2. School of Chemical Engineering & Technology, Harbin Institute of Technology, Harbin 150001, China
Polyaniline (PANI) has been extensive research due to their combined properties of organic polymers and electronic semiconductors[1-6]. Recently, much attention has been focused on the synthesis and characterization of nano-polymer composite materials for the enhanced sensing and electrocatalytic activity used in light emitting diodes, batteries, corrosion inhibition, medicine, photonics, sensors etc.[7-12]. Furthermore, among the most promising electrode material, the polyaniline composite is considered to be the best because of its high accessible surface area, low resistance and high environmental stability[13-15].
However, up to now there are very few reports exhibiting the structuring ordered nano-PANI materials that many improved properties (high conductivity, high mobility) will be expected from these ordered structures[16-17]. The possible reason is that PANI is difficult to form a uniform film. What is more, conductivity of the PANI composite membrane is low[18-20].
In order to solve these problems, a new synthesis method was reported in this work to prepare the nanostructure PANI-based membrane, which possesses unique advantages such as: (i) ordered porous structure greatly increases the diffusion and reaction speed, which makes it suited as an electro-catalyst support, (ii) more proton migrating channels can be produced, which improve response speed and reversibility of the sensors, (iii) an inductive behavior of PANI film can great improves its electrical conductivity.
In addition, polymer membrane is traditionally cured by heating treatment. However, the heating treatment usually leads to a decreased aperture of molecular imprinted. In order to hold the aperture, the curing treatment technology developed in this work is to use UV light. In this paper, we doped nano-PANI into molecular imprinted acrylate polymer membrane, which revealed an inductive behavior and pH sensitivity. The actual porous structure morphology and characteristic were determined using physical and electrochemical methods.
2 Experiment 2.1 Synthesis of Nano-PANIAniline (0.057 mol/L) was added into an aqueous solution (250 mL) mixed with polyvinyl pyrrolidone (PVP, 0.001 mol/L) and dodecylbenzene sulfonic acid (DBSA, 0.5 mol/L). The other aqueous solution (50 mL) of ammonium persulfate (APS, 0.143 mol/L) was then poured into the above solution stirring at 1 000 r/min for 24 h (IKA RW 20 digital). This operation sequence was at 0 ℃. Yet the non-PANI aqua was prepared by the same procedure without using aniline.
2.2 Phase Diagram Building for Microemulsion SystemDifferent ratio of methyl methacrylate (MMA) and acrylic acid (AA) were used to prepare a series of solutions and kept at 30 ℃ for 24 h. Nano-PANI aqua was then slowly added into the above solutions by microburet. When the solution changed from transparent to turbid, it is the point of the phase diagram. The non-PANI systematic phase diagram was made by the same procedure but used non-PANI aqua instead.
2.3 Preparation of Nano-PANI/Acrylate Gel Polymer (PAGP) MembraneThe microemulsion system consisted of MMA, AA, nano-PANI aqua, EGDMA (ethylene glycol dimethacrylate) and BP (benzophenone), and the weight ratio of them were fixed at 3:7:5.5:0.3:0.2. Among them, EGDMA and BP were used as cross-linker and initiator of the polymerization, respectively. The solution was stirred by ultrasonic vibration for 10 min to ensure well-distributed and then purged with nitrogen for 20 min to remove the oxygen. This microemulsions were injected into the 800 μm gap of two cleaned glass slides, and then were exposed to UV-light (λ= 365 nm, intensity 17 W/cm) at 0 ℃ for 1.5 h. As a comparison, the acrylate gel polymer electrolyte membranes were prepared by the identical procedures but using non-PANI aqua instead.
2.4 PAGP Membrane Characterization MeasurementThe surface morphology of the PAGP membrane was studied using scanning electronic microscopy (SEM), performed on a JSM-6330F scanning electron microscope (JEOL Ltd.). The nano-PANI particlessize' was observed by a tapping-mode using Solver Nano Atomic Force Microscopy from NT-MDT Co. The A four-point probe (FPP) was employed to measure the electronic conductivity of the PAGP membrane at 25 ℃ under atmosphere.
2.5 Electrochemical Characteristic MeasurementElectrochemical tests were conducted in a three-electrode cell. A 20×50×0.5 mm aluminum sheet was used as the working electrode. A 10×10×0.3 mm platinum foil and a saturated Hg/HgSO4 electrode (MSE) were employed as the counter and reference electrodes, respectively. Prior to measurement, the aluminum sheet was finely polished by SiC sandpaper and then was activated in 10.0% H2SO4 solution.
Electrochemical impedance spectroscopy (EIS) was performed on an electrochemical workstation Model Ref. 600 (Gamry Inc.). An ac potential (sine wave) with amplitude of 15 mV was used as imposing signal, and the measurement frequency range was set from 0.005 to 10 kHz. Defined sample area of 1.0 cm2 was exposed to the electrolyte. All electrode potentials were referred to saturated MSE, if not otherwise stated. The solution comprised of 0.5 mol/L H2SO4 at 25 ℃. The voltammogram was recorded and the scan rate was kept at 15 mV/s all the time.
2.6 pH Sensitive Characteristic MeasurementThe pH sensitive characteristic of the PAGP membrane was studied by color change and electronic conductivity measurement. The PAGP membrane was immersed in an aqueous solution with different pH for 1 min. The color change was noted, and electronic conductivity was investigated by four-point probe.
3 Results and Discussion 3.1 Microemulsion System CharacteristicThe ternary phase diagram of MMA, AA and nano-PANI aqua or non-PANI aqua is shown in Fig. 1. In this phase diagram, region D is multiphase, and the other region is single-phase microemulsion. The single-phase region can be further divided into three regions, W/O microemulsion, bicontinuous microemulsion and O/W microemulsion, marked with A, B, C in the image, respectively. Their corresponding structures are illustrated in Fig. 1(b).

|
Figure 1 Ternary phase diagram of microemulsion system made of MMA/AA/water at 25 ℃ containing either nano-PANI or not |
Although all the weight ratio of MMA, AA and nano-PANI aqua in region B can be used to prepare PAGP membranes in theory, the microemulsion region of nano-PANI aqua system is smaller than that of non-PANI aqua system due to that PANI particles result in partitioning at the interface. Besides, the addition of moderate PANI is favorable to form transparent and thermodynamically stable dispersion system of oil and water. It is a network structure, which can be used to prepare the controllable open pore polymer materials. The obtained MMA /AA/PANI latex is highly stable and no precipitation occurs. However, at high PANI content (e.g., MMA:AA:nano-PANI aqua=3:7:5.5), nano-PANI particles will reach the percolation threshold, and they tend to aggregate and collapse to globular structure. This decreased stability of microemulsion usually makes membranes preparation more difficult [21-23].
3.2 Morphology of Nano-PANI Doped Acrylate Gel Polymer MembraneThe SEM and AFM micrograph of PAGP membranes are displayed in Figs. 2 and 3.
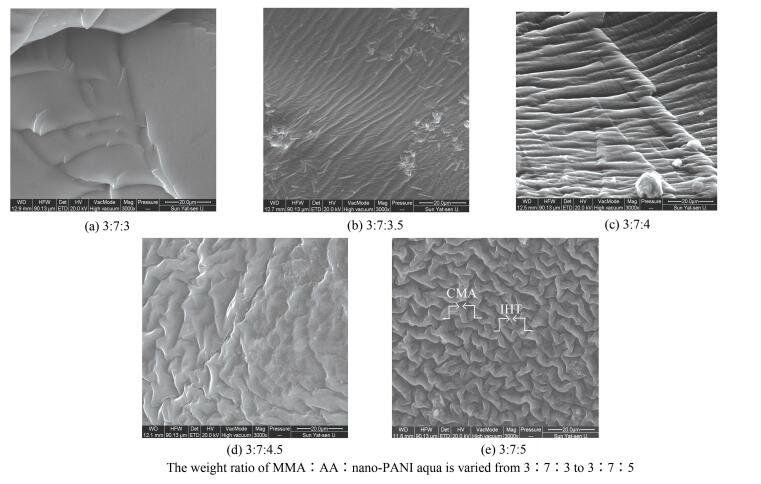
|
Figure 2 SEM micrograph of PAGP membrane |
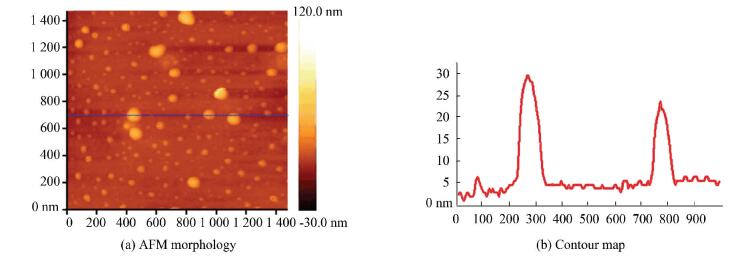
|
Figure 3 AFM morphology of nano-PANI particles on the polymer surface and contour map |
According to filled-emission SEM investigation, the resulting polymer matrix displays a unique morphology comprising of two types of proton migrating tunnels: interconnect hydrophilic trench (IHT) and continuous amphiphilic matrix (CAM). In Fig. 2(a), the IHT region can be hardly seen, while the CAM region is obvious. With the increase of content of nano-PANI aqua, the IHT region becomes more and more clearly (Figs. 2(b) and 2(c)). When the weight ratio of MMA:AA:Nano-PANI aqua is fixed at 3:7:4.5, the IHT and CAM region are clearly interconnected into a three dimensions grain-boundary structure. The grains ( < 1 μm) are primarily composed of PMMA and PAA, while the boundary region will be governed by EGDMA and BP, because such morphology is casted by the bicontinuous microemulsion. Intrinsic phase structures of the bicontinuous microemulsion are favorable of interconnection of the most hydrophilic channels in the polymer matrix. Moreover, increased nano-PANI can lead to an increase in water channels density. From the images as shown in Fig. 2(e), it can be clearly seen that PAA and PMMA hydrogels have a well-defined and interconnected 3D porous network (about 1 μm).
Compared to the porous morphology of PAA and PMMA hydrogel, lots of nano-PANI particles of the average size of 10 nm are on the surface of composite hydrogel (Fig. 3). No nano-PANI precipitation is obtained after the composite hydrogel is washed by a lot of distilled water for a long time. It shows that the nano-PANI particles are firmly embedded into the PAA and PMMA hydrogel and cannot leak out from the pore.
The twisted and interconnected network can supply electron migrating tunnels and promote the electrical conductivity and sensitivity.
3.3 Electrochemical Behavior of PAGP MembranesThe cyclic voltammograms are shown in Fig. 4(a) to explore the conversion process of PANI membrane at aluminum electrode. Two pairs anodic and cathodic peaks appearance labeled as A/A′-B/B′, corresponding to the different transition processes of PANI membrane. The peaks A (0.2 V) rises from the forming of benzoquinone (BQ) as the hydrolysis product[22-23]. The peaks B (0.5 V) is attributed to the transformation of emeraldine to pernigraniline. The reverse scan appears peak A′ at-0.1 V and a broad current peak at 0.13 V (B′). The transitions of different oxidation states reveal that the PANI composite membrane are reversible reaction process, which is greatly applicable as electrochemical capacitor and sensor.
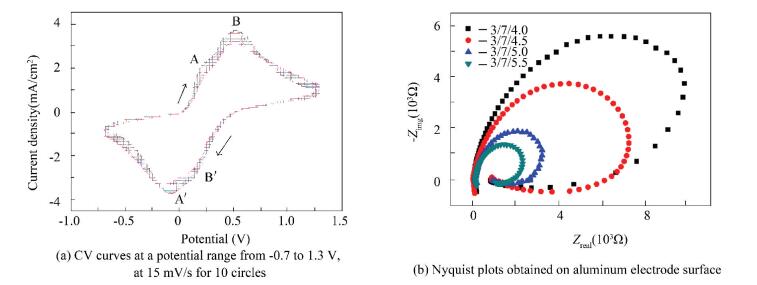
|
Figure 4 Electrochemical behavior measurement of PAGP membrane |
In addition, the amplify of the peak intensity with sweeps number is attribute to the growth of conducting polymer in each cycle.
Electrochemical behavior of PAGP membrane was measured. The Nyquist plots on aluminum electrode surface are recorded in Fig. 4(b). It can be seen that there is a capacitive loop at high frequency, which can attribute to charge and discharge of the electric double layer formed by aluminum electrode and electrolyte.
A significant phenomenon can be found from Fig. 4(b). An impedance decrease in the real part and an impedance increase in the imaginary part are simultaneously observed in the low-frequency region. Such impedance response in the low-frequency region reveals an inductive behavior of PANI membrane, which is possible for the contribution of the faradaic pseudocapacitance of the polymer materials, since PANI behaves close to a capacitor in the highly positive potential range[15, 24-25].
In the present study, the possible occurrence of simultaneous redox reactions for PANI (emeraldine to pernigraniline conversion) and BQ (conversion of BQ/HQ transportations) can develop an inductive response, which is agreement with the analysis from Fig. 4(a). The inductive behaviors can greatly improve the electrical conductivity of composite membrane.
3.4 pH Sensitive Performance of PAGP MembranesThe color change and electronic conductivity of the PAGP membranes with different pH are investigated as shown in Figs. 5 and 6.

|
Figure 5 Color change of PAGP membrane with different pH |
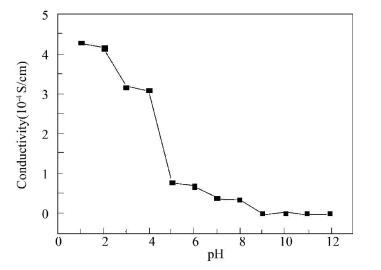
|
Figure 6 Electrical conductivity of PAGP membrane for different pH |
We can divide pH into three sections according to the measurement. Firstly, when the value of pH is within 1.0-4.0, the PAGP membranes is green, and the conductivity is greater than 3.0×10-4S/cm, which possess high conductivity. After that, when pH is 5.0-8.0, the conductivity decreases greatly and the PAGP membranes change to blue. Finally, the experiment results show that when the value of pH is within 9.0-12.0, PAGP membranes are insulator, and the purple membranes are observed.
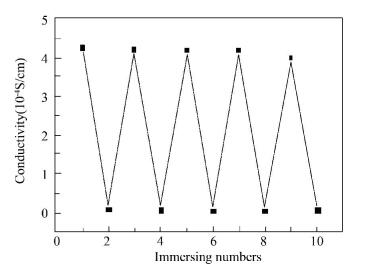
In order to survey the stability of pH sensitive behavior, the PAGP membrane is immersed in pH=1 and pH=12 aqueous solutions again and again for 1 min. The color and electrical conductivity change of the PAGP membrane show even more regularity in Figs. 7 and 8. In an acidic medium (pH 1.0), a green colored precipitate is obtained. In alkaline (pH 12.0) medium, a purple precipitate appears with poorer conductivity.

|
Figure 7 Color change of PAGP membrane immersing repeatedly in the aqueous solution for pH=1 and pH=12 |
|
Figure 8 Electrical conductivity of PAGP membrane immersing repeatedly in the aqueous solution for pH=1 and pH=12 |
The regular changes of color and conductivity are attributed to the interaction between PANI and dopant. PANI has two basic structures: a fully oxidized pernigraniline base (EB) and a half oxidized/half reduced emeraldine base (ES) state, as shown in Fig. 9. The ES has high conductivity, while EB is insulated. When PANI is doped with different dopant, its structure will transform between EB and ES, which will change PANI conductivity. The alteration is attribute to a polaron discussed through the hopping mechanism[26-28].

|
Figure 9 Sketch of polyaniline protonation |
When the PANI thin film is put in alkaline medium, alkaline take protons from PANI, which leads to the resistance increasing. It is a dedoping (deprotonation) process, shown in Fig. 9. Hence for the pernigraniline (EB), the electronic environments of all the nitrogens along the polymer chain are similar, which retrains the polaron formation. Accordingly, the purple insulating membrane is obtained.
On the contrary, in an acidic medium (pH 1.0), the PANI gets protons and varies enormously the doping structure. Therefore, the ES transition improves the conductivity and a green precipitate received. Above all, the pH sensitivity is reversible. In addition, there are two chief factors to influence the conductivity performance of PANI membrane. (i) surface morphologies of PANI nano-composite film, (ii) doping ratio and type of the dopant[26, 30].
(i) Surface morphologies of PAGP membranes. The SEM and AFM micrographs of PAGP membrane have been measured. The interconnected 3D porous network are shown in Figs. 2 and 3. The uniform porous structure contributes to decrease the response time and improve the reversibility of the sensors. The reason is that the porous structures provide more migrating tunnels to make the diffusion and reaction between ions and the film more easy.
Accordingly, the microstructure of the PAGP membranes is in good agreement with the sensitivity. Thus in this study, it is shown that the sensitive response is determined not only by the character of the polymer film, but also by the morphology of the sensitive film.
(ii) Doping ratio and type of the dopant. The doping is the most important elements for high conductivity achievement. Different type of the dopant will determine the protonation of the PANI. When the PANI is in emeraldine base, 50% doping will result in the protonation of the entire quinoid ring, which will form perfect polaron leading to a high attainable conductivity. Hence, the conductivity is improved with the increase of doping ratio. These results accord with those in Figs. 6 and 8.
4 ConclusionsIn an effort to prepare gel acrylate polymer membrane, nano-polyaniline (PANI) is successfully dispersed into methyl methacrylate (MMA) and acrylic acid (AA) microemulsions. It is found that MMA and AA can serve as a frame containing porous structure and channels, and nano-PANI particles is doped into those channels. Electrical conductivity and pH sensitivity can be promoted due to the twisted structural and interconnected hydrophilic channels. EIS results point out impedance changes reveal an inductive behavior to PANI film. These results suggest the PAGP membranes can be as an electro-catalytic materials with controllable open pores.
| [1] |
Liu C J, Hayashi K, Toko K. Electrochemical deposition of nanostructured polyaniline on an insulating substrate.
Electrochemistry Communications, 2010, 12(1): 36-39.
DOI:10.1016/j.elecom.2009.10.030 ( 0) 0)
|
| [2] |
Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries.
Nature, 2001, 414(6861): 359-367.
DOI:10.1038/35104644 ( 0) 0)
|
| [3] |
Kulkarni M V, Kale B B. Studies of conducting polyaniline (PANI) wrapped-multiwalled carbon nanotubes (MWCNTs) nanocomposite and its application for optical pH sensing.
Sensors and Actuators B, 2013, 187(10): 407-412.
( 0) 0)
|
| [4] |
Sydulu B S, Palaniappan S, Srinivas P. Nano fibre polyaniline containing long chain and small molecule dopants and carbon composites for supercapacitor.
Electrochim Acta, 2013, 95(15): 251-259.
( 0) 0)
|
| [5] |
Seki S, Kobayashi Y, Miyashiro H. Fabrication of high-voltage, high-capacity all-solid-state lithium polymer secondary batteries by application of the polymer electrolyte/inorganic electrolyte composite concept.
Chemistry of Materials, 2005, 17(8): 2041-2045.
DOI:10.1021/cm047846c ( 0) 0)
|
| [6] |
Stejskal J, Sapurina I, Trchova M. Polyaniline nanostructures and the role of aniline oligomers in their formation.
Progress in Polymer Science, 2010, 35(12): 1420-1481.
DOI:10.1016/j.progpolymsci.2010.07.006 ( 0) 0)
|
| [7] |
Ji S Z, Li Y, Yang M J. Gas sensing properties of a composite composed of electrospunpoly (methyl methacrylate) nanofibers and in situ polymerized polyaniline.
Sensors and Actuators B, 2008, 133(2): 644-649.
DOI:10.1016/j.snb.2008.03.040 ( 0) 0)
|
| [8] |
Li L, Yan G P, Wu J Y, e al. Preparation of polyaniline-metal composite nanospheres by in situ microemulsion polymerization.
Journal of Colloid and Interface Science, 2008, 326(1): 72-75.
DOI:10.1016/j.jcis.2008.07.023 ( 0) 0)
|
| [9] |
Zhao J, Wang Z, Wang J X, et al. High-performance membranes comprising polyaniline nanoparticles incorporated into polyvinylamine matrix for CO2/N2 separation.
Journal of Membrane Science, 2012, 403/404(1): 203-215.
( 0) 0)
|
| [10] |
Wang X C, Lia Y, Zhao Y, et al. Synthesis of PANI nanostructures with various morphologies from fibers to micromats to disks doped with salicylic acid.
Synthetic Metals, 2010, 160(17): 2008-2014.
( 0) 0)
|
| [11] |
Zhang H F, Huang F L, Xu S L, et al. Fabrication of nanoflower-like dendritic Au and polyaniline composite nanosheets at gas/liquid interface for electrocatalytic oxidation and sensing of ascorbic acid.
Electrochemistry Communications, 2013, 30(5): 46-50.
( 0) 0)
|
| [12] |
Virji S, Huang J, Kaner R B, et al. Polyaniline nanofiber gas sensors: Examination of response mechanisms.
Nano Letters, 2004, 4(3): 491-496.
DOI:10.1021/nl035122e ( 0) 0)
|
| [13] |
Valiavalappil S, Harinipriya S. Electrically conducting nylon 6, 6-polyaniline short composite fibres synthesised by the solvent coagulation method.
Synthetic Metals, 2012, 162(23): 2027-2032.
DOI:10.1016/j.synthmet.2012.10.008 ( 0) 0)
|
| [14] |
Cindrella L, Kannan A M. Membrane electrode assembly with doped polyaniline interlayer for proton exchange membrane fuel cells under low relative humidity conditions.
Journal of Power Sources, 2009, 193(2): 447-453.
DOI:10.1016/j.jpowsour.2009.04.002 ( 0) 0)
|
| [15] |
Zhao X Y, Zhang J B, Wang Y H, et al. Electropolymerizing polyaniline on undoped 100 nm diamond powder and its electrochemical characteristics.
Electrochemistry Communication, 2009, 11(6): 1297-1300.
DOI:10.1016/j.elecom.2009.04.029 ( 0) 0)
|
| [16] |
Xian Y Z, Liu F, Feng L J, et al. Nanoelectrode ensembles based on conductive polyaniline/poly (acrylic acid) using porous sol-gel films as template.
Electrochemistry Communication, 2007, 9(4): 773-780.
DOI:10.1016/j.elecom.2006.11.017 ( 0) 0)
|
| [17] |
Sun L, Shi Y C, He Z P, et al. Synthesis and characterization of SnO2/polyaniline nanocomposites by sol-gel technique and microemulsion polymerization.
Synthetic Metals, 2012, 162(24): 2183-2187.
DOI:10.1016/j.synthmet.2012.10.004 ( 0) 0)
|
| [18] |
Cho M S, Cho Y H, Choi H J, et al. Synthesis and electrorheological characteristics of polyaniline-coated poly (methyl methacrylate) microsphere: Size effect.
Langmuir, 2003, 19(14): 5875-5881.
DOI:10.1021/la026969d ( 0) 0)
|
| [19] |
Verma D, Dutta V. Role of novel microstructure of polyaniline-CSA thin film in ammonia sensing at room temperature.
Sensors and Actuators B, 2008, 134(2): 373-376.
DOI:10.1016/j.snb.2008.05.009 ( 0) 0)
|
| [20] |
Xiong H M, Zhao X, Chen J S. New polymer-inorganic nanocomposites: PEO-ZnO and PEO-ZnO-LiClO4 films.
The Journal of Physical Chemistry B, 2001, 105(43): 10169-10174.
( 0) 0)
|
| [21] |
Fadda P, Monduzzi M. Solid lipid nanoparticle preparation by a warm microemulsion based process: Influence of microemulsion microstructure.
International Journal of Pharmaceutics, 2013, 446(1): 166-175.
( 0) 0)
|
| [22] |
Wang Y, Shi Y C, Xu X K, et al. Preparation of PANI-coated poly (styrene-co-styrene sulfonate) nanoparticles in microemulsion media.
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 345(1): 71-74.
( 0) 0)
|
| [23] |
Mo C, Li X. Microstructure and structural transition in coconut oil microemulsion using semidifferential electroanalysis.
Journal of Colloid and Interface Science, 2007, 312(2): 355-362.
DOI:10.1016/j.jcis.2007.03.030 ( 0) 0)
|
| [24] |
Pashley R M, Rzechowicz M, Pashley L R, et al. De-gassed water is a better cleaning agent.
Journal of Physical Chemistry B, 2005, 109(3): 1231-1238.
DOI:10.1021/jp045975a ( 0) 0)
|
| [25] |
Chen W C, Wen T C, Teng H S. Polyaniline-deposited porous carbon electrode for supercapacitor.
Electrochim Acta, 2003, 48(6): 641-649.
DOI:10.1016/S0013-4686(02)00734-X ( 0) 0)
|
| [26] |
Bhadra S, Khastgir D, Singha N K, et al. Progress in preparation, processing and applications of polyaniline.
Progress in Polymer Science, 2009, 34(8): 783-810.
DOI:10.1016/j.progpolymsci.2009.04.003 ( 0) 0)
|
| [27] |
Kiattibutr P, Tarachiwin L, Ruangchuay L, et al. Electrical conductivity responses of polyaniline films to SO2-N2 mixtures: Effect of dopant type and doping level.
Reactive & Functional Polymers, 2002, 53(1): 29-37.
( 0) 0)
|
| [28] |
Luthra V, Singh R, Gupta S, et al. Mechanism of dc conduction in polyaniline doped with sulfuric acid.
Current Applied Physics, 2003, 3(2): 219-222.
( 0) 0)
|
| [29] |
Tai H L, Jiang Y D, Xie G Z, et al. Fabrication and gas sensitivity of polyaniline-titanium dioxide nanocomposite thin film.
Sensors and Actuators B, 2007, 125(2): 644-650.
DOI:10.1016/j.snb.2007.03.013 ( 0) 0)
|
| [30] |
Bhadra S, Chattopadhyay S, Singha N, et al. Improvement of conductivity of electrochemically synthesized polyaniline.
Journal of Applied Polymer Science, 2008, 108(1): 57-64.
DOI:10.1002/(ISSN)1097-4628 ( 0) 0)
|
 2017, Vol. 24
2017, Vol. 24


