Because of its superior mechanical properties and excellent thermal shock resistance, silicon carbide (SiC) has been established as a high performance material for sliding bearings and seals, and recently been used to manufacture aerospace optical mirrors[1-3] and semiconductor industry [4]. Although SiC bearings working in aqueous media can obtain very low friction coefficient and little wear through tribochemical reaction, there is a need to shorten its long running-in time to reach the polishing stage. While there is the demanding request for high surface quality for SiC mirror, it is not easy to obtain a smooth surface efficiently by traditional mechanical polishing method[5-8] and chemical mechanical polishing (CMP) techniques [9-12], due to its high hardness and chemical inertness of SiC.
Silicon carbide is electrically semi-conducting.It has shown that the applied electrical potentials influence the electrochemical corrosion behavior of SiC[13-14]. To realize more efficient polishing of SiC with minimal subsurface damage, Li et al.[15] developed a two-step electrochemical mechanical polishing(ECMP) process to polish single crystal SiC.ECMP combines anodic oxidation and mechanical polishing effects.Hydrogen peroxide and potassium nitrate were used as the electrolytes for anodic oxidation of SiC and the oxide layer was removed by polishing using colloidal silica slurry.After several repeated cycles of anodic oxidation and slurry polishing, a smooth surface was obtained.
For the polishing of sintered SiC, some researchers utilized the catalytic effect of iron oxide[16] and oxidant CrO3[1] to enhance the tribochemical reaction of SiC.Recently, Kailer et al.[17] have investigated the effects of the applied potentials on friction and wear of sintered SiC/SiC pairs, and the results showed that even for sintered SiC ceramics it was still possible to influence its tribological behaviour in aqueous electrolytes by applying different electric potentials.
The purpose of this paper is to investigate the effect of polarization potential on the friction and wear properties of SiC/HT200 pair in NaOH electrolyte, through a two-electrode electrochemical cell installment, which may provide a technique for hastening the running-in process of SiC/Fe friction pairs, as well as for SiC polishing to increase its materials removal efficiency while maintaining good surface quality.
2 ExperimentsFig. 1(a) shows the photo of the circular-translation polishing machine. As shown in Fig. 1(b), the SiC work-piece is installed in the jig 4, and the cast iron (HT200) polishing pad 6 is installed on the translational moving platform, which is driven by two servo-motors and power-screws arranged in x and y direction respectively. The rotation of servo motor under the control signal can be translated into linear reciprocating motion of sliding block in x and y directions through the screws, respectively.Through the motion control program, the polishing pad can move in a predetermined way in x-y plane. In this experiment the given translational motion along a circular path is adopted, which favors the polishing quality by changing the friction direction between the SiC/HT200 pair any time.
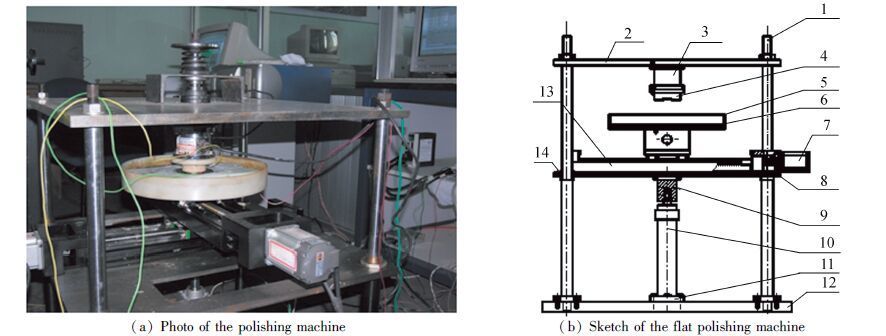
|
1-Vertical columns; 2-Upper plate; 3-Dual direction force sensor; 4-Jig; 5-Polishing cell; 6-Polishing pad; 7-Servo motor;8-Up-moving plate; 9-Coupling; 10-Cylinder; 11-Flange; 12-Base plate; 13-X-Y moving platform Figure 1 Photo and sketch of the flat polishing machine |
X-Y translational motion platform is installed on an up-moving plate, which is jacked through cylinder 10 during test, thus applying the normal load between the SiC/HT200 friction pair. Dual direction force sensor 3 can simultaneously measure the friction force and normal load, and send the analog signals to data acquisition system for processing. The measured friction coefficient is defined the ratio of measured friction force to the measure normal load.
The influence of applied electric potential on friction was studied by a two-electrode electrochemical cell system as shown in Fig. 2, in which a given voltage was directly applied between HT200 pad (work electrode) and a copper sheet(counter electrode) 1 mm above the pad. Both electrodes were immersed in the NaOH electrolyte. Since SiC piece and HT200 pad contact and rub as friction pair, they behave actually as one electrode. It should be noted that, in such a two-electrode cell system, the potential between the bulk electrode and the electrolyte adjacent to its surface cannot be known precisely.Here, for simplicity, the relative voltage between the HT200 and copper sheet is referred as the applied potential, i.e., the positive or negative potential applied on SiC/HT200 pair refers to the potential of the HT200 plate electrode relative to the upper copper sheet electrode.

|
Figure 2 Sketch of the two-electrode cell system |
The test conditions are shown in Table 1. Of course, more complete series of parameters should be used in future studies, but to show the principle effects of voltage direction, these parameters are sufficient. The sintered SiC discs had a diameter of 20 mm, with its initial surface roughness 0.1μm Ra. The surface roughness of HT200 plate was 0.8 μm Ra. All tests were done at room temperature. Before each test, the specimen were cleaned with alcohol or acetone and then dried up. The surface morphologies of SiC work-pieces before and after polishing were observed by optical microscopy and AFM.
| Table 1 Test conditions |
3 Results and Discussions
The effects of different electric potentials on friction coefficients of SiC/HT200 pair are shown in Fig. 3. From Fig. 3(a) we can see during the first 20 min when no voltage is applied, the friction coefficient of SiC/HT200 is 0.25;when +3 V voltage is applied afterwards, it is decreased to 0.20 and then fluctuates around 0.22-0.24 with time.In contrast, when -3 V voltage is applied as shown in Fig. 3(b), the friction coefficient of SiC/HT200 is increased from 0.22 to 0.30.It can be seen that the influences of negative electric potentials on the friction coefficient rise (in Fig. 3(b)) is more obvious than the effect of positive potentials on friction reduction (in Fig. 3(a)) under applied voltage shift mode.

|
Figure 3 Friction coefficient of SiC/HT200 under different applied voltages |
To eliminate the effect of applied voltage shift on the measurement accuracy of the dual-force sensor, as seen obviously for the normal load signals in Figs. 3(a) and 3(b), the experiments of applying definite voltage during the whole 1 h are done and the results are shown in Fig. 3(c). The results show that the friction coefficient of SiC/HT200 under no voltage is 0.25; but much less (even below 0.18) when +3 V voltage applied; and is increased to 0.30 in the case of -3 V voltage applied. In all the cases, the friction coefficients change little with time, especially in the positive voltage applying mode.
Fig. 4 shows the microscopic graphs of the polished SiC surfaces after the 1 h friction tests as shown in Fig. 3(c). It can be seen that under no voltage condition (Fig. 4(a)), abrasive wear occurs since there are clear fine ploughing marks on SiC surface; under the condition of -3 V voltage applied(Fig. 4(b)), adhesive wear with a smeared appearance occurs, and there are transferred iron adhering to SiC surface which makes the surface rough and SiC grains invisible; under the condition of +3 V voltage applied(Fig. 4(c)), the polished SiC surface is much smoother, the surface roughness can reach about 10 nanometers as measured by AFM (Fig. 5).

|
Figure 4 Microscopic photos of SiC after polishing under different voltages |

|
Figure 5 AFM photos of SiC after 1 h polishing under +3 V voltage |
From the friction and wear tests above, we can see the studied SiC/HT200 friction pair operates in mixed lubrication mode, and the SiC specimen and HT200 polishing disc only contacts at micro asperities. Fig. 6 shows the schematic diagram the SiC/HT200 pair under different voltages.
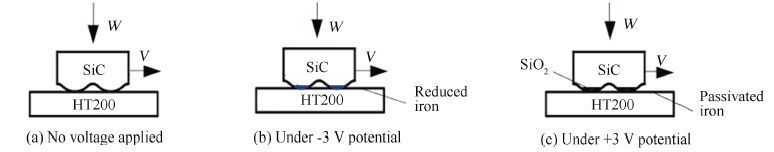
|
Figure 6 Schematic graph of tribo-electrochemical mechanism of SiC/HT200 under different voltages |
For SiC/HT200 rubbing in aqueous NaOH fluids, SiC is stable under no voltage applied as in Fig. 6(a), while HT200 can corrode to form iron oxides.
The electric voltage applied between SiC/HT200 friction pair and copper sheet electrode in NaOH electrolyte initiates an electrochemical process. As one electrode, SiC and HT200 undertake reduction process at negative potential (Fig. 6(b)) and oxidation process at anodic potential (Fig. 6(c)). During electrochemical process, the electrode potential will influence the oxidation-reduction (REDOX) reaction of SiC/HT200 pair, changing the properties and thickness of boundary film, and thus the friction and wear characteristics.
For SiC/HT200 pair in NaOH electrolyte under -3 V voltage as shown in Fig. 6(b) , SiC and cast iron plate are in reducing state due to cathodic effect, there is neither silicon dioxide formed on SiC surface nor iron oxide formed on HT200.The hard SiC asperities directly contact and plough the softer reduced iron, adhesion wear occurs and SiC surface becomes rougher due to iron transfer, friction coefficient therefore increases meanwhile.
In the case under +3 V voltage as shown in Fig. 6(c), the anodic oxidation reaction takes place on the contacting surfaces of anodic iron plate and SiC disc.The following electrochemical process of SiC dominates at contact asperities [5].
SiC+4H2O→SiO2+CO2↑+4H2↑
At SiC/HT200 interface, the SiO2-based reaction product then reacts with water to form silicon hydrooxides Si(OH)4, which is considered to have a lubricious effect and alleviates direct contact of SiC with fresh iron, making the friction coefficient decrease. Besides, this gel-like silica layer on SiC surface asperities can be easily rubbed off and the SiC surface can thus be flattened and becomes smooth.
On the anodic iron plate in NaOH electrolyte, according to Ref.[18], a compact, hard passivation FeOOH layer can be formed when the applied potential is highly positive. Some studies show that iron oxide can acts as a catalyst to the chemical reactions above[16, 19].This results in a combined synergistic process of catalyst formation and SiC oxidation in this case, facilitating the removal of contacting SiC asperities to hasten the running-in process or to obtain smoother SiC surfaces.
According to Ref.[20], the average thickness of the anodic oxide layer on single SiC after anodic oxidation statically for 10 min under voltage of 5 V is no more than 100 nm.During the ECMP process as in our experiment, oxidation and polishing are simultaneously undertaken, oxide layer is removed as soon as it is generated, it is impossible to get essential microscopic verification of the generated oxide film during test.Construction of more sophiscated experiment platform for microscopic observation of the delicate thin oxide film online is needed in future reseach.
4 Conclusions(1) When +3 V polarization potential is applied on SiC/HT200 pair, its friction coefficient is decreased from 0.25 to 0.20 or less due to polishing effects, and a much smoother SiC surface is obtained; On the contrary, under -3 V applied potential, friction coefficient of SiC/HT200 pair increases to 0.30, and the SiC surface quality deteriorates.
(2) The REDOX reaction of SiC/HT200 in NaOH electrolyte under given potential determines its tribological behavior through tribo-electrochemical process. It is believed that under anodic potentials both the SiC and HT200 oxidize, forming silicon hydrooxides on SiC surface and passivated iron oxides on HT200, the former product is soft /lubricious and therefore reduces friction, the latter has catalytic effects for SiC oxidation and thus increase materials removal of SiC.
(3) The anodic passivation technique may be used for hastening the running-in process of SiC/HT200 friction pairs in aqueous system, as well as for polishing SiC to increase its materials removal efficiency while maintaining good surface quality. References
| [1] |
Zhu Z, Murotov V, Fischer T E. Tribochemical polishing of silicon carbide in oxidant solution. Wear, 1999, 225. ( 0) 0)
|
| [2] |
Gates R S, Hsu S M. Tribochemistry between water and Si3N4 and SiC: Induction time analysis. Tribology Letters, 2004, 17(3): 399-407. ( 0) 0)
|
| [3] |
Yamaoka H, Uruga T, Arakawa E, et al. Development and surface evaluation of large SiC X-Ray mirrors for high-brilliance synchrotron radiation. Japanese Journal of Applied Physics, 1994, 33: 6718-6726. ( 0) 0)
|
| [4] |
Qian W, Skowronski M, Augustine G, et al. Characterization of polishing-related surface damage in (0001) silicon carbide substrates. Journal of the Electrochemical Society, 1995, 142(12): 4290-4294. ( 0) 0)
|
| [5] |
Zhou L, Audurier V, Pirouz P, et al. Chemomechanical polishing of silicon carbide. Journal of the Electrochemical Society, 1997, 144(6): 161-163. ( 0) 0)
|
| [6] |
Mitchel W C, Brown J, Buchanan D. Comparison of mechanical and chemomechanical polished SiC wafers using photon backscattering. Material Science Forum, 2000, 338. ( 0) 0)
|
| [7] |
Johansson S, Schweitz J-A, Lagerlog K P D. Surface defects in polished silicon studied by cross-sectional transmission electron microscopy. Journal of the American Ceramic Society, 1989, 72(7): 1136-1139. ( 0) 0)
|
| [8] |
Liang Qingrui, Zong Yanmin, Wang Xijie, et al. Effect of diamond powder on SiC mechanical polishing. Journal of Synthetic Crystals, 2015, 44(2): 295-300. ( 0) 0)
|
| [9] |
Wang Jinhu, Zhai Wenjie. Influence of polarization potential on tribo-electrochemical material removal properties of silicon wafer. Journal of Harbin Institute of Technology, 2014, 46(7): 682-687. ( 0) 0)
|
| [10] |
Zhai Wenjie, Yang Yangzhan, Wang Jinghe, et al. Inhibition effects of BTA on the electrochemical corrosion of copper in phosphoric acid electrolytes. Journal of Harbin Institute of Technology, 2012, 44(8): 67-72. ( 0) 0)
|
| [11] |
Zhang Feng. Computer-controlled chemical mechanical polishing of silicon modification layer on aspheric silicon carbide surface. Optics and Precision Engineering, 2013, 21(12): 3015-3020. ( 0) 0)
|
| [12] |
Liu Jinquan, Zhang Chaohui, Ye Wei. Simulation on temperature distribution in chemical mechanical polishing. Key Engineering Materials, 2007, 353. ( 0) 0)
|
| [13] |
Sydow U, Schneider M, Herrmann M. Electrochemical corrosion of silicon carbide ceramics. Materials and Corrosion-Werkstoffe und Korrosion, 2010, 61(8): 657-663. ( 0) 0)
|
| [14] |
Andrews A, Herrmann M, Sephton M, et al. Electrochemical corrosion of solid and liquid phase sintered silicon carbide in acidic and alkaline environments. Journal of the European Ceramic Society, 2007, 27(5): 2127-2135. ( 0) 0)
|
| [15] |
Li C, Bhat I B, Wang R, et al. Electro-chemical mechanical polishing of silicon carbide. Journal of Electronic Materials, 2004, 33(5): 481-486. ( 0) 0)
|
| [16] |
Lin Y C, Kao C H. A study on surface polishing of SiC with a tribochemical reaction mechanism. Int J Manuf Technol, 2004, 25(1): 33-40. ( 0) 0)
|
| [17] |
Kailer A, Amann T, Krummhauer O, et al. Influence of electric potentials on the tribological behaviour of silicon carbide. Wear, 2011, 271(9/10): 1922-1927. ( 0) 0)
|
| [18] |
Zhu Y Y, Kelsall G H, Spikes H A. The influence of electrochemical potentials on the friction and wear of iron and iron Oxides in aqueous systems. Tribology Transactions, 1994, 37(4): 811-819. ( 0) 0)
|
| [19] |
Zhou Y, Pan G, Shi X, et al. Chemical mechanical planarization (CMP) of on-axis Si-face SiC wafer using catalyst nanoparticles in slurry. Surface and Coatings Technology, 2014, 251: 48-55. ( 0) 0)
|
| [20] |
Deng H, Hosoya K, Imanishi Y, et al. Electro-chemical mechanical polishing of single-crystal SiC using CeO2 slurry. Electrochemistry Communications, 2015, 52: 5-8. ( 0) 0)
|
 2016, Vol. 26
2016, Vol. 26

