2. College of Life Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
The traditional prevention and control of harmful microorganisms in the environment mainly relies on using chemical pesticides. Long-term use of chemical pesticides has resulted in many problems, such as the appearance of pathogenic microorganisms that have the ability to resist such chemical pesticides, and environmental pollution and human health damage[1-3]. As the “Green Economy” and “Green GDP” theory have been proposed[4-5], the prevention and control of harmful microorganisms has fundamentally changed. Researchers have started to focus on ecological environment self-regulation ability. Ecological prevention and control was proposed to prevent and control harmful microorganisms. In the view of ecological prevention and control, occurrence of plant disease was regarded as an imbalance of biological and non-biological factors in the ecological system. So the ecological prevention and control of plant disease are the process of restoring the interacton balance and keeping the amount of the pathogenic microorganisms and hazard within a threshold value, which ensure the plant ecosystem’s healthy development[6]. This plant disease prevention and control method, breaking away from the traditional control plant disease concept, pays more attention to the initiative of the plant itself, the interaction between plants and the environment, plant micro ecology environment of dynamic balance, and the safety of the ecological environment.
Adjusting soil pH value and fertilizing organic microbial fertilizer are two effective ecological prevention and control measures of plant disease[7-12].When at the optimal value for plant growth, plant disease can be prevented, but when at the optimal value for phytopathogen, it increases risk of soil-borne plant disease. For example, when the soil pH value of the cruciferous crops is 5.7, the optimal pH value for growth, the plant disease is more severe than in other soil pH value condition. In another case, when the soil pH value is less than 5.2 for potato plants, potato scabs caused by Streptomyces scabies is rarely form.For most plant disease, the soil pH value mainly influences the growth of phytopathogen, but it also affects the change of certain nutrients in the soil.At the same time, different crops have varied the optimum growth pH values[13].So the soil pH value can affect the occurrence and development of plant disease in many ways.Organic microbial fertilizer is an organic combination of organic matter and beneficial microorganisms.The beneficial microorganisms can inhibit the growth of phytopathogen in many ways to reduce the number of pathogens in the soil.The beneficial microorganisms compete nutrition and niche with phytopathogen, and some of them can also produce antimicrobial substances, which are what mainly inhibit the growth of phytopathogen[14].In practical production processes, utilizing lime power to increase soil pH value and fertilizing organic microbial fertilizer to improve soil disease-suppressive ability, which can achieve the effective prevention and control of plant disease, especially prevent and control soil-borne disease[15]. Kelp residue microbial fertilizer has characteristics of kelp residue and bio-control bacteria. Kelp residue has a lot of organic matter and alkali metal ions that promote plant growth and increase soil pH value.Bio-control bacteria can be used as a plant disease control agent[16]. So this research is to study the eco-control effect of kelp residue microbial fertilizer on increasing soil pH value and suppressive ability, as eco-control agent to control pathogenic fungus Aspergillus parasiticus.
2 Materials and Methods 2.1 Materials 2.1.1 Kelp residue microbial fertilizerThe bio-control Bacillus amyloliquefaciens strain Hitwh-BA2, which has a broad spectrum against the growth of pathogenic fungi and has the ability to produce plant growth hormone 6-furfurylamino purine (6-KT), was inoculated into 20 mL of glucose and yeast powder (GY) liquid medium (20 g/L glucose and 5 g/L yeast powder), shaken at 150 r /min and incubated at 35 ℃ for 12 h. Kelp residue was crushed down first (d≤0.5 mm). Glass bottles were used for fermentation. Every bottle contained 100 g of fermentation substrate (37.4 g kelp residue, 2.1 g sucrose, 0.5 g KNO3 and 60 mL deionized water, nature pH value), sterilized for use. 4 mL of GY inoculation liquid was inoculated into each glass fermentation bottle, incubated at 35 ℃ for 8.25 d.
2.1.2 Pathogenic fungusA norsolorinic acid(NA) mutant of Aspergillus parasiticus 95 was used throughout this study.This A. parasiticus 95 cannot produce aflatoxin, but accumulates the first stable orange-pigmented precursor, NA, in the biosynthetic pathway of aflatoxin production, with an optimal growth pH value of about 6[17-19]. Peanuts (Huayu 17, which is sensitive to A. parasiticus 95 infection) were used for pot experimentation.
2.1.3 Culture mediumGY solid medium: 20 g/L glucose, 5 g/L yeast powder, 20 g/L agar; dichloran-glycerol (DG-18) medium: 5.0 g/L peptone, 10.0 g/L glucose, 1.0 g/L potassium dihydrogen phosphate and 0.01 g/L zinc sulfate, 0.5 g/L magnesium sulfate, 0.005 g/L copper sulfate, 0.002 g/L chloride ammonium nitrate, 0.05 g/L chloramphenicol, 0.05 g/L chlortetracycline hydrochloride, 15 g/L agar.
2.2 Methods 2.2.1 Effect of kelp residue microbial fertilizer on increasing soil pH value and soil suppressive activityAcidic soil and alkaline soil were used in this study. Acidic soil was cultivated in the lab room with a pH value of 4.30, while alkaline soil was cultivated in a field with a pH value of 7.60. The experiment was designed with two groups, namely, acidic soil treated with kelp residue microbial fertilizer and alkaline soil treated with kelp residue microbial fertilizer. Every pot contained 700 g of soil. There were four different types of treatments based on the amount of kelp residue microbial fertilizer used: 0 g, 10 g, 20 g and 30 g. Three pots were used for each treatment. The pots were kept under glasshouse condition, and 200 mL of deionized water was poured into each pot every 3 d. The soil pH value and soil suppressive ability were determined after 60 d and 90 d. The soil pH value was determined by the following methods: taking soil samples from every treatment; air-drying the soil samples; grinding the soil with mortar; sieving the soil (with a 0.15 mm sieve); taking 10 g of soil into two 50 mL beakers respectively, and adding 25 mL of distilled water, shaking for 3 min, leaving at rest for 1 min, and then determining the pH value with a pH meter. The method of using tip-culture to determine the soil leachate’s ability to counter the growth of A. parasiticus 95 was used to determine the soil suppressive ability. The process began by pouring 300 mL of deionized water to each pot and collecting the soil leachate from the tray under the pot after 12 h; 20 mL soil leachate was collected for every treatment; the leachate samples were separated by centrifugation and then filtered with microporous membrane filter; and the tip-culture (700 μL cell free soil leachate + 100 μL GY liquid +5 μL A. parasiticus 95 spores suspension) was used to determine the soil leachate’s ability to counter the growth of A. parasiticus 95.
2.2.2 To verify the effect of kelp residue microbial fertilizer on increasing the soil suppressive ability with peanut pot testCultured soil was taken from the farm and cleaned stones and other objects. Every pot was filled with 700 g of soil. The experiment was designed with three groups, namely, pot fertilized with 20 g of kelp residue microbial fertilizer, those with 20 g of kelp residue fermention medium, and those with neither the kelp residue microbial fertilizer nor the kelp residue fermention medium, which was the control group (CK). Each treatment had three replicates. Once the pots were treated, 5 peanuts were planted into each pot. The soil suppressive ability was determined after 70 d.
2.2.3 Effect of kelp residue microbial fertilizer on increasing peanut yield and inhibiting Aspergillus parasiticus 95 infectionFurther studies were performed on the effect of fertilizing kelp residue microbial fertilizer on promoting peanut yield and inhibiting A. parasiticus 95 infection under the condition of the culture soil inoculated with A. parasiticus 95. The experiment was designed with two groups: one group with drought treatment, and the other one without drought treatment. Each group had four treatments, fertilizing 0 g, 10 g, 20 g, 30 g kelp residue microbial fertilizer respectively, and every treatment had three replicates. Cultured soil was taken from the farm and cleaned stones and other objects. Then the soil was inoculated with A. parasiticus 95 to make one gram soil contain 1×104 spores. Each pot was filled with 2.1 kg of soil, and then fertilized with kelp residue microbial fertilizer. Pots were arranged according to the randomized block design. 10 peanuts were planted into each pot, and peanut seedlings were cut off to leave 4 seedlings. In the mature period, the induced infection treatment group was treated under drought condition for 30 d.
The amount of A. parasiticus 95 in peanut geocarposphere soil was determined with DG-18 medium by taking 10 g of soil sample, diluting it with sterile water in ten time dilution method, taking 100 μL into a plate with DG-18, spreading the liquid, and incubating it at 28 ℃ for 3-4 d. The amount of A. parasiticus 95 was counted by counting the orange spots. Each treatment had two replicates.
The infection rate of A. parasiticus 95 was determined by selecting 10 seed pods from every treatment, washing any soil off with water, sterlizing the surface with 75% ethanol. And washing them 5 times with sterile water. The shells and seeds were separated, and the seeds were split in half, one half was put into a plate with DG-18 medium, each dish had 10 halves and each treatment had 3 replicates. The dishes were incubated at 28 ℃ for 6 d, and the amount of A. parasiticus 95 was counted by counting the number of orange spots.
Statistical analysis was performed by using the SPSS program. Data were analyzed by one-way analysis of variance(ANOVA), and significance was set at P < 0.05.
3 Results and Discussion 3.1 Effect of Kelp Residue Microbial Fertilizer on Soil pH and Soil Suppressive ActivityThe effects of kelp residue microbial fertilizer on soil pH and soil suppressive activity were studied (as shown in Fig. 1). Results are shown in Table 1, which indicate that fertilizing 20 g of kelp residue microbial fertilizer not only has increased the alkaline soil pH significantly, but also improved alkaline soil suppressive activity significantly; fertilizing 20 g kelp residue microbial fertilizer has increased the acidic soil pH significantly, but only fertilizing 40 g kelp residue microbial fertilizer has improved the acidic soil suppressive ability obviously, with pH value of about 6.60.
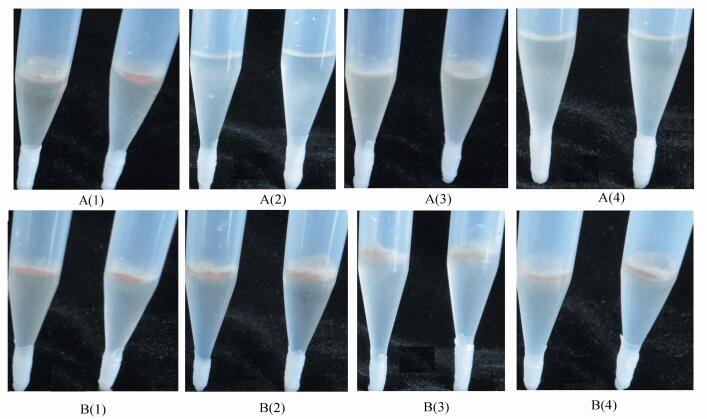
|
Figure 1 To determine soil suppressive activity with tip-culture method, to determine the soil supressive ability after 60 d |
| Table 1 Eco-preventive effect of kelp residue microbial fertilizer |
Both plant pathogenic microorganism and plants have an optimal growth pH. For example, the optimal growth pH of rice, wheat, and corn are 6.0-7.5, 6.0-7.5, 6.0-7.0, respectively[20]; and clubroot of cruciferous vegetables is severe in acidic soil. So lime powder and sulfur powder are used to adjust soil pH to control some kinds of plant disease. The pH of soil is not conducive to the occurrence of a disease, but it is conducive to plant growth. Thus, this reciprocal combination can be used to improve plants’ resistance to diseases. The conventional organic microbial fertilizer and organic compost can produce organic acids in the metabolic process, so it is also used in alkaline soil regulation. But kelp residue contains large amounts of alkali metal ions[21], so kelp residue microbial fertilizer can be used for acid soil adjustment, increasing soil pH value.
The peanut pot test was performed to verify the effect of kelp residue microbial fertilizer on improving soil suppressive ability. The results are shown in Table 2, which indicate that fertilizing kelp residue microbial fertilizer (20 g) has improved the cultivation soil suppressive ability significantly. And the difference between fertilizing kelp residue microbial fertilizer and fertilizing kelp residue fermentation medium is also extremely significant. Also, the soil suppressive ability from fertilizing kelp residue microbial fertilizer is 37.52% higher than that of the control (CK), while the soil suppressive ability from fertiliting kelp residue fermentation medium is 13.95% higher than the control.
| Table 2 Effect of kelp residue microbial fertilizer on soil suppressive ability |
3.2 Effect of Kelp Residue Microbial Fertilizer on Peanut Yield
Fertilizing organic microbial fertilizer or organic compost can reduce the amount of chemical fertilizer, and has the ability to adjust soils physical and chemical properties. It is conducive to recovery, enriching the type and amount of microbes in the soil, achieving micro ecological balance in the soil, promoting the growth of crops, and so on. The peanut yield was determined, and results are shown in Table 3, which indicate that fertilizing kelp residue microbial fertilizer has increased the peanut yield. The treatment (C3), fertilizing 20 g kelp residue microbial fertilizer with drought treatment, has increased the peanut yield by 24.71% compared with the treatment C1 without kelp residue microbial fertilizer. And the treatment (D3), fertilizing 20 g kelp residue microbial fertilizer, has increased the peanut yield by 7.43% compared with the treatment (D1) without kelp residue microbial fertilizer.
| Table 3 Effect of kelp residue microbial fertilizer on peanut yield |
3.3 To determine the Amount of Aspergillus parasiticus 95 in Peanut Geocarposphere Soil
DG-18 medium was used to determine the amount of A. parasiticus 95 in peanut geocarposphere soil (as shown in Fig. 2). Results show that the amount of A. parasiticus 95 in the C1 treatment is different from C2, C3, and C4 significantly; and the differences among C2, C3 and C4 are not significant; D1 treatment is different from D2, D3, D4 respectively significantly, and the differences among D2, D3 and D4 are not significant (as shown in Table 4).

|
Figure 2 To separate Aspergillus parasiticus 95 with DG-18 medium, the Aspergillus parasiticus 95 colony with orange colour |
| Table 4 Effect of kelp residue microbial fertilizer on the amount of Aspergillus parasiticus 95 |
3.4 Aspergillus Parasiticus 95 Infected Peanut
The effect of fertilizing kelp residue microbial fertilizer on reducing A. parasiticus 95 infection rates was also studied. Results are shown in Table 5. Results indicate that drought treatment has increased the A. parasiticus 95 infection rate; the infected peanut shell rate about C1 has increased by 142.86% compared with D1; the infected peanut kernel rate about C1 is 6.67%, while D1 experiences no infection. It is obvious that fertilizing kelp residue microbial fertilizer is effective in reducing the A. parasiticus 95 infection rates.
| Table 5 Aspergillus parasiticus 95 infecting peanuts among treatmentsCFU |
4 Conclusions
Kelp residue microbial fertilizer has the characteristics of kelp residue and biocontrol bacteria, which can be used to increase soil pH value, promote plant growth, and control plant soil borne disease. In this study, fertilizing kelp residue microbial fertilizer has increased the soil pH and soil suppressive ability significantly. The peanut potting experiment also proves that fertilizing kelp residue microbial fertilizer not only has improved the yield of peanuts significantly, but also has reduced the amount of A. parasiticus 95 in the peanut geocarposphere soil significantly. Thus, kelp residue microbial fertilizer has a good application prospect of ecological prevention and control of plant disease.
| [1] |
Faretra F, Pollastro S. Genetic basis of resistance to benzimidazole and dicarboximide fungicides in Botryotinia fuckeliana(Botrytis cinerea).
Mycological Research, 1991 , 95 : 943-951.
DOI:10.1016/S0953-7562(09)80091-9 ( 0) 0)
|
| [2] |
Zheng Xiaolan, Fu Shuai, Zheng Fucong, et al. Research advance on Fungicide resistance on plant pathogens.
Chinese Journal of Tropical Agriculture, 2011 , 31 (1) : 86-90.
( 0) 0)
|
| [3] |
Zheng Yongquan. Development and prospects of the research on pesticide residue.
Plant Protection, 2013 , 39 (5) : 90-98.
( 0) 0)
|
| [4] |
Fang Shijiao. Taking a new look at the development of low-carbon economy from the perspective of green economy.
China Population, Resources and Environment, 2010 , 20 (4) : 8-11.
( 0) 0)
|
| [5] |
Zhu Dajian. New concept and trend of green economy emerging from Rio+20.
China Population, Resources and Environment, 2012 , 22 (9) : 1-6.
( 0) 0)
|
| [6] |
Xie Lianhui, Lin Qiying, Xu Xuerong. Plant disease economy and ecologic management of plant diseases.
Journal of China Agricultural University, 2005 , 10 (4) : 39-42.
( 0) 0)
|
| [7] |
Schmidt C S, Agostini F, Simon A M, et al. Influence of soil type and pH on the colonisation of sugar beet seedlings by antagonistic Pseudomonas and Bacillus strains, and on their control of Pythium damping-off.
European Journal of Plant Pathology, 2004 , 110 : 1025-1046.
DOI:10.1007/s10658-004-1600-y ( 0) 0)
|
| [8] |
Liang Jun, Zhang Xingyao. Ecological control of forest pest: A new strategy for forest pest control.
Journal of Forestry Research, 2005 , 16 (4) : 339-342.
DOI:10.1007/BF02858204 ( 0) 0)
|
| [9] |
Fang X L, You M P, Barbetti M J. Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil Ph, soil organic amendments and crop rotation.
European Journal of Plant Pathology, 2012 , 134 : 619-629.
DOI:10.1007/s10658-012-0042-1 ( 0) 0)
|
| [10] |
Falardeau J, Wise C, Novitsky L, et al. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens.
Journal of Chemical Ecology, 2013 , 39 : 869-878.
DOI:10.1007/s10886-013-0319-7 ( 0) 0)
|
| [11] |
Ye Yunfeng, Fu Gang, Miu Jianhua, et al. Advances in application of ecological control techniques for plant diseases.
Guangxi Agricultural Science, 2009 , 40 (7) : 850-853.
( 0) 0)
|
| [12] |
Yu Fengjuan. Study on soil parasitic nematodes and its ecological control countermeasures.
Northern Horticulture, 2013 , 5 : 181-185.
( 0) 0)
|
| [13] |
Geogrge N A.
Plant Pathology. Beijing: China Agricultural Univeristy Press, 2009 : 243 .
( 0) 0)
|
| [14] |
Loon van L C. Plant responses to plant growth-promoting rhizobacteria.
European Journal of Plant Pathology, 2007 , 119 : 243-254.
DOI:10.1007/s10658-007-9165-1 ( 0) 0)
|
| [15] |
Xiao Wei, Jeong Sujing. Ecological prevention and control of plant disease in the process of vegetable production.
Journal of Changjiang Vegetables, 2019 , 9 : 50-51.
( 0) 0)
|
| [16] |
Xiao Wei, Yan Peisheng. Inhibitory activity against soil pathogenic fungi of kelp residue as additive in microbial fertilizer.
Journal of Plant Nutriton and Fertilizer, 2014 , 20 (3) : 778-782.
( 0) 0)
|
| [17] |
Yabe K, Nakamura H, Ando Y, et al. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method.
Applied and Environmental Microbiology, 1988 , 54 (8) : 2096-2100.
( 0) 0)
|
| [18] |
Yan P S, Song Y, Sakuno E, et al. Cyclo (L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus.
Appllied and Environmental Microbiology, 2004 , 70 : 7466-7473.
DOI:10.1128/AEM.70.12.7466-7473.2004 ( 0) 0)
|
| [19] |
Yan P S, Gao X J, Wu H Q, et al. Isolation and screening of biological control bacterial strains against Aspergillus parassiticus from groundnut geocarposphere.
Journal of Earth Science, 2010 , 21 : 309-311.
DOI:10.1007/s12583-010-0245-3 ( 0) 0)
|
| [20] |
Zheng Yingze. The effect of soil pH on crop growth.
Journal of Yuxi Teachers College (Natural Science Edition), 1994 , 10 (314) : 64-67.
( 0) 0)
|
| [21] |
Zhang Junji, Duan Rui, Zhao Yushan, et al. An acidic soil modifier and its application: China, 101935532A[P].2011-01-05.
( 0) 0)
|
 2016, Vol. 23
2016, Vol. 23


