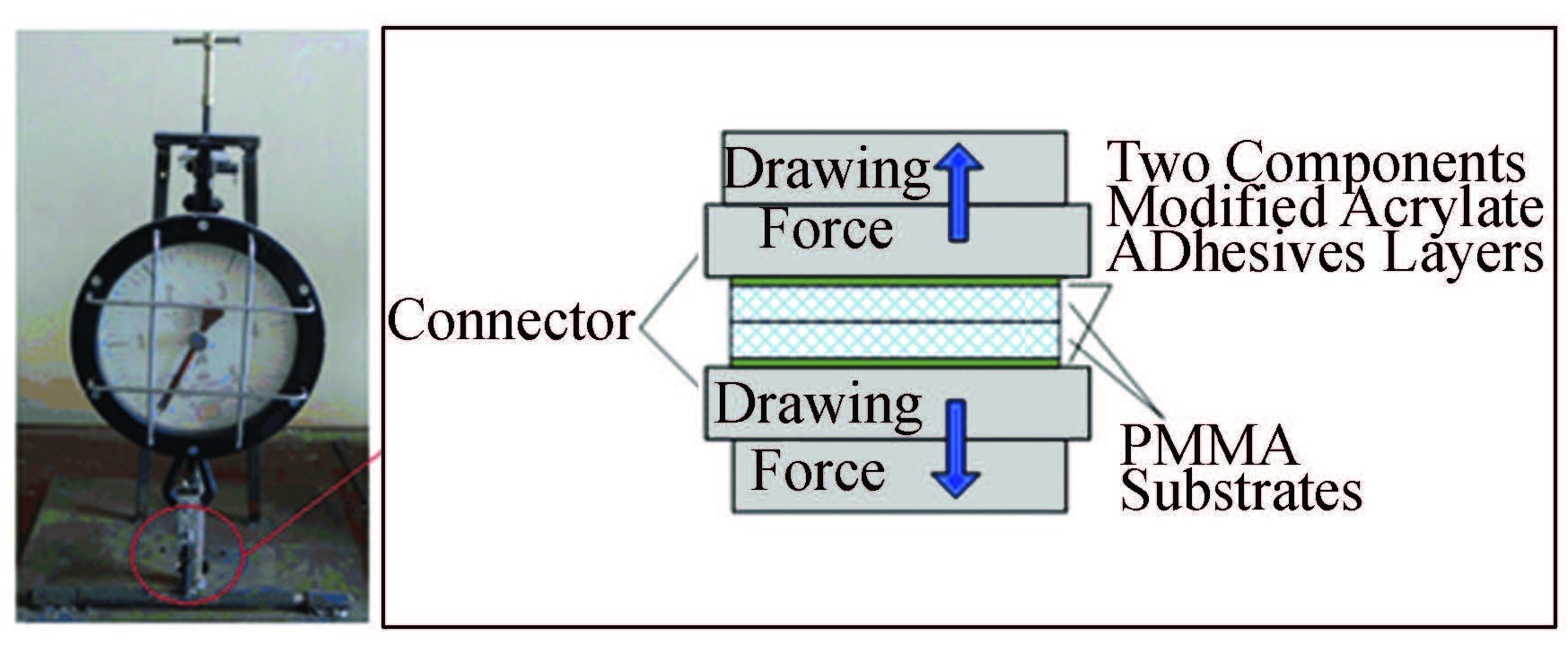
|
Figure 3 The bonding strength measure |
The relationship between bonding time and bonding strength at different microwave powers has been investigated. The experimental results are shown in Fig. 4. The maximum bonding strength is 1 215 N/cm2 at 700 W microwave power. The bonding strength increases with the increase of the microwave power. Furthermore, the bonding strength also increases with the increase of bonding time at same microwave power. However, the bonding strength increases very slowly at 350 W.

|
Figure 4 The bonding strength at different microwave power |
The VDWF (Vander Waals' force) of molecules and the covalent bonds between the atoms determine the bond strength of polymer. The dissolution of the polymer is small due to the fact that alcohol is a poor solvent for PMMA. Because the curly molecular chains are difficult to loosen, the PMMA substrates volume barely expand in the beginning stages of the bonding process. Hence, the bonding strength is mainly determined by the covalent bonds. When the bonding temperature reaches a maximum value, the bonding strength begins to decrease due to the expansion of curly molecular chains. The increased VDWF may lead to covalent bonds breakage at temperature near Tg.
3.2 Structural DeformationTo assure the fabricating quality of the micro device, the deformation of the microstructure should be decreased. The deformation of micro-channel S can be calculated by the following formula:
| $S=\frac{{{S}_{0}}-{{S}_{1}}}{{{S}_{0}}}$ | (1) |
where, S0 and S1 are the cross-section areas of the micro-channel before and after bonding, respectively.
The changes of the microchannel cross section area are calculated by using open source software Image J.The values are the averages of three measurements in Fig. 5. It is found that the deformation rate increases with the increase of microwave power and bonding time. Low deformation has been achieved due to the fast microwave heating and low bonding pressure. Fig. 6 shows a cross-section deformation of a trapezoid micro-channel before and after the bonding. The micro-channel is bonded under 700 W for 40 s. The deformation of the micro-channel is 5.1%, and the bonding strength is as high as 1 156 N/cm2 at the same time. It should be indicated that the fabricated microchannel passed through the entire PMMA substrate before bonding. Consequently, there is no error which is caused by the sample preparation during the deformation measurement.

|
Figure 5 Results of deformation measurement of microchannel at different microwave power |
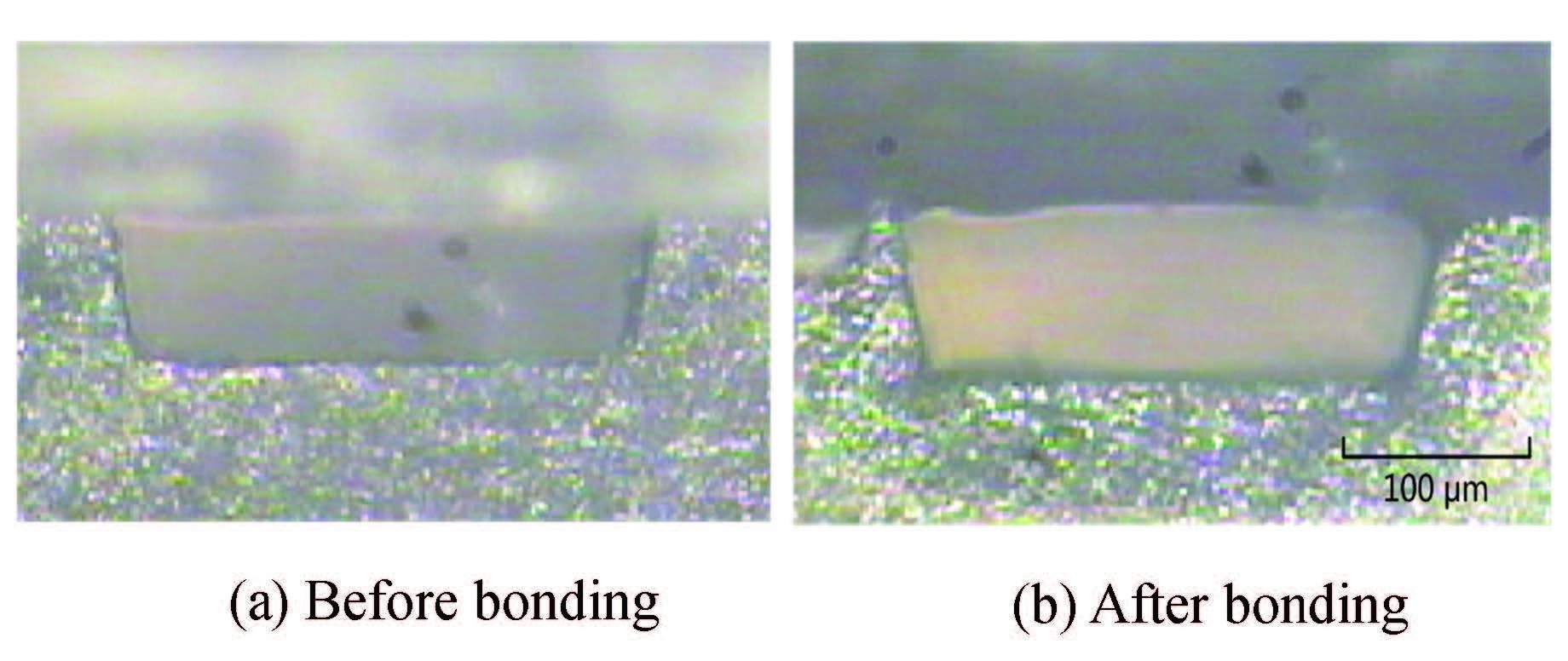
|
Figure 6 A cross-section of a trapezoid microchannel before and after bonding |
4 Application
A 4 layers 3D micro-mixer is designed and fabricated to evaluate the performance of the microwave bonding. Then, this microfluidic device is tested by employing theories of incompressible Newtonian flow and mass transport in our design. The micro-mixer contains 9 3D T-type mix units and a 2D X-type mix unit. The exploded view of the multilayer micro-mixer is shown in Fig. 7(a). The microstructures of the micro-mixer are provided in Fig. 7(b). Fig. 8(a) is the CAD drawing and Fig. 8(b) is the 4 layers of micro-mixer before bonding.

|
Figure 7 The diagram of micro-mixer |

|
Figure 8 The design and fabrication of multilayer micro-mixer |
Fig. 9(a) shows a multilayer 3D micro-mixer after bonding. The bonding of 4 layers is achievable in unit time. Moreover, there are no bubbles between adjacent layers due to the uniform heating on the bodies of PMMA substrates and bonding surfaces. The whole micro-structures were sealed without leakage.Fig. 9(b) is a scanning electron microscope (SEM) picture of the surface of the micro-mixer before and after bonding. It is found that swelling of the polymer is not involved in this microwave induced ethanol bath bonding.
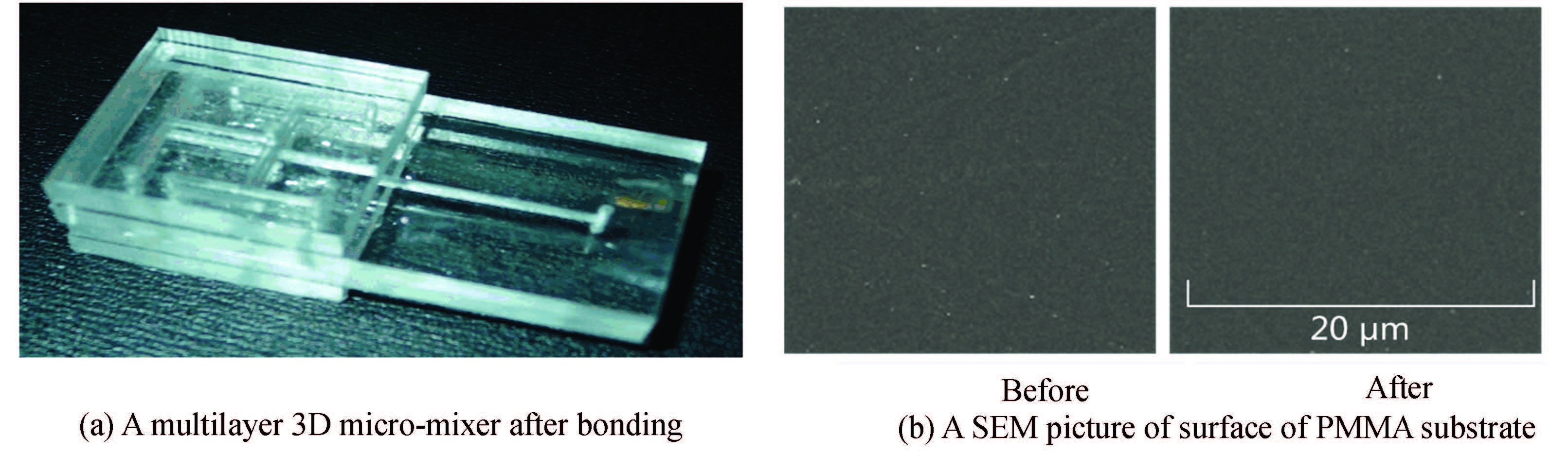
|
Figure 9 A multilayer 3D micro-mixer after bonding |
The proposed design achieves effective mix by employing two approaches. The 2D and 3D structures decrease concentration by folding micro flow of the samples. Then, the long microchannel raises mix effect by increasing diffusion distance. The reason for this is that, when the fluid velocity is extremely slow, mixing is primarily determined by the molecules diffusion, the related time and the fluid contact area. Syringe pump (SN-50F6, Sino Medical-Device Technology Co., Ltd. Shenzhen, China.) is used to inject rhodamine B (6.26 mmol/L) as sample A and methyl green (1.23 mmol/L) as sample B. After that, the mix effect is observed through the metallurgical microscope. Fig. 10 shows the contrast of the mix effects in flow rate of 0.1 mL/h. Fig. 10(a) shows the flow of rhodamine B without mixing, and Fig. 10(b) shows the flow of rhodamine B mixed with methyl green. Uniformly mixing is observed in Fig. 10(b).

|
Figure 10 Contrast of mixing effect in flow rate of 0.1 mL/h |
5 Conclusions
In this paper, an improved microwave bonding technology has been demonstrated which can overcome the conflict between bonding strength and micro structure holding. In our study, we have achieved both high bonding strength and low microstructure deformation in less than a minute by employing microwave induced ethanol bath bonding. Furthermore, we have presented our bonding method with general instrument, such as microwave oven, beakers and binder clips. Ethanol bath provides powerful microwave heating. We can achieve high bonding strength due to the covalent bonds resulted by the ethanol bath in the microwave bonding process. We have gain 1 215 N/cm2 as the maximum bonding strength at microwave power of 700 W with 35 s microwave heating time. Furthermore, low deformation of microstructure is possible due to the short bonding time, weak bonding pressure and low bonding temperature below Tg of PMMA. Thus, we can achieve 0.1% as minimum deformation at microwave power of 700 W for 10 s. Moreover, we have also gain good results with 709 N/cm2 bonding strength and 1.1% deformation under microwave power of 350 W for 35 s. Finally, we have fabricated a 4 layers micro-mixer which includes 15 rectangle micro-channels, 9 T-type mixing units and an X-type mixing unit as a validation of the microwave bonding technology. Our research has achieved the goal of providing a low cost, fast, convenient and reliable bonding technology for mass fabrication of multilayered microfluidic devices based on the polymer in the lab. The experimental results have indicated that this technology can performs well, and future investigation of it wide applications for other polymers such as polyethylene (PE) and polycarbonate (PC) will need to be continued. Our research is capable of simplifying the fabrication process of μ-TAS based on polymer. Thus, it may provide reference to other researchers and accelerate application of multifunctional μ-TAS.
| [1] |
Yang Y A, Lin C H, Wei Y C. Thread-based microfluidic system for detection of rapid blood urea nitrogen in whole blood.
Microfluidics and Nanofluidics,2014, 16 (5) : 887-894.
( 0) 0)
|
| [2] |
Huft J, Da Costa D J, Walker D, et al. Three-dimensional large-scale microfluidic integration by laser ablation of interlayer connections.
Lab on a Chip,2010, 10 (18) : 2358-2365.
( 0) 0)
|
| [3] |
Li J, Liu C, Ke X, et al. Fabrication of a thermoplastic multilayer microfluidic chip.
Journal of Materials Processing Technology,2012, 212 (11) : 2315-2320.
( 0) 0)
|
| [4] |
Chen L, Wang G, Lim C, et al. Evaluation of passive mixing behaviors in a pillar obstruction poly (dimethylsiloxane) microfluidic mixer using fluorescence microscopy.
Microfluidics and Nanofluidics,2009, 7 (2) : 267-273.
( 0) 0)
|
| [5] |
Currie C A, Shim J S, Lee S H, et al. Comparing polyelectrolyte multilayer-coated PMMA microfluidic devices and glass microchips for electrophoretic separations.
Electrophoresis,2009, 30 (24) : 4245-4250.
( 0) 0)
|
| [6] |
Land K J, Mbanjwa M B, Govindasamy K, et al. Low cost fabrication and assembly process for re-usable 3D polydimethylsiloxane (PDMS) microfluidic networks.
Biomicrofluidics,2011, 5 (3) : 036502-036502.
( 0) 0)
|
| [7] |
Farshchian B, Park S, Choi J, et al. 3D nanomolding for lab-on-a-chip applications.
Lab on a Chip,2012, 12 (22) : 4764-4771.
( 0) 0)
|
| [8] |
Zhu X, Liu G, Guo Y, et al. Study of PMMA thermal bonding.
Microsystem Technologies,2007, 13 (3/4) : 403-407.
( 0) 0)
|
| [9] |
Nayak N C, Yue C Y, Lam Y C, et al. Thermal bonding of PMMA:effect of polymer molecular weight.
Microsystem Technologies,2010, 16 (3) : 487-491.
( 0) 0)
|
| [10] |
Kelly R T, Woolley A T. Thermal bonding of polymeric capillary electrophoresis microdevices in water.
Analytical Chemistry,2003, 75 (8) : 1941-1945.
( 0) 0)
|
| [11] |
Li J M, Liu C, Qiao H C, et al. Hot embossing/bonding of a poly (ethylene terephthalate)(PET) microfluidic chip.
Journal of Micromechanics and Microengineering,2008, 18 (1) : 015008.
doi: 10.1088/0960-1317/18/1/015008 ( 0) 0)
|
| [12] |
Sun Y, Kwok Y C, Nguyen N T. Low-pressure, high-temperature thermal bonding of polymeric microfluidic devices and their applications for electrophoretic separation.
Journal of Micromechanics and Microengineering,2006, 16 (8) : 1681-1688.
( 0) 0)
|
| [13] |
Wang X, Zhang L, Chen G. Hot embossing and thermal bonding of poly (methyl methacrylate) microfluidic chips using positive temperature coefficient ceramic heater.
Analytical and Bioanalytical Chemistry,2011, 401 (8) : 2657-2665.
( 0) 0)
|
| [14] |
Liu J, Qiao H, Liu C, et al. Plasma assisted thermal bonding for PMMA microfluidic chips with integrated metal microelectrodes.
Sensors and Actuators B: Chemical,2009, 141 (2) : 646-651.
( 0) 0)
|
| [15] |
Sun Y, Luo Y, Wang X. Micro energy director array in ultrasonic precise bonding for thermoplastic micro assembly.
Journal of Materials Processing Technology,2012, 212 (6) : 1331-1337.
( 0) 0)
|
| [16] |
Ng S H, Wang Z F, De Rooij N F. Microfluidic connectors by ultrasonic welding.
Microelectronic Engineering,2009, 86 (4) : 1354-1357.
( 0) 0)
|
| [17] |
Zhang Z, Wang X, Luo Y, et al. Thermal assisted ultrasonic bonding method for poly (methyl methacrylate)(PMMA) microfluidic devices.
Talanta,2010, 81 (4) : 1331-1338.
( 0) 0)
|
| [18] |
Mani K B, Hossan M R, Dutta P. Thermal analysis of microwave assisted bonding of poly (methyl methacrylate) substrates in microfluidic devices.
International Journal of Heat and Mass Transfer,2013, 58 (1) : 229-239.
( 0) 0)
|
| [19] |
Yussuf A A, Sbarski I, Hayes J P, et al. Microwave welding of polymeric-microfluidic devices.
Journal of Micromechanics and Microengineering,2005, 15 (9) : 1692-1699.
( 0) 0)
|
| [20] |
Yussuf A A, Sbarski I, Hayes J P, et al. Single-mode microwave sealing of polymer-based microfluidic devices using conductive polymer. Photonics Europe. International Society for Optics and Photonics, 2004.74-81.
( 0) 0)
|
| [21] |
Rahbar M, Chhina S, Sameoto D, et al. Microwave-induced, thermally assisted solvent bonding for low-cost PMMA microfluidic devices.
Journal of Micromechanics and Microengineering,2010, 20 (1) : 015026.
( 0) 0)
|
 2016, Vol. 23
2016, Vol. 23


