2. State Key Lab of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China;
3. School of Municipal and Environmental Engineering, Shandong Jianzhu University, Jinan 250010, China;
4. Huainan Capital Water Co., Ltd., Huainan 232003, Anhui, China
Dissolved air flotation (DAF) is an effective particle-separation process, especially for suspended solids, algae and organic matters. Although practical application of DAF starts relatively late in drinking water treatment, it has become a matured technique and is being accepted as an alternative to sedimentation after decades of development[1-2]. Its unique advantages in low temperature and low turbidity raw water treatment have been also confirmed in our previous studies[3-4]. Over the last ten years, researches and applications of DAF in seawater treatment have been springing up all over the world. For instance, the influences of seawater chemistry on coagulation performance and micro-bubble flotation efficiency were examined by Edzwald[5-6] and the subject of "DAF for desalination pretreatment" were elaborated in his monograph as a separate chapter[7]. The impacts of salinity on bubble size, bubble bed depth and particle separation rate were also analyzed by other research groups[8]. Meanwhile, DAF is prevalently adopted as a pretreatment unit of seawater desalting system to minimize fouling of the subsequent membrane modules. And more high speed DAF with loading rates of 30-45 m/h is widely used in newly-built seawater reverse osmosis (SWRO) plants, including the world's largest SWRO plant with a capacity of 1 010 000 m3/d located at Ras Al Khair in Saudi Arabia[9]. In recent years, two large scale desalination plants have been established in China also employing DAF pretreatment, Baifa-Qingdao and Caofeidian-Tangshan.
DAF-membrane processes exhibit both rapid development and bright prospects in seawater and brackish water treatment. However, in surface water clarification, previous researches were mainly focused on the conventional coagulation/sedimentation-membrane process[10-11]. Few data are available for the coupling technique of DAF upstream of membrane filtration. Therefore, the primary purpose of this study was to evaluate the performance of DAF combined with ultrafiltration membranes in coping with the micro-polluted Huai River waters. During the 11th Five-Year Plan period, a national demonstration water treatment project was completed and put into service in July 2012 via the upgrade of a traditional waterworks along Huai River. New DAF facility was built with a feed flow of 20 000 m3/d as pretreatment ahead of ozone-based advanced oxidation and bio-filter processes. And this DAF unit was also acted as pretreatment of the pilot scale ultrafiltration membranes in this study. There were two different combination modes for them: ⅰ) DAF combined with the hollow-fiber ultrafiltration membrane (UFM) directly; ⅱ) DAF in conjunction with the up-flow membrane biological filter (MBF) realized by adding multilayer filter media between DAF and submerged curtain ultrafiltration membrane. The second combination form is based on the bio-filter process of the demonstration project which plays a crucial role in ammonia nitrogen and organic matters removal. However, there may be a risk of microbes leaking out from the biofilter due to its flourishing biofilm ecosystem and also the upward flow direction. Thus, the strategy of this work was not only to assess the elimination efficiency of typical contaminants in combined DAF-membrane systems, but also to concern with the bacterial index in membrane permeates.
2 Materials and Methods 2.1 Water QualityThe Huai River locates in the center of China, between the Yangtze and the Yellow River. Many important energy industry bases and major grain producing areas lie along Huai River Valley. The population density of the Huai River Basin also ranks first among the major rivers in China. All of these factors exert an adverse impact upon the surface water quality. Raw waters for experiments were pumped from the Huai River directly using the same water intake pipe with waterworks. Table 1 provides an overview of the basic information on feed water quality during the experimental period. The raw water was characterized by the irregular high concentration of ammonia nitrogen, organic matter and also occasional turbidity spikes, which presented great challenges to conventional water treatment processes. DAF was able to remove 25%-30% CODMn and 35%-40% UV254 of raw water, and their average contents in DAF effluent were 2.79 mg/L and 0.069 cm-1 respectively. Residual ammonia was on average 0.79 mg/L, which represents nearly 15% removal efficiency. Turbidity after DAF was generally maintained at 2.4 NTU and values as low as 0.8 NTU were even achieved. Meanwhile, a 88% reduction in suspended solid particles( > 2 μm) was easily attainable. The detailed description of DAF efficiency under various raw water conditions has been covered in previous study[12] and the emphasis in this research was mainly on the performances of two different types of membrane modules utilized as post-treatment process.
| Table 1 Quality parameters of raw water and DAF effluents (June 2013-March 2014) |
2.2 Experimental Set-up
An ultrafiltration hollow fiber membrane module (Model UA860, MANN+HUMMEL, Germany), hydrophilic modified polyacrylonitrile (PAN) material, 0.025 μm pore-size, dead-end filtration was used to remove particle pollutants from DAF effluents as shown in Fig. 1. The nominal flux rate of this membrane cartridge, 0.23 m in diameter and 1.65 m in height, was 44 L/(m2·h) subjected to its effective surface area of 45 m2. Prior to entering the membrane vessel, DAF effluent was firstly through a bag filter. One operational procedure of this system comprised three steps: 30 min filtration, 45 s air scouring (4.2 m3/h of air flow) and finally 45 s backflush cleaning (3-4 m3/h of water flow). In addition, the transmembrane pressure (TMP) was consistently maintained below 30 kPa during this experiment.
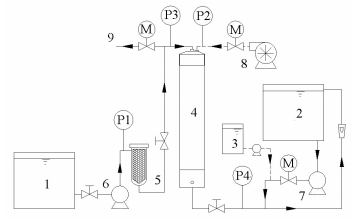
|
1.DAF effluent tank; 2.Product/Backflush tank; 3.Dosing tank; 4.Membrane module; 5.Guard-filter bag; 6.Feed pump; 7.Backflush pump; 8.Rotary air blower; 9.Drain outlet Figure 1 Schematic diagram of the DAF-UFM system |
A stainless steel MBF reactor (length 1.2 m, width 0.8 m, height 4.7 m) was designed and operated (Fig. 2). It consisted primarily of two functional partitions: membrane and fluidized biological filtration bed. A curtain type ultrafiltration membrane module (Yeanovo Co., Ltd. Guangzhou, China), polyvinylidene fluoride (PVDF) material, 0.03 μm pore-size was fixed at top of the reactor keeping a given submerged depth. The total effective filtration area of this membrane module was nearly 168 m2 corresponding to its nominal filtrate flux of 39 L/(m2·h). The multilayer filter media suspending at lower part included a granular activated carbon (GAC) layer based on bituminous coal (thickness: 1.3 m, 20×30 mesh) and also a natural zeolite layer (thickness: 0.7 m, 15×25 mesh) beneath. The upward filtration rate of multilayer media was about 7.8 m/h. Moreover, V-shaped baffles were installed in a middle position to avoid the contact between upper membrane module and lower biofilm media.
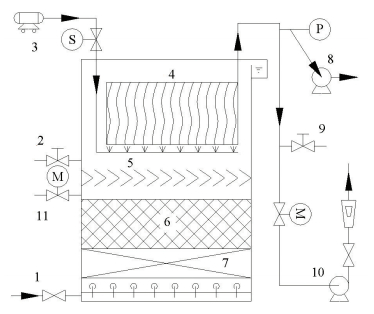
|
1.DAF effluents; 2.Sampling point after multilayer media; 3.Air pump; 4.Membrane module; 5.V-shaped baffle; 6.Granular activated carbon layer; 7.Zeolite layer; 8.Vacuum pump; 9.Sampling point for membrane permeates; 10.Suction pump; 11.Drain outlet for media backwash Figure 2 Schematic diagram of the DAF-MBF system |
DAF effluent of waterworks was pumped into the bottom of reactor and evenly distributed via filter caps. Bubble aeration pipes were laid under the membrane module and compressed air was supplied by an air pump to provide shearing force along the membrane surface. The ultrafiltration process was operated intermittently following a preset procedure (e.g., 9 min filtration and 1 min pause) and hydraulic backflushing was implemented at 24 h intervals with membrane filtrated water. In addition, the multilayer filter media was flushed nearly every two weeks using air scrubbing and subsequent water backwashing.
2.3 Materials and Analytical MethodsAll the reagents used were of analytical grade purchased from Sinopharm Chemical Reagent Co., Ltd. except for the poly-aluminum chlorides (PAC, Al2O3 content 30.5%) used as coagulants in coagulation/flocculation upstream of DAF produced by Gongyi Fuyuan Water Purification Material Co., Ltd.
UV254 was determined by DR5000 UV-Vis spectrophotometer (Hach, USA) after filtration through 0.45 μm membrane. The pH was measured using a pHS-3C pH meter (Leici, China), calibrated before using. Turbidity was measured by a HACH 2100N turbidimeter. Particle count was detected by VersaCountTM Particle Analyzer (IBR, USA), which could distinguish the particle size between 2 μm and 200 μm into eight intervals: 2-3, 3-5, 5-7, 7-10, 10-15, 15-20, 20-25, and >25 μm. Total bacterial count and total coliforms were quantified using plate count agar (PCA) and membrane filtration method respectively. Other parameters were analyzed following the standard methods recommended by APHA[13].
3 Results and Discussion 3.1 Performances of DAF-UFM System 3.1.1 Conventional contaminants controlAs shown in Fig. 3(a), the UF membrane was able to reduce CODMn by 6.4% on average, but had no effect on UV254 reduction from DAF effluents levels. Turbidity of membrane permeates remained close to 0.1 NTU, with an average value of 0.12 NTU. No bacterial or very few colony ( < 3 CFU/mL) was observed for filtrated water. Besides, no positive case of coliforms was detected throughout the tests.
The membrane removed approximately 10.7% of ammonia-nitrogen (NH3-N) accompanied with nitrite-nitrogen (NO2-N) increase, and yet on obvious change of nitrate-nitrogen (NO3-N) (Fig. 3(b)). The raising of NO2-N may be attributed to the following reasons: i) in order to achieve a high water production rate, a great quantity of hollow fibers was tightly crowded in the limited space of membrane column. Even frequent air/water backwash cannot guarantee the complete rooting out of microorganisms from membrane vessel offering favorable conditions for bacterial blooms and dead-end filtration made the situation worse; ii) DAF effluent was rich in dissolved oxygen (e.g., 2.58-3.96 mg/L in summer and 4.83-7.52 mg/L in winter), which improved the nitrogen metabolism of aerobic bacteria intercepted in membrane housing. Part of ammonia was converted into nitrite by ammonia oxidizing bacteria. However, the nitrifying bacteria were less active than the former due to its more sensitive behavior to ambient conditions, which ultimately led to the NO2-N accumulation in membrane cartridge.
In the combined process of DAF and UFM, beyond 70% of the daily NH3-N content in membrane permeates was higher than 0.5 mg/L and cannot conform to the regulatory limits of China National Standards for Drinking Water Quality (CNSDWQ GB5749-2006). On the other hand, hydrophobic fractions of aquatic humic-matters with high molecular weight could be effectively removed in the upstream DAF process according to the adsorptive fractionation behaviors of natural organic matters at bubble surfaces found in our previous research[14]. The residual organic carbons in DAF effluents were mainly composed of low molecular weight and hydrophilic components whose retention ratio via ultrafiltration alone was low. These factors resulted in most of the daily measurements of CODMn were higher than 2.7 mg/L. The chemical safety of membrane product water was still an issue.

|
Figure 3 Residual CODMn, turbidity and removal of NH3-N, NO2-N and NO3-N in UFM |
3.1.2 Development of UFM fouling
After nearly two month's operation, the negative effects of bacterial bloom on ultrafiltration efficiency were beginning to emerge. In this section, the membrane performances during two typical chemical cleaning intervals were discussed in detail. With the running time coursing, membrane operation pressure gradually rose, while permeate flow decreased steadily (Fig. 4). Major measures adopted to retard membrane fouling could be divided into two categories: self-restoring and human intervention. The membrane self-restoring methods (SRM) specifically referred to the 45 s air scouring and subsequent 45 s water backflushing after every 30 min filtration following the preset procedures via a PLC controller. And the human intervention measurements (HIM) in this test generally included compulsory physical washing (CPW) at 8:00 each morning, chemically enhanced backwash (CEB) with low concentration chemicals at 17:00 almost daily and typical chemical cleaning (NCC) nearly every month.
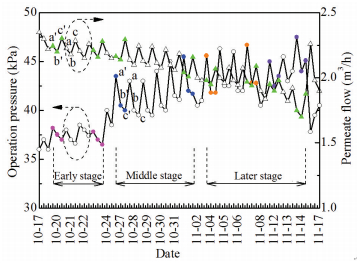
|
Figure 4 Change of membrane operation pressure and permeate flow |
Each three dots group (e.g., a, b, c or a′, b′, c′) between two vertical dashed lines represented the same day's system parameters before CPW (a), after CPW (b) and after CEB (c) respectively. The difference between c′ of the day and a of the next day indicated the effects of SRM, because there was no man-made factor except for automatic air/water backflush during night operating. In preliminary stage, SRM exhibited excellent capability of operation parameters restoration. But the effect was weakened continuously as the extending of filtration time. During the middle and later phase, SRM became nearly invalid. For example, the operation pressure increment of 0-0.9 kPa and permeate flow decrement of 0-0.08 m3/h at early stage respectively ascended to 2-3.5 kPa and 0.09-0.17 m3/h at terminal period of this production cycle.
Meanwhile, influences of HIM on system parameters can be readily obtained from comparison of each three dots group's change tendency at different periods. In term of the permeate flow, a, b and c of each days mainly showed an accordant pattern of "√", while for the operation pressure, it experienced a change profile of "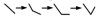
The MBF reactor started up in July 2013. In this section the performances of DAF-MBF process from mid-October to late November 2013 were elaborated as the biofilm matured and the system operation at a steady state.
3.2.1 Organic matters removalAs illustrated in Fig. 5, CODMn of DAF effluents generally maintained below 3 mg/L and only a few daily test data exceeded that. In MBF reactor, its value reduced to nearly 2 mg/L after the treatment of multilayer media and membrane can further remove the residual CODMn by 0.1-0.2 mg/L. The average removal of 33.9% CODMn was obtained by MBF with a maximum value up to 45.7% and the biological filtration contributed primarily 86.7% of the total removal efficiency.
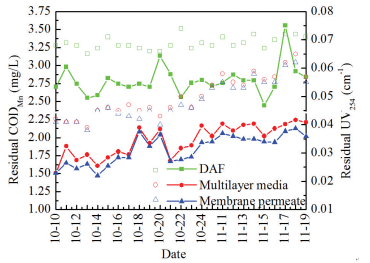
|
Figure 5 Residual CODMn (solid lines) and UV254 (scatter dots) |
UV254 of DAF effluents was varied in the range of 0.065-0.075 cm-1 and a removal efficiency of 31.6% can be accomplished in MBF. The removal rate of UV254 via multilayer media accounted for 95.1% of total value, while the ultrafiltration module was less effective. GAC is one of the most efficient and economically feasible processes in dissolved organic matters (DOM) elimination, especially for the low and intermediated molecular weight DOM residual in DAF effluent. This fraction of DOM could readily diffuse and absorb onto the GAC particles and its assimilation is also favorable for biofilm proliferation [15]. However, it should be noted that after several months running the removal efficiency of UV254 presented a decline from 37.3% to 33.1% after the middle of November as the feed water temperature dropped below 10 ℃. One reason may be regarded as the fall of water temperature interfering with the biodegradation function of biofilm, which is in agreement with the results from previous studies[16-18]. In addition, other researches found that some humic-like microbial extracellular polymeric substances (EPSs) in MBR exhibited a characteristic absorbance around 254 nm, the accumulation of which may also exert anegative effect on UV254 reduction in long-term operation [19-21].
3.2.2 Sequential removal of NH3-NPeriodic high concentration of NH3-N is a typical characteristic of the Huai River water. Although ammonia can be reduced in the upstream coagulation-DAF pretreatment to a certain extent, its content is still undesirable. It can be seen from Fig. 6, more than 50% of the daily DAF effluent NH3-N measurements was higher than 0.5 mg/L and sometimes the measured value even can reach 2.5 mg/L. In DAF-MBF process, a significant removal of 86.9% NH3-N was achieved and 95.1% of which was undertaken by the multilayer media. After that, ammonia steeply dropped to 0.1 mg/L. The mechanism may be due to the cooperation between ion exchange in the zeolite layer and biological nitrification process in the upper BAC layer. The large cation exchange capacity of zeolite media could weaken the shock loadings of ammonia and provide mild conditions for nitrogen assimilation by attached bacteria in GAC surfaces. Besides, the suspended nitrifying bacteria were conducive to zeolite regeneration.
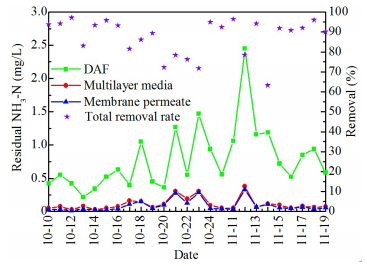
|
Figure 6 Removal of NH3-N in DAF-MBF system |
Ammonia conversion to nitrate is the dominant path in biological nitrogen cycling and there was no NO2-N accumulation in MBF reactor (Fig. 7). The DAF effluent NO2-N of 0.05-0.16 mg/L was reduced to less than 0.01 mg/L in membrane permeates. In addition, we didn't find remarkable denitrification effect in MBF as other researchers previously observed[20-21]. This phenomenon may be triggered by the extremely low carbon to nitrogen ratio (mass) of DAF effluents with an average value of only 0.7 which was significantly lower than the recommended C/N ratio around 2.0 for drinking water denitrification using MBR [20-21]. Moreover, the first consumption of readily biodegradable organic matters by aerobic heterotrophic bacteria at the bottom of the multilayer media aggravated this circumstance. Ultimately, the shortage of carbon-source hindered the development of denitrification process.

|
Figure 7 NH3-N, NO2-N and NO3-N transformation in MBF |
3.2.3 Enhanced solid particles separation
The MBF performed efficiently in turbidity elimination with an average removal efficiency of 94.9%. Turbidity of membrane permeates was always less than 0.15 NTU and beyond 60% of the daily measurements were below 0.1 NTU (Fig. 8). DAF is one of the most efficient processes in particles separation, especially for "pin point" particles with size of 10 μm similar to micro-bubbles diameter[22]. A laser based particle counter was applied to determine the particle size distribution in DAF-MBF system. DAF achieved a nearly 90% removing of particles( > 10 μm) and particle counts in DAF effluents was about 7×103CNT/mL, 96% of which were composed of 2-10 μm particles. Membrane reduced particle numbers to 190 CNT/mL and the minimum value was only 70 CNT/mL. In comparison with DAF, particles( > 10 μm) were almost completely intercepted by membrane filtration and the percentage of 2-3 μm particles also decreased by 18%. The particle counting results demonstrated that there was strong complementarity between DAF and ultrafiltration in particulate contaminants control.
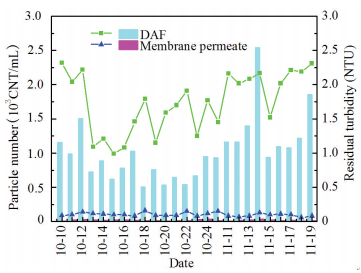
|
Figure 8 Change of turbidity (solid lines) and > 2 μm particle number (columns) |
3.2.4 UF membrane: A reliable barrier for microbes
Total bacterial count (TBC) of DAF effluents generally varied from tens to hundreds CFU/mL (Fig. 9). Although high biomass concentrations attainable in MBF reactor, bacterium-free membrane filtrates could be obtained. Even though bacterial colony was detected occasionally, its highest number was only 7 CFU/mL. Besides, no coliform was founded in membrane permeates throughout the experiment. The microbiological testing data suggested that membrane module had a high rejection rate against microbes in DAF-MBF system. The introduction of curtain UF membrane at the top of biofilter not only ensured abundant biomass for biodegradation functions, but also eliminated the risk of microorganism leakage.
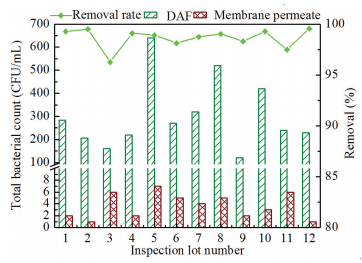
|
Figure 9 Removal of total bacteria count by curtain UF membrane |
3.2.5 A slowdown in membrane fouling rate
Fig. 10 shows the variation of membrane flux as a function of running days in MBF. The transmembrane pressure (TMP) was maintained below 30 kPa constantly. At the initial stage, the permeate flux remained stable and followed by a small decrease. After two months operation, it declined by 2.93% from 38.20 to 37.08 L/(m2·h). In the third month, the decrement rate further rose to 3.25%, manifesting a slightly accelerated fouling rate. The membrane fouling development could be explained by the varying growth phases of biofilm in MBF. Early running days, microbes were in adaptive lag phase both biomass and their released metabolites were relatively low and solid particles were the main foulants for membrane fouling. DAF efficiently lowered the particulate pollutants loads on the subsequent MBF and mitigated the membrane fouling. Moreover, in this period hydraulic backwash could effectively relieve the pore blockage and also had a pronounced effect on membrane productivity recovery. However, after the bacteria stepping into the maximal stationary phase, quantities of EPSs (e.g., soluble and loosely/tightly bounded types) were released as the ratio of live to dead cells increased which was generally acknowledged to be the dominant factor for irreversible membrane fouling increasing in MBR[23].
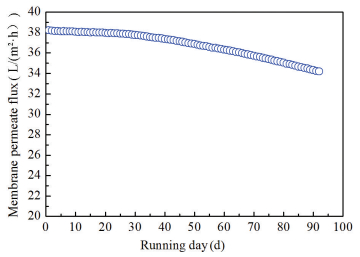
|
Figure 10 Curtain UF membrane permeate flux |
Despite the inevitability of either permeates flux decreasing or TMP rising, the membrane fouling rate was remarkably reduced for DAF-MBF system. Both the strengthened DAF pretreatment and the introduction of multilayer media ahead of the membrane module effectively alleviated membrane fouling. The water flux of membrane exhibited a nearly 6.2% decrease after three months running which maintained at an acceptable level without any types of chemical cleaning.
Many underlying factors acted in concert to ensure the smooth operation of membrane modules in MBF. Although the variation of different kinds of EPSs was not monitored in the present study, it had been reported that the introduction of biofilm carriers in MBR could efficiently reduce loosely bound EPS which exhibited more significant correlations with structure of microbial communities and membrane fouling rate compared with the tightly bound ones[24-26]. Some researchers also figured out that adding powered activated carbon in conventional MBR could promote biodegradation of refractory soluble microbial products (SMPs) and improve the reduction of membrane resistance[27-28]. Therefore, the addition of GAC and zeolite in DAF-MBF process not only improved the nitrogen and organic matters elimination, but also enhanced both biodegradation and adsorption of EPSs. In addition, for common MBR, vigorous aeration may directly lead to the increase of submicron particles (10-1 000 nm) and SPMs, both of which may cause irreversible fouling rate rising by the formation of a less permeable cake or pore blocking[29]. Distinguished from the conventional hybrid MBR, the aeration under biofilm media was no longer needed in DAF-MBF due to the dissolved oxygen enriched DAF effluents, which provided a well undisturbed environment to biofilm ecosystem and was benefit both for the bio-filtration and ultrafiltration functions.
4 ConclusionsThe remarkable control of solid particles and microbes was obtained for DAF in combination with either UFM or MBF. Compared with the DAF-UFM system, the DAF-MBF process was able to withstand the varied quality of Huai River water, especially for raw water with shock loadings of ammonia and organic matters. The finished water quality was success in meeting the more stringent regulatory limits (CNSDWQ GB5749-2006). As for DAF-UFM system, membrane biofouling was apparent after several weeks running. However, the curtain UF membrane in DAF-MBF process exhibited a decreased membrane fouling rate.
Further research would need to be conducted to improve the denitrification process in DAF-MBF system focused on denitrifying bacteria acclimation and the addition of extra nutrient substrates. In addition, the DAF-MBF system was characterized by small footprint and had a good prospect of practical application in dispersed regions for drinking water treatment or wastewater reclamation.
| [1] |
Haarhoff J. Dissolved air flotation: progress and prospects for drinking water treatment.
Journal of Water Supply: Research and Technology-Aqua, 2008, 57(8): 555-567.
DOI:10.2166/aqua.2008.046 ( 0) 0)
|
| [2] |
Edzwald J K. Dissolved air flotation and me.
Water Research, 2010, 44(7): 2077-2106.
DOI:10.1016/j.watres.2009.12.040 ( 0) 0)
|
| [3] |
Zou Jing, Zhu Juntao, Ma Jun, et al. Comparative study of dissolved air flotation and sedimentation process for treating reservoir water with low temperature, low turbidity and high natural organic matter.
Applied Mechanics and Materials, 2011, 71.
( 0) 0)
|
| [4] |
Ma Jun, Zou Jing, Shi Yulong. Effects of flocculation time and hydraulic loading on DAF treating Mopanshan reservoir water with low turbidity and low temperature. Proceeding of the 6th International Conference of Flotation in Water and Wastewater Systems. New York, 2012. 158-165.
( 0) 0)
|
| [5] |
Edzwald J K, Haarhoff J. Seawater pretreatment for reverse osmosis: Chemistry, contaminants, and coagulation.
Water Research, 2011, 45: 5428-5440.
DOI:10.1016/j.watres.2011.08.014 ( 0) 0)
|
| [6] |
Haarhoff J, Edzwald J K. Adapting dissolved air flotation for the clarification of seawater.
Desalination, 2013, 311: 90-94.
DOI:10.1016/j.desal.2012.10.035 ( 0) 0)
|
| [7] |
Edzwald J K, Haarhoff J.
Dissolved Air Flotation for Water Clarification. New York: AWWA and McGraw Hill, 2012: 1-22.
( 0) 0)
|
| [8] |
Oh S, Kim T, Han M Y. Physical characteristics of bubbles in dissolved air flotation processes in seawater reverse osmosis desalination plants. Proceeding of the 6th International Conference of Flotation in Water and Wastewater Systems. New York, 2012. 190-195.
( 0) 0)
|
| [9] |
Amato T, Park K S, Yim W, et al. The application of Enflo-DAFTM technology to SWRO pre-treatment design and operation. Proceedings of the 6th International Conference of Flotation in Water and Wastewater Systems. New York, 2012. 213-220.
( 0) 0)
|
| [10] |
Konieczny K, Bodzek M, Kopec' A, et al. Coagulation-submerge membrane system for NOM removal from water.
Desalination, 2006, 200: 578-580.
DOI:10.1016/j.desal.2006.03.451 ( 0) 0)
|
| [11] |
Ang W L, Mohammad A W, Teow Y H. Hybrid chitosan/FeCl3 coagulation-membrane processes: Performance evaluation and membrane fouling study in removing natural organic matter.
Separation and Purification Technology, 2015, 152: 23-31.
DOI:10.1016/j.seppur.2015.07.053 ( 0) 0)
|
| [12] |
Shi Yulong, Ma Jun, Yang Jiaxuan, et al. Demonstration project for upgrading water treatment plant by combined process of sedimentation and dissolved air flotation: removal of pollutants during low pollution period.
China Water & Wastewater, 2014, 30(1): 34-37.
( 0) 0)
|
| [13] |
AP HA, AW WA, W EF.
Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association, 1998.
( 0) 0)
|
| [14] |
Ma Jun, Jiang Jin, Pang Suyan. Adsorptive fractionation of humic acid at air-water interfaces.
Environmental Science & Technology, 2007, 41(14): 4959-4964.
( 0) 0)
|
| [15] |
Yan Mingquan, Wang Dongsheng, Shi Baoyou, et al. Transformations of particles, metal elements and natural organic matter in different water treatment processes.
Journal of Environmental Sciences, 2007, 19: 271-277.
DOI:10.1016/S1001-0742(07)60044-8 ( 0) 0)
|
| [16] |
Krzeminski P, Obelleiro L A, Madebo G, et al. Impact of temperature on raw wastewater composition and activated sludge filterability in full-scale MBR systems for municipal sewage treatment.
Journal of Membrane Science, 2012, 423: 348-361.
( 0) 0)
|
| [17] |
Ma Cong, Yu Shuili, Shi Wenxin, et al. Effect of different temperatures on performance and membrane fouling in high concentration PAC-MBR system treating micro-polluted surface water.
Bioresource Technology, 2013, 141: 19-24.
DOI:10.1016/j.biortech.2013.02.025 ( 0) 0)
|
| [18] |
Wang Zhiwei, Wu Zhichao, Tang Shujuan. Impact of temperature seasonal change on sludge characteristics and membrane fouling in a submerged membrane bioreactor.
Separation and Purification Technology, 2010, 45(7): 920-927.
( 0) 0)
|
| [19] |
Laspidou C S, Rittmann B E. A unified theory for extracellular polymeric substances, soluble microbial products, and active and inert biomass.
Water Research, 2002, 36: 2711-2720.
DOI:10.1016/S0043-1354(01)00413-4 ( 0) 0)
|
| [20] |
Lin Hongjun, Zhang Meijia, Wang Fangyuan, et al. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies.
Journal of Membrane Science, 2014, 460: 110-125.
DOI:10.1016/j.memsci.2014.02.034 ( 0) 0)
|
| [21] |
Kimura K, Naruse T, Watanabe Y. Change in characteristics of soluble microbial products in membrane bioreactor associated with different solid retention times: Relation to membrane fouling.
Water Research, 2009, 43: 1033-1039.
DOI:10.1016/j.watres.2008.11.024 ( 0) 0)
|
| [22] |
Nuhoglu A, Pekdemir T, Yildiz E, et al. Drinking water denitrification by a membrane bio-reactor.
Water Research, 2002, 36: 1155-1166.
DOI:10.1016/S0043-1354(01)00344-X ( 0) 0)
|
| [23] |
Ergas S J, Rheinheimer D E. Drinking water denitrification using a membrane bioreactor.
Water Research, 2004, 38: 3225-3232.
DOI:10.1016/j.watres.2004.04.019 ( 0) 0)
|
| [24] |
Mun J, Park S, Han M. Effects of Al3+ and hydraulic characteristics on the removal and behaviour of particles in dissolved air flotation.
Water Science and Technology: Water Supply, 2006, 6(3): 89-95.
DOI:10.2166/ws.2006.743 ( 0) 0)
|
| [25] |
Khan T M, Takizawa S, Lewandowski Z, et al. Combined effects of EPS and HRT enhanced biofouling on a submerged and hybrid PAC-MF membrane bioreactor.
Water Research, 2013, 47: 747-757.
DOI:10.1016/j.watres.2012.10.048 ( 0) 0)
|
| [26] |
Liu Yongjun, Liu Zhe, Zhang Aining. The role of EPS concentration on membrane fouling control: Comparison analysis of hybrid membrane bioreactor and conventional membrane bioreactor.
Desalination, 2012, 305: 38-43.
DOI:10.1016/j.desal.2012.08.013 ( 0) 0)
|
| [27] |
Ramesh A, Lee D J, Lai J Y. Membrane biofouling by extracellular polymeric substances or soluble microbial products from membrane bioreactor sludge.
Applied Microbiological Biotechnology, 2007, 74(3): 699-707.
DOI:10.1007/s00253-006-0706-x ( 0) 0)
|
| [28] |
Wang Zhiwei, Wu Zhichao, Tang Shujuan. Extracellular polymeric substances (EPS) properties and their effects on membrane fouling in a submerged membrane bioreactor.
Water Research, 2009, 43(9): 2504-2512.
DOI:10.1016/j.watres.2009.02.026 ( 0) 0)
|
| [29] |
N g, C A, Sun D, Bashir M J K, et al. Optimization of membrane bioreactors by the addition of powdered activated carbon.
Bioresource Technology, 2013, 138: 38-47.
DOI:10.1016/j.biortech.2013.03.129 ( 0) 0)
|
| [30] |
Ma Defang, Gao Yue, Gao Baoyu, et al. Impacts of powdered activated carbon addition on trihalomethane formation reactivity of dissolved organic matter in membrane bioreactor effluent.
Chemosphere, 2014, 117: 338-344.
DOI:10.1016/j.chemosphere.2014.07.070 ( 0) 0)
|
| [31] |
Temmermana L D, Maereb T, Temmink H, et al. The effect of fine bubble aeration intensity on membrane bioreactor sludge characteristics and fouling.
Water Research, 2015, 76: 99-109.
DOI:10.1016/j.watres.2015.02.057 ( 0) 0)
|
 2017, Vol. 24
2017, Vol. 24


