2. School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
In the late 1960s, pyrotechnics with high radiation energy in the infrared wavelength has been developed at abroad. It is called MT pyrolant that is mainly composed of Magnesium (Mg) and Teflon (PTFE). For ease of processing and enhancing combustion stability, Viton A copolymer is added to the MT mix as a binder, so called MTV.
A large number of studies have been conducted on the thermal decomposition of MT based pyrolant by researchers. Griffiths[1] had studied the thermal decomposition of PTFE by using simultaneous TG/DTA in the anaerobic and aerobic environments firstly. They had found that the decomposition of PTFE is endothermic in inert and nitrogen environments but exothermic and complete consumed in the air environment. Ksiażczak[2] had confirmed the above conclusion and obtained the kinetic parameters of PTFE in nitrogen environment. Meanwhile, Griffiths[1] had also observed the decomposition process of PTFE added Mg and found thermal decomposition characteristics become exothermic in argon atmosphere which has shown the occurrence of exothermic reaction. Kubota[3] and Kuwahara[4] had published papers on experimental studies of the thermal decomposition of Mg/PTFE materials (containing 3% fluororubber) at the temperature range from 127 ℃ to 727 ℃. Davis and Liu[5-6] had recently performed ab-initio calculations on the reaction between PTFE and Mg. They had postulated a Grignard-type reaction between PTFE and Mg which was to be part of the preignition reaction (PIR) in the condensed phase. Based on the FTIR spectrum of DSC residue of MT composition, Koch[7] had proved the Davis' hypothesis and confirmed the intermediate reaction process which occurs below 600 ℃, then the intermediate products degrades at about 700 ℃. According to the temperature measuring experiments, the solid surface temperature of MT based pyrolant can reach about 850 ℃[4, 8-9], so there may be other decomposition processes existing in the temperature range of 700-850 ℃. However, it has not been concerned in previous thermal analysis experiments, and so far limited studies on systematic analysis of decomposition of MT based pyrolant have been conducted in different environments.
In this paper, focusing on MT and MTV pyrolants, the TG/DTA experiments are carried out under nitrogen and air environments.The effect of different atmosphere environments on the thermal decomposition process is analyzed, and then combined with the results of DSC experiments, the solid phase thermal decomposition mechanism is revealed.
2 Experiment 2.1 Materials and CharacterizationPolytetrafluoroethylene (PTFE), the average diameter is (30±5)μm; Mg powder, particle size is 100-200 mesh, active Mg content exceeds 99.0%; fluororubber (FPM26) is provided by Zhonghao Chenguang Research Institute which is equal to Viton A (Dupont) in domestic. The MT pyrolants are mixtures of Mg and PTFE with a mass ratio of 5:4 through dry mix and well-stirred. The MTV pyrolants are made of Mg, PTFE and fluororubber with a mass ratio of 5:4:1. In production of MTV pyrolant, acetone is used to solve the binder, providing good processability during the mixing period. A solution of binder in acetone is poured onto a solid premix of Mg and PTFE in a beaker and stirred. The solvent is evaporated while mixing until the mixture is obtained in the granular forms, and then dried. The experiment samples are shown in Fig. 1.

|
Figure 1 Experimental samples |
2.2 Experimental Instruments and Methods
The thermal analysis method is TG/DTA and DSC. The simultaneous thermal analyzer: SDT Q600 (TA, USA). The test parameter is set as: heating rate at 10 ℃/min, the temperature range at 50-850 ℃, samples and gas environment as shown in Table 1, and flow rate at 100 mL/min. The mass of samples is about 1 mg which is put into a corundum crucible and the reference object is Al2O3.
| Table 1 Samples and gas environment of simultaneous TG/DTA |
The DSC analyzer: HP DSC827E (METTLER TOLEDO, Switzerland). The test parameter is set as: heating rate at 10 ℃/min with a nitrogen dynamic atmosphere, the temperature range at 50-600 ℃, and the experimental samples are MT and MTV pyrolants.
3 Results and Discussion 3.1 Thermal Decomposition 3.1.1 Thermal decomposition of PTFEThe TG-DTG/DTA curves of PTFE in air and nitrogen environments are shown in Fig. 2. It can be seen that, PTFE is basically in the thermal stability below 300 ℃. The DTA result presents a clear endothermic peak at 325.6 ℃[1-2] which represents the melting of PTFE. There is no obvious change in the melting temperature of different atmospheres. In air environment, at 450 ℃ an exothermic decomposition process accompanied by weight loss starts and the peak temperature is at 566.5 ℃. But in the nitrogen environment, it shows an endothermic process with 907.3 J/g of heat absorption. The initial decomposition temperature of 500 ℃ and the peak temperature of 576.5 ℃[10] are both lagging behind the air environment. It indicates that oxygen in the air is not only involved in the decomposition reaction[11], but also leads to the shift of the characteristic temperature. Continuing to heat up to 850 ℃, no other phenomenon happens.

|
Figure 2 TG-DTG/DTA curves of PTFE in air and nitrogen environments |
3.1.2 Thermal decomposition of fluororubber
Fig. 3 describes the TG-DTG/DTA curves of fluororubber in nitrogen environment. The onset of the weight loss is at 425 ℃ and completes at~500 ℃ where all of materials have been consumed (100% weight loss). The DTA curves show two endothermic peaks: the smaller one is at 452.4 ℃ which results from the melting of fluororubber and the other is at 476.7 ℃ which is corresponding to the position of maximum weight loss rate[12-13].
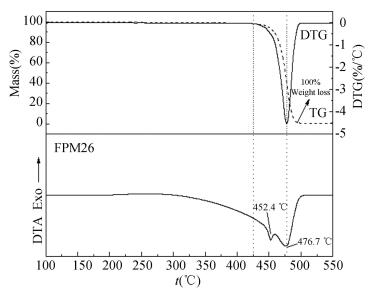
|
Figure 3 TG-DTG /DTA curves of fluororubber in nitrogen environment |
3.1.3 Thermal decomposition of MT based pyrolant
1) The thermal decomposition of MT pyrolant in nitrogen and air environments.The TG-DTG curves of MT in nitrogen and air environments are shown in Fig. 4(a). It is apparent from Fig. 4(a) that MT pyrolant degrades in two weight loss stages in nitrogen environment. In the first stage, the weight loss is shown at 500-596 ℃ and the peak temperature is at 570 ℃. The DTA presents an exothermic process (Fig. 5), and the weight loss is 38.95% which indicates a reaction between Mg and PTFE. In the second stage, an cendothermi process results from a second decomposition of component at 684-740 ℃. The peak is at 728.1 ℃, and the weight loss is 12.1%.
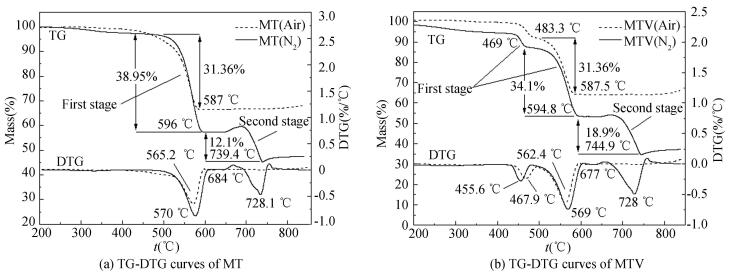
|
Figure 4 TG-DTG curves of MT and MTV in air and nitrogen environments |
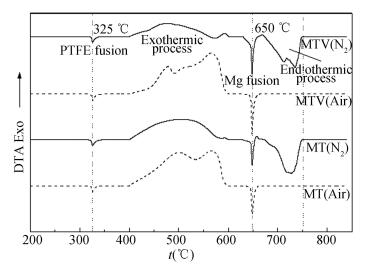
|
Figure 5 DTA curves of MT and MTV in air and nitrogen environments |
However, in air environment, there only one weight loss stage at 450-587 ℃ exits in the decomposition of MT pyrolant. The peak is at 565.2 ℃ and the weight loss is 31.36%. The DTA curve shows an exothermic process (Fig. 5). Compared with the same stage in nitrogen environment, the weight loss is decreased by 7.59%, and no second stage exists. In Fig. 5, the melting process of PTFE and Mg is represented by the endothermic peak at 325 ℃ and 650 ℃, respectively.
2) The thermal decomposition of MTV pyrolant in nitrogen and air environments. As shown in Fig. 4(b). In the decomposition of MTV pyrolant, there are also two weight loss stages in nitrogen environment. The first stage contains two consecutive weight loss processes. The onset of a first reaction is at 435 ℃. The tentative maximum of this reaction is a shoulder at 455.6 ℃. The onset of a second reaction, which superimposes the first reaction, is at 469 ℃ and the process complete at 595 ℃. The DTA results both present an exothermic process (Fig. 5) and the weight losses are 6.32% and 34.1%, respectively. In the second stage, the endothermic process is at 677-745 ℃, the peak is at 728 ℃, and the weight loss is 18.9%. Compared with the MT pyrolant in nitrogen environment, there come to two differences. Firstly, a process at 435-469 ℃ is added in MTV. Secondly, the temperature range of the second stage is enlarged, and the weight loss is increased.
In air environment, only one stage exists that also contains two consecutive weight loss processes in the decomposition of MTV pyrolant. The processes are at 435-483 ℃ and 483-588 ℃, and the weight losses are 6.64 % and 28.3 % respectively. No second stage exists as well.
3.2 Analysis on the Decomposition Characteristics of MT Based Pyrolant 3.2.1 Grignard-type reaction hypothesisIt is obviously shown from the weight loss curves in the nitrogen environment (Fig. 4) that MT and MTV pyrolants degrade in two weight loss stages. The first stage of decomposition is completed within 600 ℃ and the initial temperature of the second stage is at 680 ℃ that verify the spectrum result of Grignard-type reaction[7]. Koch found the presence of Mg-C-F units in the FTIR spectrum when the DSC experiment stops at 600 ℃. Then the temperature reaches 700 ℃, the intermediate product is decomposed and shown the signal of MgF2. The reaction mechanism is as follows. Q1 and Q2 are represented the released heat or the absorbed heat:
| $ \begin{array}{l} n{\rm{Mg}}{_{\left( {\rm{s}} \right)}} + {\left( { - {\rm{C}}{{\rm{F}}_2} - {\rm{C}}{{\rm{F}}_2} - } \right)_n} \to \\ \;\;\;{\left( { - {\rm{C}}{{\rm{F}}_2} - {\rm{CFMgF}} - } \right)_n} + {Q_1} \end{array} $ | (1) |
| $ \begin{array}{l} {\left( { - {\rm{C}}{{\rm{F}}_2} - {\rm{CFMgF}} - } \right)_n} \to \\ n{\rm{Mg}}{{\rm{F}}_2} + {\left( { - {\rm{CF = CF - }}} \right)_n} + {Q_2} \end{array} $ | (2) |
According to the results of thermal analysis, the temperature ranges of these two stages can be confirmed: MT displays an exothermic process of Grignard-type reaction at 500-596 ℃ that part of Mg reacts with PTFE, Eq.(1), and the intermediate products are produced. The decomposition of the intermediate products occurs at 684-740 ℃ and shows an endothermic process, Eq.(2). In the MTV pyrolant, the intermediate reaction process is at 435-595 ℃, and then the intermediate products degrades at 677-745 ℃. The weight loss of the second stage in MTV is greater than MT in nitrogen environment. The reason may be that fluororubber is also involved in the process of intermediate reaction.
3.2.2 Parameters estimation of intermediate reactionThe DSC experiments were carried out to estimate the reaction parameters. The resultant curves of MT and MTV pyrolants in nitrogen environment are shown in Fig. 6. The temperature range is at 50-600 ℃. From the DSC investigation of MT pyrolant, it is evident that 1 318.57 J/g is released in the intermediate reaction in the range up to 600 ℃. There are two exothermic peaks in the decomposition of MTV. The smaller peak corresponds to the thermal decomposition process of fluororubber, and the temperature is about 480 ℃. The other exothermic peak temperature is at about 541.2 ℃, and the total heat release is increased to 1 723.25 J/g. It shows that fluororubber is involved in the exothermic reaction and increases the release heat. The characteristic values of DSC are shown in Table 2.
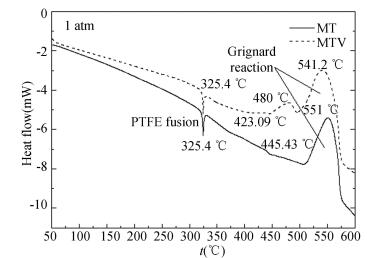
|
Figure 6 DSC curves of MT and MTV in nitrogen environment (atmospheric pressure) |
| Table 2 DSC parameters of MT and MTV in nitrogen environment |
Since the weight loss in the second stage of MT is about 12.1%, given the reaction mechanism (1) and (2), it has followed that about 8.71% Mg is consumed in the intermediate reaction by computation. It is further assumed that at the temperature of 50-600 ℃, it includes the intermediate reaction between part of Mg and PTFE, and the decomposition of residual PTFE in nitrogen environment (Heat absorption is 907.3 J/g). According to the above, it can be estimated that the heat release of the intermediate reaction of MT is about 31.93 kJ/g (Q1). Owing to the lack of the reaction mechanism of fluororubber, no more information is obtained from the decomposition of MTV pyrolant.
3.2.3 Influence of air atmosphereFrom the comparison of the TG-DTG curves in air environment with nitrogen environment (Fig. 4), it can be clearly seen that, the thermal decomposition of MT and MTV in air environment shows different mechanisms from nitrogen environment. There is no second decomposition process in air environment which means the absent of intermediate reaction. In view of these, it is reasonable to explain that the existence of oxygen in the air has an inhibitory effect on the intermediate reaction process. According to X-ray analysis of MT combustion products, the main components are MgO, MgF2, C[8, 14], and the reaction mechanism is as follows[15]:
| $ 2n\text{Mg}+{{\left( -\text{C}{{\text{F}}_{2}}-\text{C}{{\text{F}}_{2}}- \right)}_{n}}\xrightarrow{\text{Air}}2n\text{Mg}{{\text{F}}_{2}}+2n\text{C} $ | (3) |
| $ \text{2Mg+}{{\text{O}}_{2}}\to 2\text{MgO} $ | (4) |
In addition, the decomposition of MT and MTV is at 450-587 ℃ and 435-588 ℃ in air environment, respectively. Compared with the same stage in nitrogen environment, the temperature range is slightly shrunk, and the weight loss is decreased that is related to the reaction of oxygen with Mg.
4 ConclusionsFrom the studies of the thermal decomposition of MT and MTV pyrolants in the nitrogen and air environments, the thermal analysis experiments are carried out at the heating rate of 10 ℃/min. The experimental results can be concluded as follows:
1) In nitrogen environment, the spectrum result of Grignard-type reaction is verified by the thermal decomposition characteristics of MT and MTV pyrolants. Then the temperature ranges of decomposition stage can be confirmed. In the decomposition of MT, the exothermic intermediate reaction is at 500-596 ℃, and the decomposition of the intermediate products occurs at 684-740 ℃ which tends to be an endothermic process. In MTV, the intermediate reaction is at 435-595 ℃, and then the intermediate products degrade at 677-745 ℃.
2) In nitrogen environment, the consumption of Mg is 8.71% in the intermediate reaction, and the heat release is 31.93 kJ/g which is increased by the addition of fluororubber.
3) In air environment, the decomposition of MT and MTV shows the different reaction mechanism from the nitrogen environment. The presence of oxygen not only is involved in the reaction of Mg, but also inhibits the intermediate reaction of Mg and PTFE.
| [1] |
Griffths T T, Robertson J, Hall P G, et al. Thermal decomposition of PTFE in the presence of magnesium.
16th International Annual ICT Conference.Karlsruhe, 1985: 1-10.
( 0) 0)
|
| [2] |
Ksiażczak A, Boniuk H, Cudziło S. Thermal decomposition of PTFE in the presence of silicon, calcium, silicide, ferrosilicon and iron.
Journal of ThermalAnalysis and Calorimetry, 2003, 74(2): 569-574.
DOI:10.1023/B:JTAN.0000005195.46390.48 ( 0) 0)
|
| [3] |
Kubota N, Serizawa C. Combustion of magnesium/ polytetrafluoroethylene.
Journal of Propulsion and Power, 1987, 3(4): 303-307.
DOI:10.2514/3.22990 ( 0) 0)
|
| [4] |
Kuwahara T, Matsuo S, Shinozaki N. Combustion and sensitivity characteristics of Mg/TF pyrolants.
Propellants Explosives and Pyrotechnics, 1997, 22(4): 198-202.
DOI:10.1002/(ISSN)1521-4087 ( 0) 0)
|
| [5] |
Davis S R, Liu L. Ab initio study of the insertion reaction of Ma into a C-F bond of tetrafluoroethylene.
Journal of Molecular Structure (Theochem), 1994, 304(3): 227-232.
DOI:10.1016/0166-1280(94)80019-7 ( 0) 0)
|
| [6] |
Liu L, Davis S R. Ab initio study of the Grignard reaction between magnesium atoms and fluoroethylene and chloroethylene.
The Journal of Physical Chemistry, 1991, 95(22): 619-625.
( 0) 0)
|
| [7] |
Koch E C. Metal-Fluorocarbon-Pyrolants Ⅳ:Thermochemical and combustion behaviour of Magnesium/Teflon/Fluororubber(MTV).
Propellants Explosives and Pyrotechnics, 2002, 27(6): 340-351.
DOI:10.1002/prep.200290004 ( 0) 0)
|
| [8] |
Chen D M, Hsieh W H, Snyder T S, et al. Combustion behavior and thermophysical properties of metal-based solid fuels.
Journal of Propulsion and Power, 1991, 7(2): 250-257.
DOI:10.2514/3.23318 ( 0) 0)
|
| [9] |
Kubota N. Combustion process of Mg/TF pyrotechnics.
Propellants Explosives and Pyrotechnics, 1987, 21(5): 145-148.
( 0) 0)
|
| [10] |
Xia Ruiquan, Zhang Xiaoping. Experimental study on waste polytetrafluoroethylene pyrolysis.
Chemcal Industry and Engineering Progress, 2008, 27(1): 98-103.
( 0) 0)
|
| [11] |
Baker B B, Kasparyk D J. Thermal degradation of commercial fluoropolymers in air.
Polymer Degradation and Stability, 1993, 42: 181-188.
DOI:10.1016/0141-3910(93)90111-U ( 0) 0)
|
| [12] |
Gao Dayuan, He Bi, He Songwei, et al. Thermal decomposition kinetics of F2314 bonder.
Chinese Journal of Explosives & Propellants, 2006, 29(5): 29-31.
( 0) 0)
|
| [13] |
Burnham A K, Weese R K. Kinetics of thermal degradation of explosive binders Fluororubber A. Estane, and Kel-F.
Thermochimica Acta, 2005, 426(1/2): 85-92.
( 0) 0)
|
| [14] |
Koch E C.
Metal-Fluorocarbon Based Energetic Materials. Germany: Wiley-VCH Verlag GmbH & Co, 2012: 119-148.
( 0) 0)
|
| [15] |
Koch E C.
Handbook of Combustion Vol.5: New Technologies. Germany: Wiley-VCH Verlag GmbH & Co, 2010: 355-396.
( 0) 0)
|
 2017, Vol. 24
2017, Vol. 24


