Molybdenum is an essential trace element for both plants and animals, and it plays an important role in the redox reactions of enzymes [1]. However, excessive amount of molybdenum can be potentially toxic. Research has shown that subchronic and chronic oral exposure to molybdenum can result in gastrointestinal disturbance, growth retardation, anemia, hypothyroidism, bone and joint deformities, sterility, liver and kidney abnormalities and death [2]. Nowadays molybdenum is widely used as an alloying element in steel, cast iron, super alloys and also plays an important role in the electronics industry [3]. This has advanced the exploitation of molybdenum mines. Near the molybdenum mining areas, the molybdenum concentration in surface water can reach 200 to 400 μg/L and in ground water it can be as high as 25 mg/L[4]. A high concentration of molybdenum may negatively impact human health so it is important to develop proper technology to remove Mo (Ⅵ) from water.
In natural water, molybdenum forms molybdate Mo (Ⅵ) oxyanions and the dominant Mo (Ⅵ) species is MoO42- at most pH values[3]. Some researchers have investigated the removal of molybdenum from water, but these efforts are mainly focused on the adsorption of molybdenum by variety of absorbents such as hematite[5], pyrite[6-7], goethite[7-8], ferrihydrite[9], γ-Al2O3[10-11], kaolinite[12-13], soil[14-16], and carbon cloth[17]. Most of these processes are costly and the initial concentration of molybdenum is always too high to simulate the situation in the practical water treatment engineering. Coagulation-filtration is a traditional water treatment process which has been proven to be effective in the removal of some oxoanions [18-20]. Iron salt is frequently used as a coagulant which can interact with water molecules to form a series of positively charged hydrolysis products like, Fe(OH)2+, Fe(OH)2+, Fe2(OH)24+, Fe4(OH)66+, etc. These hydrolysis products can interact with negatively charged ions through electrostatic adsorption, surface complexation, coprecipitation, etc. and lead to the reduction of target ions in water. Since molybdenum exists mostly as negatively charged ions, iron salt should be effective on its removal. This has been shown by many types of iron based adsorbents in the adsorption process[5-9]. Though the use of iron salt for coagulation-filtration removal of molybdenum has not been reported, its potential effectiveness can be reasonably assumed. Since coagulation-filtration is low cost and easy to operate, it can be appealing for practical engineering use in the removal of molybdenum once shown to be feasible. Therefore, this study was initiated to investigate the effect of FeCl3 coagulation-filtration process on the removal of molybdenum from water and the influence of co-existing background constituents common in natural water were also considered.
2 Materials and MethodsAll chemicals used in this study were reagent grade and used without further purification. All solutions were prepared with ultrapure water. The stock solution of Mo (Ⅵ) containing 1.4 mg/L Mo was freshly prepared each week by dissolving Na2MoO4·2H2O in ultrapure water. A FeCl3 solution containing 20 g/L Fe was prepared weekly and 0.5 mL hydrochloric acid was added into 1 L FeCl3 solution to inhibit the hydrolysis of Fe3+. The Na2SO4, Na2SiO3·9H2O and NaH2PO4·2H2O solutions were prepared every week with a concentration of 1 mol/L SO42-, 0.1 mol/L SiO32- and 0.1 mol/L PO43-, respectively. Background electrolyte solutions were prepared using NaCl and NaHCO3.
Jar tests were performed with a jar testing device (TA2-2) to simulate the coagulation process.Experiments about the influence of pH on the removal of Mo (Ⅵ) were carried out as follows: the jar testing procedure began with a rapid mixing at 150 r/min for 1 min, followed by 80 r/min for 10 min, 40 r/min for 10 min, consecutively and then a 20 min settling period. 10 mg FeCl3·6H2O as Fe was added to 1 L ultrapure water before the rapid mixing step. Before the addition of FeCl3·6H2O, 0.7 mg Na2MoO4·2H2O as Mo was added to the ultrapure water to provide Mo (Ⅵ), and 0.01 mol NaCl was added to the ultrapure water to provide ionic strength and 0.001 mol NaHCO3 was added to the ultrapure water to provide alkalinity. NaOH and HCl were used to adjust the pH of the solution to the pre-determined value before addition of FeCl3·6H2O and the pH was kept constant during the experiment. After the settling step, the supernatant was sampled and filtered through a cellulose acetate membrane (MFS) of 0.45 μm pore size to simulate the filtration process. Then the sample was acidified with concentrated HNO3 for the determination of Mo and Fe.
The procedure of experiments about the influence of co-existing background constituents on the removal of Mo (Ⅵ) was approximately the same as the experiments about the influence of pH but added predetermined aliquots of certain constituent right after the addition of NaCl and NaHCO3. The aliquots of constituents are as follows: SO42-: 0, 1 and 5 mmol; SiO32-: 0, 0.5 and 1 mmol; PO43-: 0, 0.05 and 0.1 mmol; Humic acid (HA): 0, 1 and 5 mg.
All the experiments were maintained at (20±1) ℃ using a temperature control device (THD-0515) and each experiment was conducted in (at least) duplicate. All the experiments were carried out open to the atmosphere and before use, all the glassware was cleaned by soaking in 10% HNO3 for at least 6 hours and rinsed for five times with tap water followed by washing for three times with distilled water. The concentration of Mo was determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) method and the concentration of Fe was determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) method. The solution pH was measured using a high performance pH meter (PHS-3C) which was calibrated daily using proper buffer solutions (pH 4.00, 6.86 and 9.18) to ensure its accuracy. The zeta potential of the iron flocs was measured at 20 ℃ with a zetasizer Nano-ZS90 (Malvern Instruments Led., UK). The species distribution of molybdenum and phosphate at different pHs were calculated by Visual Minteq (Ⅱ) software, and the species distribution of silicate at different pHs was calculated according to the data:pKa1, H2SiO3=9.77, pKa2, H2SiO3=11.8.
To examine the effect of FeCl3 coagulation-filtration process on the removal of Mo (Ⅵ) from natural water, raw water from Songhua River was prepared as the test water and the characteristic of the songhua water was tested as shown in Table 1.
| Table 1 hemical composition of water from Songhua River |
Since there was no Mo (Ⅵ) in the Songhua water, 0.7 mg Na2MoO4·2H2O as Mo was added into 1 L of the Songhua water to provide Mo (Ⅵ). The procedure of experiments about the effect of FeCl3 on the removal of Mo (Ⅵ) from Songhua water was the same as the experiments about the effect of FeCl3 on the removal of Mo (Ⅵ) from the synthetic ultrapure water.
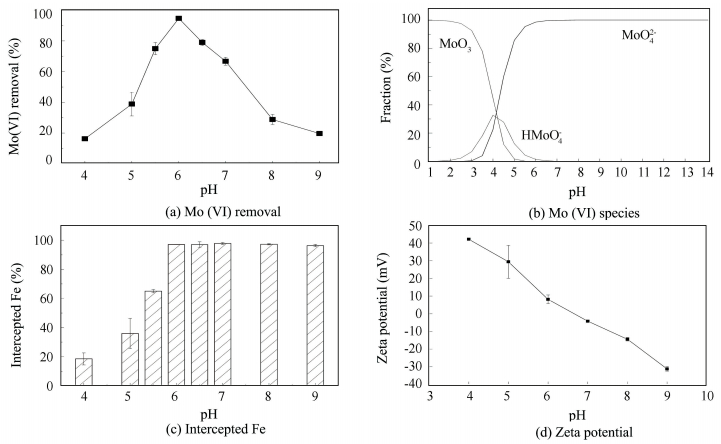
3 Results and Discussion 3.1 Mo (Ⅵ) Removal by FeCl3 as Function of pHFig. 1(a) shows the effect of pH on the removal of Mo (Ⅵ), and it can be seen that the removal of Mo (Ⅵ) is highly dependent on the pH. When the pH changes from 4.00 to 6.00, Mo (Ⅵ) removal increases from 14% to 95% and, thereafter, drops sharply to 20% at pH 9.00.
|
Figure 1 Effect of pH on the removal of Mo (Ⅵ), species of molybdenum, content of Fe intercepted from water and zeta potential of the FeCl3 flocs |
Many researchers have investigated the effect of pH on the removal of Mo (Ⅵ) by different kinds of adsorbents[21-24]. Though the adsorbents were varied, one phenomenon was consistent: The removal efficiency of Mo (Ⅵ) by the adsorbents was high at low pH attributing to the neutralization of negatives charges on the surface of adsorbent by excess hydrogen ions, there by facilitating the diffusion of Mo (Ⅵ) and their subsequent adsorption [17, 21]; while the removal of Mo (Ⅵ) decreased notably as pH increased over the point of zero charge (PZC). And the reason is that the negatively charged adsorption surface sites increased the electrostatic repulsion between Mo (Ⅵ) and adsorbents [21, 25]. Former researchers [8-9, 26-27] also reported that Mo (Ⅵ) interacts with iron oxides through ligand exchange[14, 28-29] and forms inner sphere surface complexes.
The iron flocs derived from the hydrolysis of FeCl3 in the coagulation process are a series of iron (hydro) oxides, and it can be reasonably speculated that Mo (Ⅵ) may also interact with the iron flocs through ligand exchange and form inner sphere surface complexes. As shown in Fig. 1(d), the PZC of the iron flocs was 6.66. At pH 7.00 to 9.00, the surface charge of the iron flocs was negative, and the electrostatic force between negatively charged Mo (Ⅵ) (As shown in Fig. 1(b)) and the iron flocs was repulsion. However, there were still 67%, 29% and 20% Mo (Ⅵ) were removed at pH 7.00, pH 8.00 and pH 9.00, separately. This means that other interactions besides electrostatic force occurred between Mo (Ⅵ) and the iron flocs: ligand exchange, coprecipitation or both. Considering that the coprecipitation effect was affected by the content of iron flocs precipitated but not the surface charge of the flocs, the decrease in the removal of Mo (Ⅵ) and little change in the content of Fe intercepted from water as pH increased from 7.00 to 9.00 indicated that ligand exchange did happen between Mo (Ⅵ) and the iron flocs in the coagulation-filtration process. The coprecipitation effect between the iron flocs and Mo (Ⅵ) may also existed, but it cannot be certified in the present study.
According to the discussion above, the following factors are speculated to influence the removal of Mo (Ⅵ) as the pH varied: firstly, the species of Mo (Ⅵ) and the surface charge of the iron flocs which may influence the affinity (electrostatic force or specific adsorption force) between Mo (Ⅵ) and the iron flocs; secondly, the content of Fe intercepted from water which may influence the amount of adsorption sites and the effect of coprecipitation (if occurred).
Fig. 1(b) shows the species distribution of molybdenum with pH. Mo (Ⅵ) existed mainly as MoO42- from pH 6.00 to 9.00, indicating that the large decrease of Mo (Ⅵ) removal from pH 6.00 to 9.00 had nothing to do with the shift of the molybdenum species. But at pH 4.00, Mo (Ⅵ) existed as three dominant species: MoO3, HMoO4- and MoO42- and the fractions of these three species were generally the same. As the pH increased from 4.00 to 6.00, the fraction of MoO3 and HMoO4- decreased gradually, while the fraction of MoO42- increased, which meant that the Mo (Ⅵ) species became more negatively charged. As the iron (hydro) oxides derived from the hydrolysis of Fe3+ were positively charged at pH 4.00 to 6.00 (Fig. 1(d)), the negative charge-shift of Mo (Ⅵ) species appeared to improve its adsorption on the iron flocs, which led to the increase of Mo (Ⅵ) removal.
The content of Fe intercepted from water as function of pH was investigated, as illustrated in Fig. 1(c). When the pH increased from 4.00 to 6.00, the content of Fe intercepted from water increased from 18% to 97%, in conformity with the removal of Mo (Ⅵ). More intercepted iron flocs offered more adsorption sites, which was probably the main reason for the large increase in Mo (Ⅵ) removal when the pH changed from 4.00 to 6.00. By the way, the increase in the content of Fe intercepted from water also strengthened the coprecipitation effect if this occurred in the coagulation-filtration study. However, at pH 6.00 to 9.00, the content of Fe intercepted did not change markedly, which meant there must be some other reason for the sharp decrease of Mo (Ⅵ) removal.
Fig. 1(d) shows the zeta potential of the Fe flocs at various solution pH values. When the pH increased from 4.00 to 9.00, the zeta potential of the Fe flocs decreased almost linearly from 42.2 mV to-31.2 mV. At pH 4.00 to 6.00, the zeta potential of the Fe flocs was positive and the decrease of the zeta potential promoted the agglomeration of the iron (hydro) oxide particles, because the electrostatic repulsion between the particles reduced as the zeta potential decreased. This might be one of the reasons for the increase in the content of Fe intercepted as the pH increased from 4.00 to 6.00. At pH 6.00 to 9.00, the zeta potential of the Fe flocs was negative and the decrease of the negative zeta potential raised the electrostatic repulsion between the iron flocs and Mo (Ⅵ) species. This might be why the Mo (Ⅵ) removal decreased sharply when pH increased from 6.00 to 9.00.
In summary, the change of Mo (Ⅵ) removal as the pH increased from 4.00 to 9.00 could be interpreted as follows: As the pH increased from 4.00 to 6.00, the negative-charge shift of Mo (Ⅵ) enhanced the adsorption affinity between Mo (Ⅵ) and the iron flocs. The decrease in the positive surface charge of the iron flocs reduced their adsorption of Mo (Ⅵ) but obviously facilitated the agglomeration of the iron flocs and increased the content of Fe intercepted from water. The combined effect was the obviously enhancement in the removal of Mo (Ⅵ). As the pH increased from 6.00 to 9.00, the electrostatic force between the iron flocs and Mo (Ⅵ) was repulsion, and the increase in the negative surface charge of the iron flocs improved the repulsion between the iron flocs and Mo (Ⅵ), leading to the obviously reduction of Mo (Ⅵ) removal.
3.2 Influence of Co-Existing Background Constituents on Mo (Ⅵ) Removal by FeCl3The presence of co-existing back ground constituents may influence the removal of Mo (Ⅵ) by competing with molybdenum species for adsorption sites, influencing the agglomeration of the iron (hydro) oxides or changing the surface charge of the iron flocs. Different constituents may have different effect and this study examined the influence of sulfate, silicate, phosphate and HA which are commonly present in natural water.
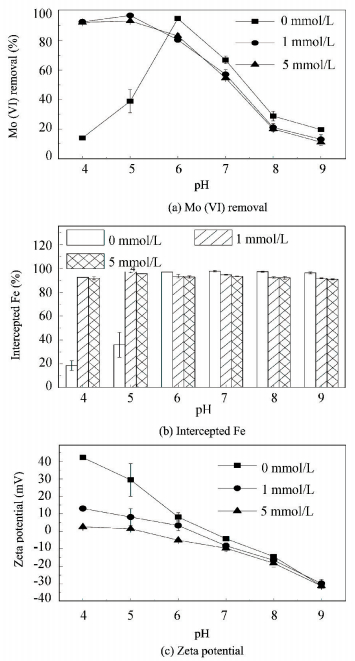
3.2.1 Influence of sulfateFig. 2(a) shows the influence of sulfate on the removal of Mo (Ⅵ) during the pH range of 4.00 to 9.00. The content of Fe intercepted from water and the zeta potential of the iron flocs are also determined as shown in Figs. 2(b) and 2(c), respectively.
|
Figure 2 Effect of sulfate on the removal of Mo (Ⅵ), content of Fe intercepted from water and the zeta potential of the FeCl3 flocs |
It can be seen that the influence of sulfate on the removal of Mo (Ⅵ) had a high dependence on the pH inthe FeCl3 coagulation-filtration process.The removal of Mo (Ⅵ) was greatly increased from 14% to 93% and 93%, separately, in the presence of 1 and 5 mmol/L sulfate at pH 4.00. And at pH 5.00, the removal of Mo (Ⅵ) increased from 39% to 97% and 93%. Both exhibited an obvious facilitating effect, which was different from previous reported studies[30-31]. The enhanced coagulation effect of sulfate might be the reason for its facilitation of Mo (Ⅵ) removal at pH 4.00 and pH 5.00, because small content of Fe were intercepted from water when no sulfate was added to the system; but addition of 1 and 5 mmol/L sulfate caused the interception ratio to increase from 18% to 93% and 92% at pH 4.00, from 36% to 97% and 96% at pH 5.00, as shown in Fig. 2(b). As a negatively charged ion, sulfate could compete with the target ion for adsorption sites if the target ion is also negatively charged [30-31]. Similar effects also occurred in the case of Mo (Ⅵ) removal by FeCl3, which can be seen at pH 6.00 to 9.00. Fig. 2(a) shows that the addition of 1 mmol/L sulfate decreased the removal of Mo (Ⅵ) from 95%, 67%, 29%, 20% to 80%, 57%, 21%, 13%, respectively, at pH 6.00, 7.00, 8.00 and 9.00. In addition, increasing the sulfate concentration from 1 to 5 mmol/L did not further decrease Mo (Ⅵ) removal. Since most of the Fe added to the system in the absence of sulfate had already been intercepted at pH 6.00 to 9.00, the addition of sulfate, either 1 or 5 mmol/L, did not change the content of Fe intercepted. So the decrease in Mo (Ⅵ) removal caused by the presence of sulfate can be attributed to the competitive adsorption between the sulfate and Mo (Ⅵ) species. Additionally, a similar reduction effect in the presence of 1 and 5 mmol/L sulfate on the removal of Mo (Ⅵ) indicated that the mechanisms for the adsorption of sulfate and Mo (Ⅵ) were not completely consistent; otherwise the removal of Mo (Ⅵ) should decrease further when the concentration of sulfate was increased from 1 to 5 mmol/L.
In addition, as seen from Fig. 2(c), at pH 4.00 and 5.00, with addition of sulfate, the zeta potential of the iron flocs decreased substantially from high positive values to nearly zero, which meant that the electrostatic repulsion between the iron (hydro) oxides particles would be much weaker. This might be one of the reasons for the enhanced coagulation effect of sulfate.
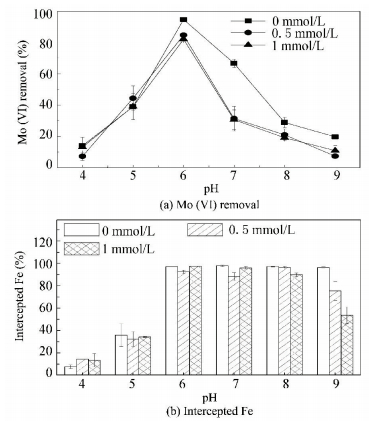
Fig. 3(a) shows the influence of silicate on the removal of Mo (Ⅵ) and experimental results showed that the influence of silicate was strongly dependent on the pH. The presence of either 0.5 or 1 mmol/L silicate did not obviously affect the removal of Mo (Ⅵ) at pH 4.00 and pH 5.00, but greatly decreased Mo (Ⅵ) removal at pH 6.00 to 9.00. The negligible effect at low pH and reduction effect at high pH of silicate on the removal of target contaminant have also been reported by other researchers[31-32]. Two reasons were proposed to explain the more noticeable effect of silicate with increasing pH: (1) more inhibition of further agglomeration of small ferric-hydroxide colloids, which could pass through the 0.45 μm pore-size membrane filters at higher pH[33]; (2) the characteristics of different silicate species at different pHs[34].
|
Figure 3 Effect of silicate on the removal of Mo (Ⅵ)and the content of Fe intercepted from water |
3.2.2 Influence of silicate
Fig. 3(b) shows the content of Fe intercepted from water as a function of pH. The presence of silicate had no obvious influence on the content of Fe intercepted at pH 4.00 to 8.00, so the drop of Mo (Ⅵ) removal in the presence of silicate at pH 7.00 and pH 8.00 should be ascribed to the competition of silicate for adsorption sites. At pH 9.00, the presence of silicate hindered the agglomeration of the iron (hydro) oxides and led to the reduction of the intercepted Fe content. This was consistent with the results reported by Meng et al. in which more than 97% of the iron formed hydroxide precipitation in the presence of 5 mg/L silicate as Si between pH 4.00 and 8.60, but the efficiency decreased to 65% at pH 9.40. So the drop of Mo (Ⅵ) removal at pH 9.00 can be ascribed to the reduction of the intercepted Fe content. In addition, the competition for adsorption sites was also responsible for the drop of Mo (Ⅵ) removal at pH 9.00.
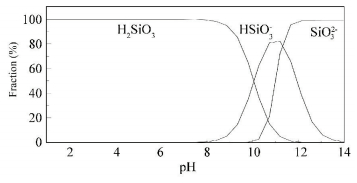
H2SiO3 is the dominant species of silicate at pH 4.00 to 8.00, as shown in Fig. 4, while the dominant species of Mo (Ⅵ) is MoO42- at pH 6.00 to 8.00, but mixture of MoO3, HMoO4- and MoO42- at pH 4.00 and pH 5.00. So the discrepancy between the removal of Mo (Ⅵ) at pH 4.00 to 5.00 and pH 6.00 to 8.00 in the presence of silicate might be due to the change in the chemistry of the Mo (Ⅵ) species. At pH 4.00 to 5.00, the silicate had less affinity for the iron flocs in comparison to the Mo (Ⅵ) species and had a minor effect on the removal of Mo (Ⅵ). While at pH 6.00 to 8.00, the affinity of the silicate for the iron flocs was sufficiently strong to compete with the Mo (Ⅵ) species and led to the reduction of Mo (Ⅵ) removal.
|
Figure 4 Species distribution of silicate with pH |
3.2.3 Influence of phosphate
Pre-determined aliquots of phosphate were added to investigate its influence on the removal of Mo (Ⅵ), as shown in Fig. 5(a). The content of Fe intercepted from water and the species distribution of phosphate at different pHs were also determined as shown in Figs. 5(b) and 6, respectively.
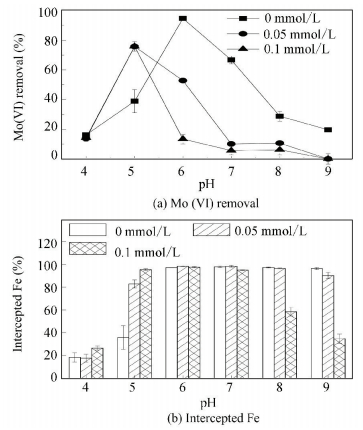
|
Figure 5 Effect of phosphate on the removal of Mo (Ⅵ) and the content of Fe intercepted from water |
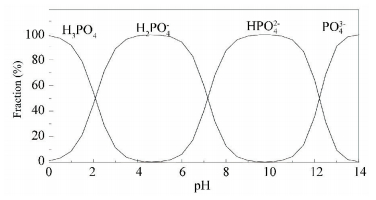
|
Figure 6 Species distribution of phosphate with pH |
At pH 4.00, the presence of phosphate, either 0.05 or 0.1 mmol/L, had a negligible effect on the removal of Mo (Ⅵ). Considering that the content of Fe intercepted from water was not influenced by the presence of phosphate, the negligible effect of phosphate on the removal of Mo (Ⅵ) indicated that H2PO4-, the main species of phosphate at pH 4.00, had a much weaker affinity for the surface of the iron flocs in comparison to the Mo (Ⅵ) or H2PO4- and Mo (Ⅵ) did not share the same adsorption sites. At pH 5.00, the presence of phosphate increased the removal of Mo (Ⅵ) remarkably and this could be attributed to the increase of the intercepted Fe content since more adsorption sites were formed. The facilitating effect of phosphate on the agglomeration of iron (hydro) oxides was also observed by Dong et al. [32]. At pH 6.00 to 9.00, the presence of phosphate had a reducing effect on the removal of Mo (Ⅵ) and this effect became more pronounced as the concentration of phosphate was increased from 0.05 to 0.1 mmol/L. At pH 6.00 and pH 7.00, the sharp decrease of Mo (Ⅵ) removal was due to the competitive adsorption between the phosphate and Mo (Ⅵ) since the content of Fe intercepted did not show a correspondingly change in the presence of phosphate. At pH 8.00 and pH 9.00, the presence of 0.05 mmol/L phosphate had a negligible effect on the content of Fe intercepted and reduced the removal of Mo (Ⅵ) through competition for adsorption sites. But the presence of 0.1 mmol/L phosphate hindered the agglomeration of iron flocs and led to a decrease in Mo (Ⅵ) removal by competition for adsorption sites and by reducing the quantity of the adsorption sites.
3.2.4 Influence of HAFig. 7(a) shows the effect of HA on the removal of Mo (Ⅵ). It was observed that 1 mg/L HA had a negligible effect on the removal of Mo (Ⅵ) over the whole pH range, while the effect of 5 mg/L HA was conspicuous. At pH 4.00 and pH 5.00, 5 mg/L HA increased the removal of Mo (Ⅵ) remarkably. At pH 6.00, the influence of 5 mg/L HA was negligible. And at pH 7.00 to 9.00, the presence of HA decreased the removal of Mo (Ⅵ). Fig. 7(b) shows the effect of HA on the content of Fe intercepted from water at pH 4.00 to 9.00. The facilitating effect of 5 mg/L HA on the removal of Mo (Ⅵ) at pH 4.00 and pH 5.00 could be attributed to the increase in the content of Fe intercepted, which was similar to the case of SO42-. At pH 6.00 and pH 7.00, the presence of 5 mg/L HA had little impact on the content of Fe intercepted and the discrepancy on the removal of Mo (Ⅵ) could be due to the different affinity of HA for the iron flocs. This means that the affinity of HA at pH 6.00 was too weak to compete with Mo (Ⅵ) for adsorption sites while the affinity of HA for the iron flocs at pH 7.00 was strong enough to occupy parts of adsorption sites which would otherwise adsorb Mo (Ⅵ). At pH 8.00 and pH 9.00, the agglomeration of the iron flocs was hindered in the presence of 5 mg/L HA and the decrease of Mo (Ⅵ) removal could be reasonably ascribed to the reduction of adsorption sites. In addition, a competitive effect of HA may also exist, which had been concluded by some researchers under basic pH conditions [19, 31], though the competition of HA with Mo (Ⅵ) for adsorption sites at pH 8.00 and pH 9.00 could not be concluded in this study.
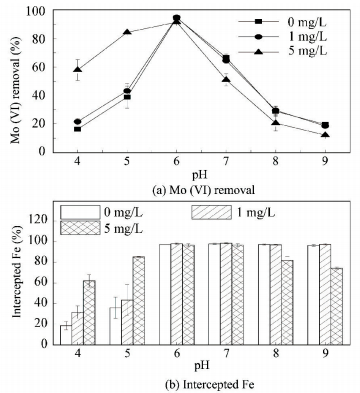
|
Figure 7 Effect of HA on the removal of Mo (Ⅵ) and the content of Fe intercepted from water |
3.3 Application of FeCl3 for Mo (Ⅵ) Removal from Natural Water 3.3.1 Effect of pH on the removal of Mo (Ⅵ) by FeCl3 from natural water
The effect of FeCl3 on the removal of Mo (Ⅵ) from Songhua water was examined and the results are shown in Fig. 8(a).
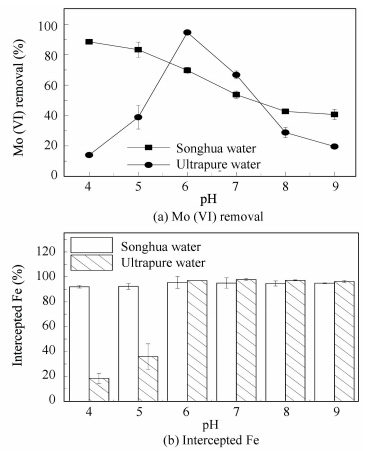
|
Figure 8 Effect of FeCl3 on Mo (Ⅵ) removal from Songhua water and the content of Fe intercepted from water |
It could be seen that the removal ratio of Mo (Ⅵ) from natural water by FeCl3 decreased as the increase of pH during the pH range of 4.00 to 9.00, showing a different tendency as in the synthetic ultrapure water. Seeing from the composition of Songhua water, we can find that the turbidity of the Songhua water was 11.4, which meant there were a lot of particles in the Songhua water. Particles in water can act as the core of the flocs, facilitate the collision of the flocs and act as a bridge between the flocs. So the Songhua water may show a better agglomeration behavior than the ultrapure water. By the way, the particles can also facilitate the coprecipitation effect on the removal of Mo (Ⅵ). What’s more, there are also some co-existing ions in the Songhua water like silicate, phosphate, HA, etc. And these co-existing ions will compete with Mo (Ⅵ) for adsorption sites and lead to the decrease of the Mo (Ⅵ) removal. So the discrepancy of the Mo (Ⅵ) removal between the Songhua water and the ultrapure water could be explained like this: At pH 4.00 and pH 5.00, particles in Songhua water facilitated the removal of Mo (Ⅵ) by facilitating the agglomeration of the iron flocs (as shown in Fig. 8(b)) and enhancing the coprecipitation effect. While some of the co-existing ions decreased the Mo (Ⅵ) removal by competing with the Mo (Ⅵ) for adsorption sites. The facilitation effect preceded, and the removal ratio of the Mo (Ⅵ) in Songhua water was higher than in ultrapure water. At pH 6.00 and 7.00, the intercepted Fe in Songhua water and in ultrapure water were approximately the same. Particles in Songhua water facilitated the removal of Mo (Ⅵ) by facilitating the coprecipitation effect while some of the co-existing ions decreased the Mo (Ⅵ) removal by competing with the Mo (Ⅵ) for adsorption sites. The decreasing effect preceded, so the removal ratio of Mo (Ⅵ) in Songhua water was lower than in ultrapure water. At pH 8.00 and pH 9.00, the effect of particles and co-existing ions in Songhua water was the same as the case at pH 6.00 and 7.00, but the facilitation effect preceded and the removal ratio of Mo (Ⅵ) in Songhua water was higher than in ultrapure water.
3.3.2 Effect of FeCl3 dosage on the removal of Mo (Ⅵ) from natural waterFig. 8(a) shows that in natural water the Mo (Ⅵ) removal ratio at acidic pH was much higher than at neutral pH, which indicated that it was better to adjust the pH of the water to be acidic during the coagulation process in the practical water treatment engineering. Considering that the Mo (Ⅵ) removal ratio at pH 5.00 was only a little lower than at pH 4.00 but much higher than at pH 6.00, to guarantee the high Mo (Ⅵ) removal efficiency and decrease the dosage of pH adjustment agent (NaOH and NaCl, for example), it is better to adjust the pH of the coagulation process to 5.00 in the practical water treatment engineering.
Fig. 9 shows the effect of FeCl3 dosage on the removal of Mo (Ⅵ) from Songhua water at pH 5.00 and pH 7.00. It could be seen that at pH 5.00, the Mo (Ⅵ) removal ratio of 10 mg/L FeCl3 as Fe was 83%, much higher than in the ultrapure water. But the corresponding residual Mo (Ⅵ) was 0.119 mg/L, higher than the permitted value (≤0.07 mg/L) regulated by the “Stands for drinking water quality (GB5749-2006)” (China). When the dosage was increased to 15 mg/L FeCl3 as Fe at pH 5.00, the removal ratio of Mo (Ⅵ) increased to 95%, and the corresponding residual Mo (Ⅵ) was 0.035 mg/L, meeting the “Stands for drinking water quality (GB5749-2006)”. While at pH 7.00 the Mo (Ⅵ) removal ratio was much lower than at pH 5.00, and to meet the demand of the “Stands for drinking water quality (GB5749-2006)”, the FeCl3 dosage should be 25 mg Fe/L at least. So to save the consumption of FeCl3, it is better to operate the coagulation process at pH 5.00 in the practical water treatment engineering.
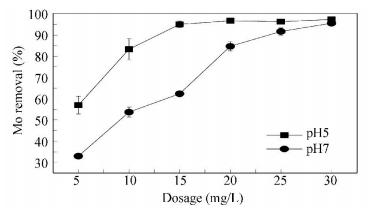
|
Figure 9 Effect of FeCl3 dosage on Mo (Ⅵ) removal from Songhua water |
4 Conclusions
1) The removal of Mo (Ⅵ)was highly dependent on the pH during the FeCl3 coagulation-filtration process. The removal efficiency increased as the pH was increased from 4.00 to 6.00 and decreased as the pH was increased from 6.00 to 9.00, which meant that the optimum Mo (Ⅵ) removal in FeCl3 coagulation-filtration process was achieved at pH 6.00.
2) Reasons for the increase of Mo (Ⅵ) removal when pH increased from 4.00 to 6.00 were the negative charge-shift of the molybdenum species and the increase of the content of Fe intercepted from water. The reason for the decrease of Mo (Ⅵ) removal when the pH was increased from 6.00 to 9.00 was the large drop in the surface charge of the iron flocs.
3) The influence of co-existing background constituents on the removal of Mo (Ⅵ) was determined by two factors: the affinity of co-existing background constituents for the surface of the iron flocs and the influence of co-existing background constituents on the content of Fe intercepted from water. At pH 4.00 and pH 5.00, where the agglomeration of the iron flocs was limited, the content of Fe which could be intercepted from water dominated the removal of Mo (Ⅵ). The presence of sulfate, phosphate (pH 5.00 only) and HA could facilitate the agglomeration of the iron flocs and had a positive effect on the removal of Mo (Ⅵ). At pH 6.00 to 9.00, where the agglomeration of the iron flocs was fully developed, the competition of co-existing background constituents became the dominant factor and all the co-existing background constituents including sulfate, silicate, phosphate and HA showed a reduction effect on the removal of Mo (Ⅵ). Among these, silicate, phosphate and HA could hinder the agglomeration of the iron flocs at certain pHs, while sulfate didn’t exhibit the same characteristic.
4) The Mo (Ⅵ) removal efficiency of FeCl3 in natural water decreased as the pH increased from 4.00 to 9.00, and it was better to operate the coagulation process at pH 5.00 in the practical water treatment engineering.
| [1] |
Sun Y C, Mierzwa J, Lan C R. Direct determination of molybdenum in seawater by adsorption cathodic stripping square-wave voltammetry.
Talanta, 2000 , 52 (3) : 417-424.
DOI:10.1016/S0039-9140(00)00391-X ( 0) 0)
|
| [2] |
Namasivayam C, Sangeetha D. Removal of molybdate from water by adsorption onto ZnCl2 activated coir pith carbon.
Bioresource Technology, 2006 , 97 (10) : 1194-1200.
DOI:10.1016/j.biortech.2005.05.008 ( 0) 0)
|
| [3] |
Xu N, Braida W, Christodoulatos C, et al. A review of molybdenum adsorption in soils/bed sediments:Speciation, mechanism, and model applications.
Soil and Sediment Contamination: An International Journal, 2013 , 22 (8) : 912-929.
DOI:10.1080/15320383.2013.770438 ( 0) 0)
|
| [4] |
Barceloux D G. Molybdenum.
J Toxicol Clin Toxicol, 1999 , 37 (2) : 231-237.
DOI:10.1081/CLT-100102422 ( 0) 0)
|
| [5] |
Reyes E, Jurinak J. A mechanism of molybdate adsorption on α-Fe2O3.
Soil Science Society of America Journal, 1967 , 31 (5) : 637-641.
DOI:10.2136/sssaj1967.03615995003100050010x ( 0) 0)
|
| [6] |
Bostick B C, Fendorf S, Helz G R. Differential adsorption of molybdate and tetrathiomolybdate on pyrite (FeS2).
Environmental Science & Technology, 2003 , 37 (2) : 285-291.
( 0) 0)
|
| [7] |
Xu N, Christodoulatos C, Braida W. Adsorption of molybdate and tetrathiomolybdate onto pyrite and goethite: Effect of pH and competitive anions.
Chemosphere, 2006 , 62 (10) : 1726-1735.
DOI:10.1016/j.chemosphere.2005.06.025 ( 0) 0)
|
| [8] |
Zhang P C, Sparks D L. Kinetics and mechanisms of molybdate adsorption/desorption at the goethite/water interface using pressure-jump relaxation.
Soil Science Society of America Journal, 1989 , 53 (4) : 1028-1034.
DOI:10.2136/sssaj1989.03615995005300040007x ( 0) 0)
|
| [9] |
Gustafsson J P. Modelling molybdate and tungstate adsorption to ferrihydrite.
Chemical Geology, 2003 , 200 (1) : 105-115.
( 0) 0)
|
| [10] |
Wu C H, Lo S L, Lin C F. Competitive adsorption of molybdate, chromate, sulfate, selenate, and selenite on γ-Al2O3.
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2000 , 166 (1) : 251-259.
( 0) 0)
|
| [11] |
Wu C H, Lo S L, Lin C F, et al. Modeling competitive adsorption of molybdate, sulfate, and selenate on γ-Al2O3 by the triple-layer model.
Journal of Colloid and Interface Science, 2001 , 233 (2) : 259-264.
DOI:10.1006/jcis.2000.7223 ( 0) 0)
|
| [12] |
Phelan P J, Mattigod S V. Adsorption of molybdate anion (MoO42-) by sodium saturated kaolinite.
Clays Clay Miner, 1984 , 32 (1) : 45-48.
DOI:10.1346/CCMN ( 0) 0)
|
| [13] |
Motta M M, Miranda C. Molybdate adsorption on kaolinite, montmorillonite, and illite:Constant capacitance modeling.
Soil Science Society of America Journal, 1989 , 53 (2) : 380-385.
DOI:10.2136/sssaj1989.03615995005300020011x ( 0) 0)
|
| [14] |
Goldberg S, Forster H, Godfrey C. Molybdenum adsorption on oxides, clayminerals, and soils.
Soil Science Society of America Journal, 1996 , 60 (2) : 425-432.
DOI:10.2136/sssaj1996.03615995006000020013x ( 0) 0)
|
| [15] |
Barrow N. Comparison of the adsorption of molybdate, sulfate and phosphate by soils.
Soil Science, 1970 , 109 (5) : 282-288.
DOI:10.1097/00010694-197005000-00004 ( 0) 0)
|
| [16] |
Gonzalez B, Appelt H, Schalscha E, et al. Molybdate adsorption characteristics of volcanic-ash-derived soils in Chile.
Soil Science Society of America Journal, 1974 , 38 (6) : 903-906.
DOI:10.2136/sssaj1974.03615995003800060021x ( 0) 0)
|
| [17] |
Afkhami A, Conway B E. Investigation of removal of Cr (Ⅵ), Mo (Ⅵ), W (Ⅵ), V (Ⅳ), and V (Ⅴ) oxy-ions from industrial waste-waters by adsorption and electrosorption at high-area carbon cloth.
Journal of Colloid and Interface Science, 2002 , 251 (2) : 248-255.
DOI:10.1006/jcis.2001.8157 ( 0) 0)
|
| [18] |
Han B, Runnells T, Zimbron J, et al. Arsenic removal from drinking water by flocculation and microfiltration.
Desalination, 2002 , 145 (1) : 293-298.
( 0) 0)
|
| [19] |
Hering J G, Chen P Y, Wilkie J A, et al. Arsenic removal from drinking water during coagulation.
Journal of Environmental Engineering, 1997 , 123 (8) : 800-807.
DOI:10.1061/(ASCE)0733-9372(1997)123:8(800) ( 0) 0)
|
| [20] |
Qin G, McGuire M J, Blute N K, et al. Hexavalent chromium removal by reduction with ferrous sulfate, coagulation, and filtration:A pilot-scale study.
Environmental Science & Technology, 2005 , 39 (16) : 6321-6327.
( 0) 0)
|
| [21] |
Lian J, Xu S, Yu C, et al. Removal of Mo (Ⅵ) from aqueous solutions using sulfuric acid-modified cinder:Kinetic and thermodynamic studies.
Toxicological & Environmental Chemistry, 2012 , 94 (3) : 500-511.
( 0) 0)
|
| [22] |
Afkhami A, Norooz-Asl R. Removal, preconcentration and determination of Mo (Ⅵ) from water and wastewater samples using maghemite nanoparticles.
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009 , 346 (1) : 52-57.
( 0) 0)
|
| [23] |
Moret A, Rubio J. Sulphate and molybdate ions uptake by chitin-based shrimp shells.
Minerals Engineering, 2003 , 16 (8) : 715-722.
DOI:10.1016/S0892-6875(03)00169-9 ( 0) 0)
|
| [24] |
El-Moselhy M M, Sengupta A K, Smith R. Carminic acid modified anion exchanger for the removal and preconcentration of Mo (Ⅵ) from wastewater.
Journal of Hazardous Materials, 2011 , 185 (1) : 442-446.
DOI:10.1016/j.jhazmat.2010.09.052 ( 0) 0)
|
| [25] |
Namasivayam C, Sureshkumar M V. Removal and recovery of molybdenum from aqueous solutions by adsorption onto surfactant-modified coir pith, a lignocellulosic polymer.
CLEAN-Soil, Air, Water, 2009 , 37 (1) : 60-66.
DOI:10.1002/clen.v37:1 ( 0) 0)
|
| [26] |
Verbinnen B, Block C, Hannes D, et al. Removal of molybdate anions from water by adsorption on zeolite-supported magnetite.
Water Environment Research, 2012 , 84 (9) : 753-760.
DOI:10.2175/106143012X13373550427318 ( 0) 0)
|
| [27] |
Rietra R P, Hiemstra T, Riemsdijk van W H. The relationship between molecular structure and ion adsorption on variable charge minerals.
Geochimica et Cosmochimica Acta, 1999 , 63 (19) : 3009-3015.
( 0) 0)
|
| [28] |
Jones L. The solubility of molybdenum in simplified systems and aqueous soil suspensions.
Journal of Soil Science, 1957 , 8 (2) : 313-327.
DOI:10.1111/ejs.1957.8.issue-2 ( 0) 0)
|
| [29] |
Ferreiro E, Helmy A, Bussetti de S. Molybdate sorption by oxides of aluminium and iron.
Zeitschrift für Pflanzenernhrung und Bodenkunde, 1985 , 148 (5) : 559-566.
DOI:10.1002/(ISSN)1522-2624 ( 0) 0)
|
| [30] |
Qiao J, Jiang Z, Sun B, et al. Arsenate and arsenite removal by FeCl3: Effects of pH, As/Fe ratio, initial As concentration and co-existing solutes.
Separation and Purification Technology, 2012 , 92 : 106-114.
DOI:10.1016/j.seppur.2012.03.023 ( 0) 0)
|
| [31] |
Guan X, Dong H, Ma J, et al. Removal of arsenic from water: Effects of competing anions on As (Ⅲ) removal in KMnO4-Fe (Ⅱ) process.
Water Research, 2009 , 43 (15) : 3891-3899.
DOI:10.1016/j.watres.2009.06.008 ( 0) 0)
|
| [32] |
Dong H, Guan X, Wang D, et al. Individual and combined influence of calcium and anions on simultaneous removal of chromate and arsenate by Fe (Ⅱ) under suboxic conditions.
Separation and Purification Technology, 2011 , 80 (2) : 284-292.
DOI:10.1016/j.seppur.2011.05.007 ( 0) 0)
|
| [33] |
Laky D, Licsko I. Arsenic removal by ferric-chloride coagulation-effect of phosphate, bicarbonate and silicate.
Water Science & Technology, 2011 , 64 (5) : 1046-1055.
( 0) 0)
|
| [34] |
Dong H, Gao B, Yue Q, et al. Effect of Fe (Ⅲ) species in polyferric chloride on floc properties and membrane fouling in coagulation-ultrafiltration process.
Desalination, 2014 , 335 (1) : 102-107.
DOI:10.1016/j.desal.2013.12.018 ( 0) 0)
|
 2016, Vol. 23
2016, Vol. 23


