Benzothiazole (BTH) containing a benzene ring fused with a thiazole ring, is a good starting material of many industrial chemicals for various kinds of applications[1-2]. Released from diverse industrial products or BTH production plants, these compounds have been detected not only in effluent of industrial wastewater but also in various environmental compartments[3-4]. They are of concern for the aquatic environment due to their potential toxicity[5]. In terms of their limited biodegradability and toxicity, degradation of BTH and its derivatives can be hardly realized completely by biotechnology and related treatments. In addition, the conventional physical and chemical treatment obtain low efficiency of organic compounds degradation, high energy consumption and cost[6].
As a process of water purification, three-dimensional electrocatalytic technology has numerous advantages over many existing technologies. It is a suitable technology for refractory organics degradation by merit of its environmental friendliness, catalytic efficiency, power saving and simplicity of operation[7-9]. The key to make high efficiency of the system is precisely the function of the particle electrodes.
Heretofore, three-dimensional electrochemical system has been successfully applied into the field of contaminants degradation including metal ions[10], dyes[11], cyanide[12], oil refinery wastewater, phenolic compounds and derivatives[13-17]. However, little attention has been paid to its application in BTH degradation. Moreover, in present reported researches, only a few amount of materials have been functioned as particle electrodes, including inexpensive activated carbon with nice adsorbability and catalytic metals with high catalytic effectiveness[18-21]. Nevertheless, these frequently-used materials have their own weakness respectively. Activated carbon(AC) has low catalytic efficiency as well as high electric consumption. While, the application of metals, metallic oxides or alloys is limited by high price, waste of superfluous catalyst, complexity for preparation and low adsorption ability[22-25]. To overcome these drawbacks, an improvement of electrocatalytic particle electrodes is essential for lowering electric consumption, inexpensiveness and enhancing catalytic efficiency, thus promoting its practice in BTH degradation.
In this work, iron species-impregnated granular activated carbon particle electrodes were prepared by impregnation, and then characterized for adsorbability and electrocatalysis. After that, the particle electrodes were applied for adsorption-electrocatalytic degradation of BTH in a self-designed three-dimensional eletrocatalytic system under natural conditions. In comparison with pure AC, the variation of modified particle electrodes in physical porous structure, surface chemical property and catalytic activation energy was investigated to further illustrate the mechanism of BTH degradation improvement.
2 Experiments 2.1 Materials and Chemicals0.2-0.4 mm granular activated carbon made from coconut shell was obtained from Luyuan Company(Zhengzhou, China). BTH was purchased from Aladdin Industrial Corporation(Shanghai, China). Fe(NO3)3·9H2O was purchased from Tianli Chemical Reagent Co., Ltd.(Tianjin, China). All other reagents were of analytical grade and used without further purification. All the aqueous solutions were prepared with deionized water.
2.2 Particle Electrodes PreparationFirstly, the commercial activated carbon material should be stewed in the deionized water keeping continuously stirred at 60 ℃ for 6 h, and cleaned by ultrasonic wave for 20 min. Then, the activated carbon was dried at 105 ℃ for 12 h.
Iron species-impregnated particle electrodes were prepared by incipient wetness impregnation of the cleaned activated carbon 12 g with an aqueous solution of 0.74 mol/L iron nitrate nonahydrate(Fe(NO3)3·9H2O). The stage of impregnation lasted 18 h at room temperature. Subsequently, all the particle electrodes were dried at 105 ℃ for 2.5 h and finally heat-treated at 380 ℃ for 4 h in muffle furnace.
2.3 Characterization and Analysis 2.3.1 Porous structure measurementProperties of porous structure including surface area and pore volume were determined in a Micromeritics BET surface apparatus(ASAP 2020), from the N2 adsorption-desorption isotherms at 77 K. Prior to the analysis, samples were degassed 10 h at 150 ℃. Pore width was measured by Barrett, Joyner and Halenda algorithm. Micropore size distribution was measured using HK.
2.3.2 Fourier transform infrared (FTIR) analysisFourier transform infrared analysis was used to perform the surface functional groups by using FTIR spectroscope(Spectrum One), where the FTIR spectra were recorded from 4 000 to 400 cm-1 with the KBr pellet method. The particle electrode samples were primarily mixed with dried KBr(Merk, spectroscopy grade)(about 0.5% samples in KBr) and ground into approximately 1 mm thick KBr pellets. The spectra were charted by the relationship between the percent of transmittance and wave number(cm-1).
2.3.3 Scanning electron microscopy(SEM) with energy dispersive spectrometry(EDS)SEM(EVO 18) was applied to analyze the surface of samples. EDS(X-Max), as an attachment, was performed to study the component distribution.
2.3.4 Powder X-ray diffraction(XRD)Powder X-ray diffraction patterns of fresh samples were carried out with a D/max-rB diffractometer using Cu Kα radiation(λ=1.540 6 ×10-10m) in the 2θ range of 10°-90° at the rate of 2(°)/min. X-ray patterns were compared to X-ray powder reference to confirm the phases using Joint Committee on Powder Diffraction Standard(JCPDS) files.
2.3.5 X-ray photoelectron spectroscopy(XPS)X-ray photoelectron spectra were recorded with a SPECS system equipped with an Al anode and a PH1-5700 detector. Prior to the analysis, the samples were reduced at 350 ℃ and atmospheric pressure in a SPECS high pressure cell integrated. Binding energies(accuracy±0.1 eV) were referred to the C1s signal.
2.3.6 Thermogravimetric(TG) analysisThermogravimetric analyses were performed in a NETZSCH STA 499C equipped with a thermal conductivity detector(TCD). About 5 mg of the samples was laid in a crucible reactor and reduced in a stream of 20 cm3/min of air(O2 as the reactive gas; N2 as the protective gas) at a heating rate of 10 ℃/min. The air consumption on account of the sample reduction was continuously monitored by the TCD. DTG, the derived function of TG, was applied to show the consumption of samples clearly.
2.3.7 Compounds measurementHigh performance liquid chromatography(HPLC) analyses were performed using a Waters Acquity Ultra Performance LC fitted with a reserved-phase column(Acquity UPLC BEH C18; 1.7 μm, 2.1×50 mm) at 20 ℃. The mobile phase was methanol-water(55:45 in adsorption experiment; 75:25 in degradation experiment, by volume), with a flow rate of 0.1 mL/min. Detection was performed with a Waters TUV Detector set at 254 nm.
Total organic carbon(TOC) was measured with a TOC-V CPH/CPN Analyzer(Shimadzu) at 200 Pa with a stream rate of 130 mL/min.
The chemical oxygen demand(COD) concentration was measured according to Standard Methods.
Liquid chromatography mass spectrum(LC-MS) analyses were performed with a Triple Quard Agilent 6430 series mass spectrometer with a Zorbax XDB-C18(150 nm×4.6 mm, 5 μm) column. The mobile phase was methanol-water(55:45), with a flow rate of 0.1 mL/min. The conditions of mass spectrometer were as follows: capillary voltage of 4.5 kV in the positive mode, nebulizer pressure of 45 psi, drying gas flow rate of 10 L/min, drying gas temperature of 350 ℃.
Iron ion dissolution concentration was measured by inductively coupled plasma and atomic emission spectrum(ICP-AES) Optima 5300DV analyzer(Perkin Elmer).
2.3.8 Electrochemical analysesElectrochemical characterizations of open circuit potential(OCPT), linear scan voltammetry(LSV) and electrochemical impedance spectroscopy(EIS) were performed on a CHI 700E series electrochemical station with a working electrode of Ir/Ti(ϕ 8 mm×20 cm), a counter electrode of annular stainless steel mesh(ϕ 6 cm×10 cm), a reference electrode of Ag/AgCl(CH111, Shanghai Chen Hua Instrument Co., Ltd.). The particle electrodes were AC and modified particle electrodes, respectively. The electrolyte solution was 0.1 mol/L NaCl+100 mg/L BTH. OCPT was conducted within the scan range from -10 to 10 V with scan time of 400 s. LVS was conducted within the scan range from 0 to 3.5 V with a rate of 10 mV/s. EIS was conducted within the frequency range from 100 to 50 mHz with the amplitude of 5 mV/s.
2.4 Experimental Design 2.4.1 Static adsorption of BTHThe static adsorption process was conducted in 2 L BTH solutions with the concentration of 100 mg/L. About 20 g of the prepared particle electrodes and non-modified activated carbons were placed into the solutions above at room temperature, respectively. The BTH concentration was measured by HPLC every 3 min for first hour and every 30 min for the rest until the concentration was hardly decreasing which was regarded to reach the adsorption saturation. Adsorption capacity of the samples was calculated as follows[26-27]:
| $ q = \frac{{\left( {{\rho _0} - {\rho _t}} \right)}}{m}V $ | (1) |
where q is the static adsorption capacity of the adsorbent, mg/g; ρ0 is the initial concentration of BTH, mg/L; ρt is the adsorption-saturated concentration of BTH, mg/L; V is the volume of the BTH solution, L; m is the mass of the adsorbent, g.
2.4.2 Dynamic adsorption of BTHThe prepared particle electrodes and non-modified activated carbons were tested for the adsorption removal of BTH in water through a continuous adsorption column operating at atmospheric conditions(25 ℃, 1 atm). The internal diameter and height of the adsorption column were 4 cm and 15 cm, respectively. The adsorption column was filled with about 10 g of adsorbent. 100 mg/L BTH solution was introduced into the adsorption column at the flow rate of 0.88 mL/min.
The concentration of BTH was measured by HPLC every 3 min for the first hour and every 30 min for the rest until the BTH removal efficiency was above 95% which was set as the "breakthrough point" of adsorbent. Adsorption capacity of the samples of BTH was calculated as follows[28-30].
| $ q = \frac{{{\rho _0}t - \int\limits_0^t {{\rho _t}{\rm{d}}t} }}{m}Q \times {10^{ - 3}} $ | (2) |
where q is the dynamic adsorption capacity of the adsorbent, mg/g; Q is the BTH solution flow rate, mL/min; t is the adsorption time, min; ρ0 is the influent BTH concentration, mg/L; ρt is the effluent BTH concentration at time t, mg/L; m is the mass of adsorbent, g.
2.4.3 Electrocatalytic degradation of BTHThe electrocatalytic degradation experiment was carried out in a cylindrical glass reactor(1 L) at atmospheric conditions(25 ℃, 1 atm) with Ir/Ti anode(ϕ 8 mm×20 cm) and the annular stainless steel mesh cathode(ϕ 6 cm×10 cm). The electrode distance between the anode and the cathode is 3 cm. The particle electrodes filled 60% of the reactor volume. Prior to the electrocatalytic experiment, particle electrodes have to be soaking in the 100 mg/L BTH solution for 72 h in order to eliminate the adsorption of particle electrodes. An experimental setup diagram of the electrocatalytic system is presented in Fig. 1. Constant voltage of 5 V was provided by a DC power supply(HSPY-36-03). For the experiment of electrocatalysis, the mixture only contained 100 mg/L concentration of BTH and 0.1 mol/L NaCl functioned as the electrolyte. The process of electrocatalytic reaction lasted 2 h. Samples were collected periodically at intervals and filtered by 0.22 μm polytetrafluoroethylene(PTFE) membrane for chemical analysis by HPLC and TOC. For comprehensive contrast of the consumption between AC and modified particle electrodes, mineralization current efficiency(EMC) and energy consumption(CE) were used.
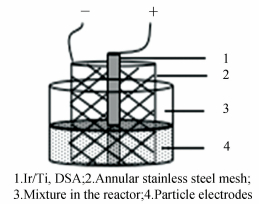
|
Figure 1 Experimental setup diagram of the electrocatalytic system |
EMC was calculated as follows[31]:
| $ {E_{{\rm{MC}}}} = \frac{{nFV\Delta \rho \left( {{\rm{TOC}}} \right)}}{{4.32 \times {{10}^7}mIt}} \times 100\% $ |
where n is the number of electrons consumed in the mineralization process; F is the Faraday constant, 96 487 C/mol; V is the solution volume, L; Δρ(TOC) is the experimental TOC decay, mg/L; 4.32×107 is a conversion factor(= 3 600 s/h× 12 000 mg); m is the number of carbon atoms in a BTH molecule, 7; I is the applied current, A.
CE was calculated as follows[32-33]:
| $ {C_{\rm{E}}} = \frac{{UIt}}{{\Delta \rho \left( {{\rm{COD}}} \right) \cdot V}} \times 1000 $ |
where U is voltage, V; I is the applied current, A; t is the reaction time, h; Δρ(COD) is the experimental COD decay, mg/L; V is the solution volume, L; 1 000 is a conversion factor.
3 Results and Discussion 3.1 Optimization of Particle Electrodes PreparationImpregnation concentration and time have effects on Fe-containing catalysts loading, adsorbability and electrocatalytic performance of the particle electrodes. To optimize the conditions of preparation, TOC removal and iron ion dissolution concentration in solution were detected during a 2 h electrocatalytic reaction. The results are processed as shown in Fig. 2. When impregnation concentration is 0.74 mol/L, TOC removal reaches a maximum value of 66.7%. As for the impregnation time, the TOC removal of 18-hour impregnation approaches the highest one of 24-hour impregnation. But iron ion dissolution concentration is 9 μg/L under 18-hour impregnation, which is far less than that of 21.6 μg/L under 24-hour impregnation. According to the comprehensive investigation of TOC removal and iron dissolution concentration, the optimized impregnation concentration and time are 0.74 mol/L and 18 h, respectively.
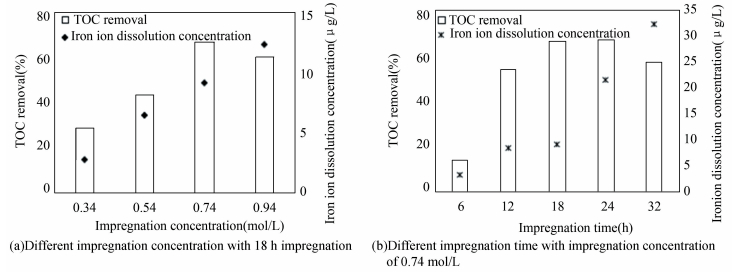
|
Figure 2 TOC removal and iron ion dissolution concentration |
3.2 Effects of Modified Particle Electrodes for BTH Adsorption Removal
BTH adsorption removal curves for the modified particle electrodes and the unmodified AC are shown in Fig. 3. After a series of calculations based Eqs. (1) and (2), the adsorption capacity of AC and the particle electrodes are obtained. The static adsorption capacities of AC and the particle electrodes are 4.68 mg/g and 5.89 mg/g, respectively; while, the dynamic adsorption capacities of AC and particle electrodes are 1.67 mg/g and 1.79 mg/g, respectively. The modified particle electrodes show a significant improvement in BTH removal than the unmodified AC.

|
Figure 3 BTH static adsorption removal curves and dynamic adsorption removal curves of different adsorbents |
The adsorption ability improvement of the modified particle electrodes attributes to their variation in porous structure and surface chemical property.
3.2.1 Variation in porous structureFig. 4 reveals the N2 adsorption-desorption isotherms for the modified particle electrodes and unmodified AC. According to the International Union of Pure and Applied Chemistry classification, the isotherms for the particle electrodes and AC are in correspondence with the type Ⅳ curve which indicates the mesoporous nature of the samples. While, both of the isotherms show hysteresis loops at a relative pressure range of 0.4-0.5, which is the result of capillary condensation-evaporation from the mesopores[34]. It is clear that the porous textural properties of the modified particle electrodes increase obviously in comparison with those of pure AC (Table 1), suggesting that impregnation preparation influences the porous structure significantly. Impregnation and high temperature heat treatment could make the pores sheltered before open. Meanwhile, introduction of Fe(NO3)3 and other iron oxides attributes to the results above due to their activation. In addition, variation of the modified particle electrodes in micropore size distribution (Fig. 5) is another important factor leading to the improvement in adsorption ability. The average micropore width of the modified particle electrodes is 0.603 nm(analyzed by HK method)and far higher than 0.368 nm of AC, which is closer to the optimal dynamic diameter for N2 adsorption based on Poludisperse Slit Pore Model[35-36].
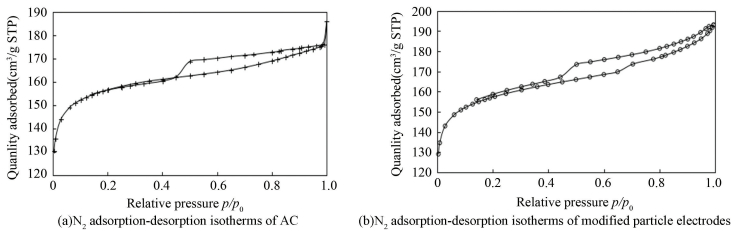
|
Figure 4 N2 adsorption-desorption isotherms of AC and modified particle electrodes |
| Table 1 Textural and structural properties of the samples |
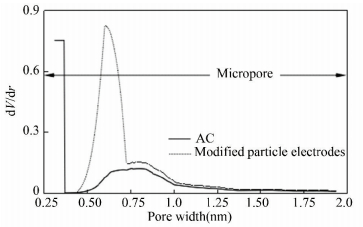
|
Figure 5 Comparison of micropore size distribution of AC and modified particle electrodes |
3.2.2 Variation in surface chemical property
Surface chemical property is majorly determined by the functional groups on the surface of samples. In FT-IR spectra,
surface functional groups are measured by the characteristic vibration to individual peaks[36-38]. Fig. 6 shows the surface functional groups of pure AC and the modified particle electrodes. Oxygen-containing functional groups including hydroxyl group and carbonyl group provide the modified particle electrodes with stronger polarity than pure AC, which makes the modified particle electrodes easier to adsorb polar compounds. Because of the introduction of nitrogen-heteroatom, the increase of nitrogen content varies the adsorption selectivity of the modified particle electrodes. The surface chemical element content (Table 2) indicates that oxygen-containing functional groups play a more crucial role in the improvement of adsorption ability than nitrogen-containing groups.
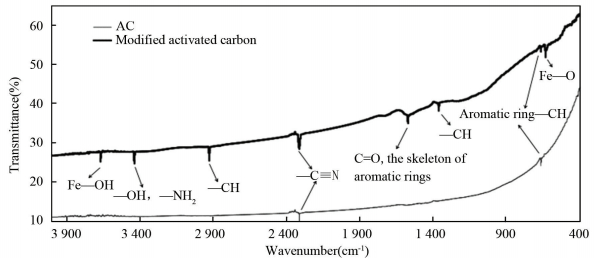
|
Figure 6 Comparison of surface functional groups of AC and modified particle electrodes by FT-IR spectra |
| Table 2 Surface chemical element content of AC and the modified particle electrodes by EDS |
3.2.3 Adsorption kinetics of BTH in the solution
Both physisorption and chemisorption occurred during the process of adsorption of BTH. Physisorption ability is mainly determined by the porous structure of samples; while, chemisorption ability is mainly determined by the surface chemical property. According to the experimental results, pseudo-first order adsorption kinetic model(suitable to describe the physisorption behavior that adsorbates take mass transference with liquid phase) and Elovich adsorption kinetic model(suitable for chemisorption) are chosen to represent the process of BTH adsorption[39].
Pseudo-first order adsorption kinetic model is presented as follows[39-41]:
| $ \frac{{{\rm{d}}{q_t}}}{{{\rm{d}}t}} = k\left( {{q_e} - {q_t}} \right) $ | (3) |
where qe is the adsorption capacity of the adsorbent for adsorption saturation, mg/g; qt is the adsorption capacity of the adsorbent at time t, mg/g; k is the adsorption rate constant of pseudo-first order kinetics.
Eq. (3) is integrated as follows.
| $ \int\limits_0^{{q_t}} {\frac{{{\rm{d}}{q_t}}}{{\left( {{q_e} - {q_t}} \right)}}} = k\int\limits_0^t {{\rm{d}}t} $ |
Then, plotting log(qe-qt) to t can get the data of k and qe.
Elovich adsorption kinetic model is presented as follows[39-41]:
| $ \frac{{{\rm{d}}{q_t}}}{{{\rm{d}}t}} = \alpha \exp \left( { - \beta {q_t}} \right) $ | (4) |
where qt is the adsorption capacity of the adsorbent at time t, mg/g; α is the initial adsorption rate constant; β is the adsorption rate constant of Elovich adsorption kinetics.
Eq.(4) is simplified (assuming that αβt ≥ 1[42]) and integrated.
| $ {q_t} = \frac{1}{\beta }\ln \left( {\alpha \beta } \right) + \frac{1}{\beta }\ln t $ |
Then, plotting qt to t can get the data of α and β.
Table 3 shows the results of kinetic parameters for pure AC and modified particle electrodes of BTH adsorption. Compared with pure AC, the modified particle electrodes' adsorption rate constants are bigger in the process of static as well as dynamic adsorption. Moreover, for the same sample, adsorption rate constants of chemisorption are much bigger than those of physisorption, suggesting that chemisorption has played a major role in the BTH adsorption.
| Table 3 Kinetic parameters for pure AC and modified particle electrodes of BTH adsorption |
3.3 Effects of Modified Particle Electrodes for BTH Electrocatalytic Degradation 3.3.1 Characterization of iron species-impregnated granular activated carbon particle electrodes
In order to have an internal microstructure cognition of the modified particle electrodes, the external morphology was observed through SEM. The modified particle electrodes and pure AC show noticeable differences in morphology including major deformation and fracturing[43](Figs. 7(a) and 7(c)). In addition, it is obvious that the modified particle electrodes have a rougher surface with porous structure than pure AC(Figs. 7(b) and 7(d)), which might greatly strengthen the mass transference and accelerate the adsorption rate. This is in accordance with the result of textural and structural properties by BET. Furthermore, the surface chemical element content programmed by EDS(Table 2) indicates that the introduction of Fe-containing compounds is the main reason for the variation in surface morphology.

|
Figure 7 SEM images |
The XRD data(Fig. 8) is to characterize the existence status of the Fe-containing catalysts by the corresponding diffraction angle of 2θ. It suggests that the Fe-containing catalysts is coated with the modified particle electrodes in the form of α-Fe(JCPDS 06-0696), α-Fe2O3(JCPDS 13-0534), γ-Fe2O3(JCPDS 39-1346), α-FeOOH(JCPDS 29-0713) and Fe3O4(JCPDS 88-0866)[44-47]. In the preparation, the Fe3O4 phase is theoretically oxidized to γ-Fe2O3 which subsequently is partly turned into the hexagonal phase hematite α-Fe2O3[48]. An approximate weight ratio of 18.5% Fe, 39% Fe2O3 and 42.5% Fe3O4 calculated by Fe2p spectral regions of XPS.

|
Figure 8 XRD for the modified particle electrodes |
3.3.2 Variation in activation energy
The samples were analyzed by TG-DTG to determine the weightlessness and activation energy by thermal analysis kinetics. Compared with pure AC, the modified particle electrodes have another weightless at 340-460 ℃ that signifies the catalysts weightlessness stage of the whole(Fig. 9).
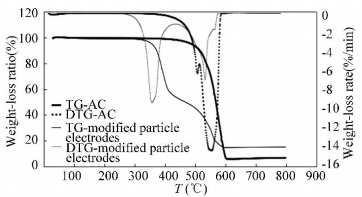
|
Figure 9 TG-DTG curve of the modified particle electrodes and pure AC |
In this study, Coats-Redfern thermal analysis kinetics model(suitable for the single heating rate curve) is used to calculate the activation energy as follows[49-51].
| $ \frac{{{\rm{d}}\alpha }}{{{\rm{d}}t}} = A\exp \left( { - \frac{E}{{RT}}} \right){\left( {1 - \alpha } \right)^n} $ | (5) |
where α=(m0-mt)/(m0-m′), m0 is initial mass of the sample, g; mt is the mass of the sample at temperature T, g; m′ is the ablation mass of the sample, g; R is ideal gas constant, 8.314 J/(mol·K).
When heating up with the constant rate of β, dT=βdt and Eq. (5) can be presented as follows.
| $ \int\limits_0^a {\frac{{{\rm{d}}\alpha }}{{{{\left( {1 - \alpha } \right)}^n}}}} = \frac{A}{\beta }\int\limits_0^T {\exp \left( { - \frac{E}{{\beta T}}} \right){\rm{d}}T} $ | (6) |
Hypothesis
| $ \ln \left[ {\frac{{G\left( \alpha \right)}}{{{T^2}}}} \right] = \ln \left[ {\frac{{AR}}{{\beta E}}\left( {1 - \frac{{2RT}}{E}} \right)} \right]\left( { - \frac{E}{{RT}}} \right) $ |
where G(α)=1-(1-α)1/3, which is suitable for the reaction mechanism of phase boundary reaction[49-50].
For general internal of reaction temperature and mostly E, E/RT ≫ 1, so that ln [
The result shows the activation energy of AC and the modified particle electrodes were 383 kJ/mol and 261 kJ/mol, respectively. For the modified particle electrodes, the activation energy decreases by 32%, which means the catalytic reaction occurs more easily. In this way, it contributes to high reaction efficiency of the three-dimensional electrocatalytic system.
3.3.3 Electrocatalytic performance of modified particle electrodes-based three-dimensional systemBTH has been degraded completely in 30 min in the modified particle electrodes-based system(Fig. 10). This removal rate is nearly 10% higher than that of the AC-based system. As total organic carbon is measured to indicate the degree of mineralization[52], so its removal is a pretty important parameter for evaluation of the system's efficiency. The TOC removal of modified particle electrodes-based system(66.7% removal) greatly surpasses that of AC-based system(43.2% removal), suggesting that the former has the advantages of high removal efficiency for BTH and its intermediate products.

|
Figure 10 Electrocatalytic degradation ability of BTH removal and TOC removal |
Comparison of mineralization current efficiency(EMC) and energy consumption(CE) between AC and modified particle electrodes are shown in Fig. 11. EMC of AC is much less than modified particle electrodes, while CE is higher. The reaction constant of AC is less than modified particle electrodes. Since a low reaction constant is not conductive to the chemical equilibrium moving forward, therefore, more electric energy has to be consumed for the AC system to improve the reaction.
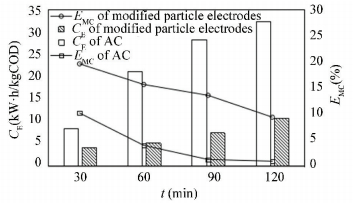
|
Figure 11 Comparison of EMC and CE between AC and modified particle electrodes |
The open circuit potential(OCPT) is the electrode potential at current density of zero, reflecting the stability of electrode surface. The higher OCPT is, the better corrosion resistance is. The OCPT of AC and modified particle electrodes are -10.06 V and -9.13 V, respectively(Fig. 12(a)) suggesting that introduction of modified particle electrodes promotes corrosion resistance of electrocatalytic system.
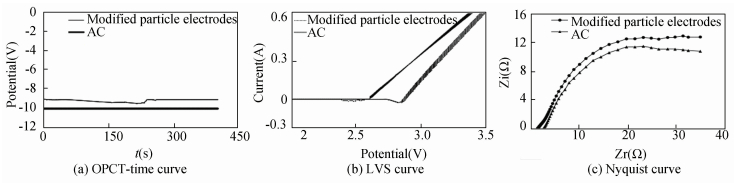
|
Figure 12 Electrochemical analyses of AC and modified particle electrodes |
It should be noted that several by-reactions have occurred during the actual reaction process such as oxygen and ydrogen evolution reaction. Generally, when the electrodes get a higher oxygen evolution potential, fewer by-reactions will be arisen [23]. When the potential of AC system reaches 2.6 V, correspondingly the current sharply increases, suggesting that oxygen evolution has happened at the surface of anode and particle electrodes. The oxygen evolution potentials of AC and modified particle electrodes are 2.6 V and 2.8 V(Fig. 12(b)), respectively. So the modified particle electrodes-based system takes less energy consumption but higher current efficiency in accordance with the results of EMC and CE above.
For the bipolar three-dimensional electrocatalytic reactor, the ideal particle electrodes should have bigger impedance to avoid the occurence of short-circuit current[53]. In contrast to the Nyquist curve of AC, the curve radius of modified particle electrodes system is bigger(Fig. 12(c)) suggesting modified particle electrodes have bigger impedance.
The degradation products of BTH were conducted by LC-MS analysis, and the results are shown in Fig. 13. Analogues of benzothiazole were detected in the degradation, a phenomenon that has been discussed previously[5, 54-55]. A possible degradation pathway of BTH using modified particle electrodes is illustrated preliminarily.
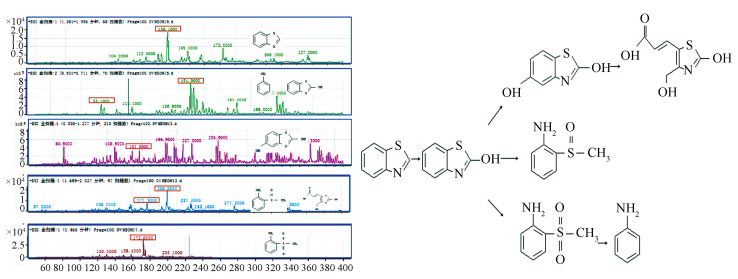
|
Figure 13 Electrocatalytic degradation of BTH by modified particle electrodes |
4 Conclusions
In this study, iron species-impregnated granular activated carbon particle electrodes with 18.5%Fe, 39%Fe2O3 and 42.5%Fe3O4, were synthesized by Fe(NO3)3 impregnation for 18 h at room temperature and then heat-treatment for 4 h at 380 ℃. When applied to BTH degradation, the modified particle electrodes imply higher efficiency than pure AC in adsorption as well as electrocatalytic degradation, resulting in 25.9% higher in static adsorption capacities and 54.4% higher in TOC removal. The static adsorption capacities of AC and the particle electrodes are 4.68 mg/g and 5.89 mg/g respectively for 100 mg/L BTH in the volume of 2 L at room temperature. The TOC removal of electrocatalytic degradation is 66.7%, when dealing with a given BTH concentration of 100 mg/L under the conditions of 0.1 mol/L NaCl functioned as the electrolyte, 60% modified particle electrodes in volume, electrode distance of 3 cm, constant voltage of 5 V in 1 L reactor for 2 h at atmosphere conditions. The improvement of adsorption is determined by porous structure(physical adsorption) and surface chemical property(chemical adsorption). According to the adsorption rate constant of adsorption kinetics, chemisorption plays the major role in BTH adsorption. The improvement of electrocatalytic degradation is determined by the Fe-containing catalysts on the modified particle electrodes. These Fe-containing catalysts make the activation energy decreased by 32% so as to take degradation reaction more easily.
| [1] |
Ginsberg G, Toal B, Kurland T. Benzothiazole toxicity assessment in support of synthetic turf field human health risk assessment.
Journal of Toxicology and Environmental Health, Part A: Current Issues, 2011, 74(17): 1175-1183.
DOI:10.1080/15287394.2011.586943 ( 0) 0)
|
| [2] |
Bellavia V, Natangeolo M, Fanelli R, et al. Analysis of benzothiazole in Italian wines using headspace solid-phase microextraction and gass chromatography mass spectrometry.
Journal of Agricultral and Food Chemistry, 2000, 48: 1239-1242.
DOI:10.1021/jf990634t ( 0) 0)
|
| [3] |
Fiehn O, Reemtsma T, Jekel M. Extraction and analysis of various benzothiazoles from industrial wastewater.
Journal of Analytica Chimica Acta, 1994, 295: 297-305.
DOI:10.1016/0003-2670(94)80235-1 ( 0) 0)
|
| [4] |
Reemtsma T, Fiehn O, Kalnowski G, et al. A 1H/15N NMR study of nitrogen metabolism in cultured mammalian cells.
Journal of Biochemical, 1993, 291: 485-492.
DOI:10.1042/bj2910485 ( 0) 0)
|
| [5] |
Besse P, Combourieu B, Boyse G, et al. Long-Range 1H-15N heteronuclear shift correlation at natural abundance: a tool to study benzothiazole biodegradation by two Rhodococcus strains.
Journal of Applied and Environmental Microbiology, 2001, 67: 1412-1417.
DOI:10.1128/AEM.67.4.1412-1417.2001 ( 0) 0)
|
| [6] |
Qin Yinghua, Sun Meng, Liu Huijuan, et al. AuPd/Fe3O4-based three-dimensional electrochemical system for efficiently catalytic degradation of 1-butyl-3-methylimidazolium hexafluorophosphate.
Journal of Electrochimica Acta, 2015, 186: 328-336.
DOI:10.1016/j.electacta.2015.10.122 ( 0) 0)
|
| [7] |
Gu Lin, Wang Bo, Ma Hongzhu, et al. Catalytic oxidation of anionic surfactants by electrochemical oxidation with CuO-Co2O3-PO43- modified kaolin.
Journal of Hazard Mater, 2006, 137: 842-848.
DOI:10.1016/j.jhazmat.2006.03.012 ( 0) 0)
|
| [8] |
Xiong Ya, He Chun, Karlsson H T, et al. Performance of three-phase three-dimensional electrode reactor for the reduction of COD in simulated wastewater-containing phenol.
Journal of Chemosphere, 2003, 50: 131-136.
DOI:10.1016/S0045-6535(02)00609-4 ( 0) 0)
|
| [9] |
Li Ming, Zhao Feiping, Sillanpää M, et al. Electrochemical degradation of 2-diethylamino-6-methyl-4-hydroxypyrimidine using three-dimensional electrodes reactor with ceramic particle electrodes.
Journal of Separation and Purification Technology, 2015, 156: 588-595.
DOI:10.1016/j.seppur.2015.10.053 ( 0) 0)
|
| [10] |
Issabayeva G, Aroua M K, Sulaiman N M. Electrodeposition of copper and lead on palm shell activated carbon in a flow-through electrolytic cell.
Journal of Desalination, 2006, 194: 192-201.
DOI:10.1016/j.desal.2005.09.029 ( 0) 0)
|
| [11] |
Xu Lina, Zhao Huazhang, Shi Shaoyuan, et al. Electrolytic treatment of C.I. Acid Orange 7 in aqueous solution using a three-dimensional electrode reactor.
Journal of Dyes and Pigments, 2008, 77: 158-164.
DOI:10.1016/j.dyepig.2007.04.004 ( 0) 0)
|
| [12] |
EI-Ghaoui E A, Jansson R E W, Moreland C. Application of the trickle tower to problems of pollution control. Ⅱ. The direct and indirect oxidation of cyanide.
Journal of Applied Electrochemistry, 1982, 12: 69-73.
DOI:10.1007/BF01112066 ( 0) 0)
|
| [13] |
Wang Yi, Liu Yuhui, Liu Tianfu, et al. Dimethyl phthalate degradation at novel and efficient electro-Fenton cathode.
Journal of Applied Catalysis B: Environmental, 2014, 156/157: 1-7.
DOI:10.1016/j.apcatb.2014.02.041 ( 0) 0)
|
| [14] |
Kong Wuping, Wang Bo, Ma Hongzhu, et al. Electrochemical treatment of anionic surfactants in synthetic wastewater with three-dimensional electrodes.
Journal of Hazardous Materials, 2006, 137: 1532-1537.
DOI:10.1016/j.jhazmat.2006.04.037 ( 0) 0)
|
| [15] |
Zhang Chao, Jiang Yonghai, Li Yunlin, et al. Three-dimensional electrochemical process for wastewater treatment: A general review.
Journal of Chemical Engineering, 2013, 228: 455-467.
DOI:10.1016/j.cej.2013.05.033 ( 0) 0)
|
| [16] |
Karthikeyan S, Viawanathan K, Boopathy R, et al. Three dimensional electro catalytic oxidation of aniline by boron doped mesoporous activated carbon.
Journal of Industrial and Engineering Chemistry, 2015, 21: 942-950.
DOI:10.1016/j.jiec.2014.04.036 ( 0) 0)
|
| [17] |
He Wenyan, Ma Qingliang, Wang Jing, et al. Preparation of novel kaolin-based particle electrodes for treating methyl orange wastwater.
Journal of Applied Clay Science, 2014, 99: 178-186.
DOI:10.1016/j.clay.2014.06.030 ( 0) 0)
|
| [18] |
Jung K W, Hwang M J, Park D S, et al. Performance evaluation and optimization of a fluidized three-dimensional electrode reactor combining pre-exposed granular activated carbon as a moving particle electrode for greywater treatment.
Journal of Separation and Purification Technology, 2015, 156: 414-423.
DOI:10.1016/j.seppur.2015.10.030 ( 0) 0)
|
| [19] |
Jinnouchi R, Kodama K, Suzuki T, et al. DFT calculations on electro-oxidations and dissolutions of Pt and Pt-Au nanoparticles.
Journal of Catalysis Today, 2016, 262: 100-109.
DOI:10.1016/j.cattod.2015.08.020 ( 0) 0)
|
| [20] |
Xie Man, Huang Yongxin, Xu Menghao, et al. Sodium titanium hexacyanoferrate as an environmentally firendly and low-cost cathode material for sodium-ion batteries.
Journal of Power Sources, 2016, 302: 7-12.
DOI:10.1016/j.jpowsour.2015.10.042 ( 0) 0)
|
| [21] |
Herrmann-Geppert I, Bogdanoff P, Emmler T, et al. Cold gas spraying-A promising technique for photoelectrodes: The example TiO2.
Journal of Catalysis Today, 2016, 260: 140-147.
DOI:10.1016/j.cattod.2015.06.007 ( 0) 0)
|
| [22] |
WeiJinzhi. Construction of Electro Catalytic-biological Oxidation Combined Process and Treatment of Herbicides Wastewater. Harbin: Harbin Institute of Technology, 2011.
( 0) 0)
|
| [23] |
Song Yuehai. Preparation of Insoluble Catalytic Electrode on Stainless Steel and Its Electro-catalysis for Degrading of Organic Wastewater with Low Biodegradability. Beijing: Beijing University of Chemical Technology, 2007.
( 0) 0)
|
| [24] |
Qiao N, Ma H, Hu M. Design of a neutral three-dimensional electro-Fenton system with Various bentonite-based Fe particle electrodes: A comparative study.
Journal of Materials Research Innocations, 2015, 19: S2-137-S2-141.
DOI:10.1179/1432891715Z.0000000001315 ( 0) 0)
|
| [25] |
Jung K W, Hwang M J, Park D S, et al. Combining fluidized metal-impregnated granular activated carbon in three-dimensional electrocoagulation system: Feasibility and optimization test of color and COD removal from real cotton textile wastewater.
Journal of Separation and Purification Technology, 2015, 146: 154-167.
DOI:10.1016/j.seppur.2015.03.043 ( 0) 0)
|
| [26] |
Abdel-Ghani N T, Rawash E S A, El-Chaghaby G A. Equilibrium and kinetic study for the adsorption of p-nitrophenol from wastewater using olive cake based activated carbon.
Global Journal of Environment Science and Management, 2016, 2: 11-18.
( 0) 0)
|
| [27] |
Shan Danna, Dong Shubo, Zhao Tianning, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling.
Journal of Hazardous Materials, 2016, 305: 156-163.
DOI:10.1016/j.jhazmat.2015.11.047 ( 0) 0)
|
| [28] |
Jiang Ming, Bai Yangwei, Ning Ping, et al. Adsorption removal of arsine by modified activated carbon.
Journal of Adsorption, 2015, 21: 135-141.
DOI:10.1007/s10450-015-9656-x ( 0) 0)
|
| [29] |
Ning Ping, Wang Xiangyu, Bart H J, et al. Removal of phosphorus and sulfur from yellow phosphorus off-gas by metal-modified activated carbon.
Journal of Cleaner Production, 2011, 19(13): 1547-1552.
DOI:10.1016/j.jclepro.2011.05.001 ( 0) 0)
|
| [30] |
Wang Xueqian, Ma Yixing, Ning Ping, et al. Adsorption of carbonyl sulfide on modified activated carbon under low-oxygen content conditions.
Journal of Adsorption, 2014, 20(4): 623-630.
DOI:10.1007/s10450-014-9607-y ( 0) 0)
|
| [31] |
Guinea E, Arias C, Cabot P L, et al. Mineralization of salicylic acid in acidic aqueous medium by electrochemical advanced oxidation processes using platinum and boron-doped diamond as anode and cathodically generated hydrogen peroxide.
Journal of Water Research, 2008, 42: 499-511.
DOI:10.1016/j.watres.2007.07.046 ( 0) 0)
|
| [32] |
Li Peng.
Characterization of the Effective Current and the Phase-reaction Kinetics Mechanism for Organic Matters Electro-catalytic Oxidation. Beijing: China University of Mining Technology, 2015.
( 0) 0)
|
| [33] |
Bi Qiang.
Investigation on Preparation and Properties of Electrode Materials for Electrochemical Oxidation of Organic Wastewater. Xi'an: Xi'an University of Architecture and Technology, 2014.
( 0) 0)
|
| [34] |
Juráez J M, Gómez M B, Anunziatz O A. Preparation and characterization of activated CMK-1 with Zn and Ni species applied in hydrogen storage.
Journal of Energy Research, 2015, 39: 941-953.
DOI:10.1002/er.v39.7 ( 0) 0)
|
| [35] |
Kluson P, Scaife S, Quirke N. The design of microporous graphitic adsorbents for selective separation of gases.
Journal of Separation and Purification Technology, 2000, 20(1): 15-24.
DOI:10.1016/S1383-5866(00)00070-8 ( 0) 0)
|
| [36] |
Zhao Guofeng.
The Modification and Characterization of Activated Carbons and the Application in Enriching Coal-well Methane. Qingdao: China University of Petroleum(East China), 2008.
( 0) 0)
|
| [37] |
Sun Yuanyuan.
Preparation, Characterization and Adsorption Properties of Activated Carbon from Arundo Donax L. Ji'nan: Shandong University, 2014.
( 0) 0)
|
| [38] |
Yuan Lei, Shen Jimin, Chen Zhonglin, et al. Role of Fe/pumice composition and structure in promoting ozonation reactions.
Journal of Applied Catalysis B: Environmental, 2016, 180: 707-714.
DOI:10.1016/j.apcatb.2015.07.016 ( 0) 0)
|
| [39] |
Ho Y S, Mckay G. Pseudo-second order model for sorption processes.
Journal of Process Biochemistry, 1999, 34(5): 451-465.
DOI:10.1016/S0032-9592(98)00112-5 ( 0) 0)
|
| [40] |
You Xiangyu.
Fundamental Research on Treatment of EDTA-containing Wastewater Using Three-dimensional Electrode Method. Changsha: Central South University, 2013.
( 0) 0)
|
| [41] |
Li Xinyang.
Development and Experimental Study of a Three-dimensional Electrode Reactor Packed with Catalyst-coated Granular Activated Carbons. Beijing: Tsinghua University, 2013.
( 0) 0)
|
| [42] |
Chien S H, Clayton W R. Application of Elovich equation to the kinetic of phosphate release and sorption on soil.
Journal of Soil Science Society of America, 1980, 44(2): 265-268.
DOI:10.2136/sssaj1980.03615995004400020013x ( 0) 0)
|
| [43] |
Gao Jie, Wang Wei, Rondinone A J, et al. Degradation of trichloroethene with a novel ball milled Fe—C nanocomposite.
Journal of Hazardous Materials, 2015, 300: 443-450.
DOI:10.1016/j.jhazmat.2015.07.038 ( 0) 0)
|
| [44] |
Park Y, Misol O h, Park J S, et al. Electrochemically deposited Fe2O3 nanorods on carbon nanofibers for free-standing anodes of lithium-ion batteries.
Journal of Carbon, 2015, 94: 9-17.
DOI:10.1016/j.carbon.2015.06.031 ( 0) 0)
|
| [45] |
Orellana-García F, ílvarez M A, López-Ramón M V, et al. Photoactivity of organic xerogels and aerogels in the photodegradation of herbicides from waters.
Journal of Applied Catalysis B: Environmental, 2016, 18: 94-102.
( 0) 0)
|
| [46] |
Miao Hua.
Study of the Preparation and Properties of α-FeOOH Nanoparticles and the Colloids. Chongqing: Southwest University, 2011.
( 0) 0)
|
| [47] |
Zhang Xin.
Preparation, Self-assembly and Optical Properties of the F2O3@SiO2 Ellipsoid Nanoparticles. Harbin: Harbin Institute of Technology, 2014.
( 0) 0)
|
| [48] |
Cunha I T, Teixeira I F, Albuquerque A S, et al. Catalytic oxidation of aqueous sulfide in the presence of ferrites(MFe2O4, M=Fe, Cu, Co).
Journal of Catalysis Today, 2015, 259: 222-227.
( 0) 0)
|
| [49] |
Wang Wenzhao.
Study on Thermogravimetric Analysis and Pyrolysis Kinetics of Cellulose. Chongqing: Chongqing University, 2008.
( 0) 0)
|
| [50] |
Liu Guowei, Dong Peng, Han Yafen, et al. Experimental study on combustion characteristics of coals under enriched-oxygen condition by thermo-gravimetric analysis.
Journal of Harbin Institute of Technology, 2011, 43(1): 2011.
( 0) 0)
|
| [51] |
Othman M B H, Khan A, Ahmad Z, et al. Kinetic investigation and lifetime prediction of Cs-NIPAM-MBA-based thermo-responsive hydrogels.
Journal of Carbohydrate Polymers, 2016, 136: 1182-1193.
DOI:10.1016/j.carbpol.2015.10.034 ( 0) 0)
|
| [52] |
Cai Chun, Zhang Zhuoyue, Liu Jin, et al. Visible light-assisted heterogeneous Fenton with ZnFe2O4 for the degradation of orange Ⅱ in water.
Journal of Applied Catalysis B: Environmental, 2016, 182: 456-468.
DOI:10.1016/j.apcatb.2015.09.056 ( 0) 0)
|
| [53] |
Wang Ce.
Study on Electrocatalysis of EE2 and Estrogenic Activity of Oxidation Products. Harbin: Harbin Institute of Technology, 2008.
( 0) 0)
|
| [54] |
Wever H D, Vereecken K, Stolz A, et al. Initial transformations in the biodegradation of benzothiazoles by Rhodococcus isolate.
Journal of Applied and Environmental Microbiology, 1998, 64(9): 3270-3274.
( 0) 0)
|
| [55] |
Liu Chunmiao.
Degradation Efficiency of Benzothiazole in Antibiotic Wastewater by Microbial Electrolysis Cell. Harbin: Harbin Institute of Technology, 2014.
( 0) 0)
|
 2017, Vol. 24
2017, Vol. 24


