2. Department of Chemistry, Washington State University, Pullman, WA 99164, United States
Sustainable energy supply is critical for global economy. Rapid development of portable electronics, electric vehicles and stationary storage systems calls for urgent demands for next-generation energy storage devices (ESDs) as well as new energy economy. Lithium ion batteries (LIBs) and electrochemical capacitors are among the most of important ESDs due to their high performances in energy/power densities and life span[1-3]. These ESDs are typically composed of two electrodes including a cathode and an anode, an electrolyte that allows the transportation of the ions, a separator that separates two electrodes to prevent short-circuit, and highly electrically conductive current collectors allowing current flow out of the cells. According to the cell configuration shown in Fig. 1 (LIB as an example), one can see that the working mechanism of LIB relies on electrochemical reactions enabling transportation of lithium ions between the two electrodes. Herein, it can also be seen that the electrolyte is the only medium that connects all the components and plays a vital role in controlling the dynamics of the lithium ions inside of the battery.
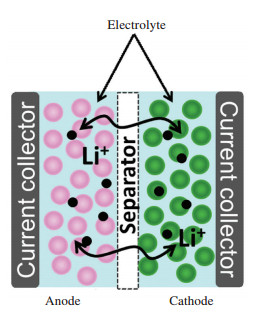
|
Figure 1 Illustration of typical configuration of LIBs |
Electrolyte is a critical component with basic functionality as conducting ions, but not electrons for ESDs. A desired electrolyte should possess such properties as high ionic conductivity to satisfy the ion transportation process, good contact/interfacial property to effectively construct stable ion/electron transport pathways, and good thermal and electrochemical stabilities to survive in the environment during service. Currently, the most successfully commercialized electrolytes are organic liquid electrolytes (OLEs) based on carbonate liquids, e.g. polycarbonate (PC), ethylene carbonate (EC), dimethoxyethane (DME), etc.[4] owing to their high ionic conductivity and superior contact/interfacial property. However, the safety issues (e.g. thermal runaway, electrolyte leakage, explosion, etc.) caused by their flammable property have been intensively concerned, which hinder their broader applications[5-6]. Therefore, studies on electrolytes for safe and high performance LIBs are obligatory and urgently needed. At the same time, the demands for development of next-generation ESDs, especially wearable or flexible electronic devices, have been significantly raised[7-9]. Currently, liquid electrolytes are still the most popular electrolyte materials for fabrication of ESDs, although they have limitations because of their extremely poor mechanical properties. In this case, advanced solid or gel electrolytes are very much desired as they are safer and enable design of fully flexible and safe LIBs and more importantly, they can maintain structural integrity when undergoing various deformations. Therefore, the desire for safe and flexible batteries and diversity of ESDs make solid or gel electrolytes promising candidates for substituting liquid electrolytes.
Over the past decades, towards achieving safe and flexible LIBs, best electrolyte candidates include polymers[10-11], ceramics[12-13], hybrids[14-15] and gels[16-17]. Among various types of electrolyte materials, polymer electrolytes have drawn considerable attention owing to their obvious ease of application to LIBs particularly as compared with ceramic electrolytes. Based on polymers, gel electrolytes are defined as one type of polymer electrolytes, which are the combinations of solid polymer electrolyte and liquid component such as liquid electrolytes. The comprehensive attributes and basic advantages/disadvantages of the two types of polymer electrolytes including solid and gel polymer electrolytes are summarized as below.
1.2 Solid Polymer ElectrolytesSolid polymer electrolytes (SPEs) refer to the solid polymers that are able to transport ions, such as lithium ions, sodium ions, and protons. SPEs are believed to be the most promising candidates to replace liquid electrolytes for applications of wearable electronics and microelectronics because of their remarkable advantages such as excellent mechanical properties, high safety and good flexibility[18-19]. In addition, it is also found that SPEs are of great potential to suppress the growth of lithium dendrites due to their high modulus[20-21]. Similar to the composition of liquid electrolytes, for SPEs, the polymer hosts act as "solid solvents" that dissolve the lithium salts. The lithium salts can be dissociated by the polymer hosts due to the coupling interaction between the electrolyte ions and the polymer hosts, and thus, the ions are movable in the free volume of the polymer hosts. The most critical property for electrolytes is their ionic conductivity. It is generally accepted that the ionic conductivity of SPEs is confined in the amorphous phase of the polymer hosts above the glass transition temperature. Due to the long-chain effect and slow segment movement, the ionic conductivity of SPEs (< 10-4 S/cm at room temperature) is far lower than that of the liquid electrolytes, which severely hinder the practical applications of SPEs.
In the past years, focus has been mainly concentrated on many kinds of polar polymers such as poly(ethylene oxide) (PEO)[22-24], poly(propylene oxide)[25-27], polyacrylonitrile (PAN)[14, 28-29] and so on. The most classical SPE system is PEO-based electrolytes. PEO is a typical semi-crystalline polymer and shows very good solubility for various lithium salts. The typical ionic conductivity of PEO-based SPEs is in the range of 10-8-10-4 S/cm at room temperature. PEO can form complexes with lithium salts primarily due to the coupling interactions between lithium ions and oxygen atoms of the PEO chains[5, 30-31]. On one hand, the strong coupling interaction facilitates the dissociation of lithium salts. However, on the other hand, it impedes the ion transport simultaneously. The coupling effect between PEO and lithium ions can be well reflected by several aspects. For instance, the ionic conductivity can be increased by decreasing the molecular weight of PEO or by increasing flexibility of PEO chains via adding plasticizers[32-34].
The most challenging and critical issue for SPEs is to improve their ionic conductivity to approach the level of liquid electrolytes. Diverse strategies have been investigated such as introducing nanoparticles (e.g. Al2O3[23, 35-36], TiO2[37-38]), adding plasticizers[33-34], blending with polymers[24, 39] and so forth. The primary purpose of the reported strategies is to increase the percentage of amorphous phase in the polymer hosts. Among these approaches, addition of nanoparticles is found to be very attractive because of several aspects. Firstly, the ion transport can be facilitated due to the conductive interfaces formed between the polymer matrix and nanoparticles, as well as formation of Lewis acid-base interaction between the electrolyte ions and the nanoparticles[40-41]. Secondly, the nanoparticles can act as reinforcement additives to enhance the mechanical properties that are deteriorated by the reduced crystallinity of the polymer hosts. The effects of ceramic fillers[23, 42], zeolites[43] and hybrids[44] on SPEs have been well investigated. It is also established that addition of nanofillers may improve the compatibility between electrolyte and lithium electrode as well as thermal/electrochemical stabilities of the electrolytes. Moreover, the nanofillers can be designed with various dimensions and functionalities to involve in the ion conduction. For example, some fillers having high aspect ratio with such as a fiber-like structure[45] can easily construct continuous conductive networks. Also, some fillers can be chemically modified to introduce atoms (e.g. Boron[46]) that can actively interact with ions in order to increase ionic conductivity as well as lithium transference number that is defined as the fraction of current contributed by lithium ions.
Copolymers with conductive blocks are another type of promising candidates for high-performance SPEs[47-50]. In specific, the conductive block (e.g. EO block) is responsible for the ion transportation, and at the same time, the other blocks, such as polyethylene (PE) or polystyrene (PS), enable to form 3D frameworks to provide strong mechanical strength. Their properties can be precisely patterned by adjusting simultaneously their block fractions and polymer architectures. It is usually found that the ionic conductivity and mechanical properties are significantly dependent on the specific microstructures of the blocks. For instance, Devaux et al.[51] reported a PEO-PS block polymer electrolyte, and found that, compared with the linear PEO, comb PEO showed slightly better ionic conductivity owing to absence of PEO crystallization, but the linear PEO presented the best compromise between high conductivity and good mechanical properties. Although copolymers enable a fine design of ion conduction pathways, the mechanism of the ion conduction in the conductive blocks is generally the same as that of conventional SPEs. Therefore, the ionic conductivity of copolymer-based SPEs is usually at the same level as it is for common SPEs.
1.3 Gel Polymer ElectrolytesAll solid lithium batteries with SPEs have never reached the stage of large-scale commercialization due to the unsatisfactory ionic conductivity and poor interfacial property of SPEs. Concerned by these critical issues, people paid increasingly attention to gel polymer electrolytes (GPEs), in which advantages of both liquid electrolytes (e.g. high ionic conductivity, good interfacial property) and SPEs (e.g. good mechanical properties, high safety) can be taken. Although the introduction of a liquid component inevitably deteriorates the mechanical properties, the combination of the attributes from different states of components is still an attractive approach to achieving moderate to high performances all around. Therefore, GPEs are still taken as high-performance electrolytes for fabrication of advanced LIBs with enhanced flexibility and safety.
Many polar polymers have been studied as the hosts for fabrication of GPEs, such as PEO[33, 52], poly(vinylidene fluoride) (PVDF)[53-54], poly(methyl methacrylate) (PMMA)[49, 55] and so on. These polymers usually have good affinity with the liquid electrolytes. It is also noted that in addition to OLEs, new electrolytes such as ionic liquid electrolytes (ILEs) have also been of great interest for fabrication of GPEs recently. Studies on GPEs are mainly focusing on optimization of their structures and compositions to enhance ionic conductivity, mechanical performance, interfacial property, etc. For GPEs, to achieve high ionic conductivity and good mechanical properties is the primary challenge. The liquid component induces swelling of the polymer host, and therefore the mechanical properties of GPEs are unavoidably sacrificed to some degree. The key to alleviate this issue is to design structures that can adsorb large amount of liquid and at the same time retain good structural integrity.
Various strategies have been employed to keep a good balance between ionic conductivity and mechanical strength. For instance, Shin et al.[16] introduced functionalized SiO2 nanoparticles as both cross-linking sites and reinforcement additive to fabricate high-performance composite GPEs with high ionic conductivity and good mechanical properties. The cross-linked polymer matrix, e.g. PAN in their study, provided strong structural integrity to absorb a large amount of liquid electrolytes while the introduction of SiO2 nanoparticles promotes the mechanical strength and creates good contacts at the interfaces for smooth ion transportation. This work represents a significantly effective approach via cross-linking and addition of nanofillers to greatly improving the ionic conductivity and mechanical properties.
2 Naturally Bio-Based Polymer ElectrolytesAlthough tremendous efforts have been made for development of synthetic polymer-based electrolytes, effective progress appears stagnant. The primary issue is that the ionic conductivity is limited to the ranges of 10-8-10-4 S/cm, which is fairly unsatisfactory for practical applications. Researchers have been pursuing breakthroughs and switched to some feasible natural materials. Particularly in recent years, motivated by sustainable development, people conducted more and more studies with a focus on exploiting environmental friendly materials to substitute synthetic polymer-based electrolytes. Naturally derived polymeric materials have received considerable attention because of their intrinsic advantages such as eco-benignity, low-cost and ease of accessibility. More importantly, they possess versatile functional groups in their more complicated structures so that they have notable advantages in multi-functionalities developed from nature. At the same time, it is well known that the development of new electrolytes to replace the traditional synthetic polymer electrolytes is desired from natural resources. Therefore, in this context, the significance of sustainable economy and diversity of polymer resources imply that natural polymers should be very promising substitutes for synthetic polymers.
As illustrated in Fig. 2, one of the important development trends of next-generation LIBs is towards "green" and flexible designs. It is believed that, electrolytes are the most possible component to be replaced with eco-friendly materials. In this case, naturally bio-based solid or gel electrolytes are expected to enable fabrication of safer and "greener" or flexible LIBs. Meanwhile, the employment of solid or gel electrolytes can avoid the use of separator, which would not only significantly reduce production costs but also can potentially enhance the electrochemical properties for the batteries. Therefore, in this review, state-of-the-art studies of employing various natural materials to fabricate polymer electrolytes for LIBs are summarized.
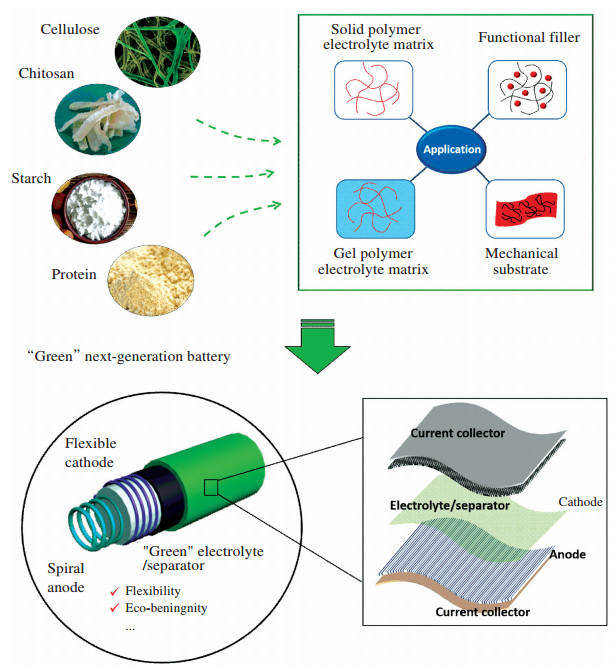
|
Figure 2 Illustration of the "green" next-generation flexible battery with specific applications of natural materials |
For application in LIBs, natural biopolymers such as cellulose[56-59], starch[60-63], protein[64-66], chitosan[67-70], etc. were found to be the most potential polymer electrolyte candidates. These biopolymers possess good mechanical properties (e.g. high modulus) due to their high molecular weight and special molecular structures, in which tremendous interactions exist within and among their molecular chains. More importantly, such natural biopolymers usually contain abundant functional groups (e.g. amine and carboxyl groups of protein) that might have great potential of being reactive to lithium salts or show good affinity with liquid electrolytes. In addition, most natural biopolymers contain active moieties that can be physically or chemically modified to enable the resulting electrolytes to exhibit good properties. Based on their unique functionalities, reported studies on applying natural polymers for making polymer electrolytes are summarized as follow (see Fig. 2). Firstly, natural polymers can act as polymer hosts for fabrication of polymer electrolytes. In regard of those natural biopolymers that can well dissolve lithium salts, SPEs can be directly fabricated via applying them as hosts. In addition, some of the natural polymers show good affinity with liquid electrolytes, which make them good candidates for GPEs. Secondly, the natural polymers can be applied as reinforcement additives to enhance the mechanical strength for conventional polymer electrolytes. With this aim, the natural polymers are dispersed in the polymer matrix as fillers or blended with the polymers to fabricate composite polymer electrolytes. Thirdly, the natural polymers can be used as mechanical support frames for fabricating high-performance polymer electrolytes. In this case, such polymers are usually employed as porous substrates where conventional polymer electrolytes are grown onto. In the following sections, several natural polymer materials that have been widely investigated for LIB electrolyte applications are introduced and significant findings are emphasized.
2.1 CelluloseCellulose is an abundant biomaterial on earth with outstanding properties such as biodegradability, chemical stability, thermal stability, good mechanical strength and so on[71-74]. Cellulose can be chemically modified via substituting the hydroxyl groups in the backbones to generate various derivatives. Over the past years, many cellulose derivatives have been studied for polymer electrolyte applications, e.g. carboxymethyl cellulose (CMC)[56, 75-76], hydroxyethyl cellulose (HEC)[77-78], etc. Besides their excellent mechanical properties, benefits also come from their loose fiber-like structures. Generally, cellulose usually possesses porous structures that are conducive to adsorb liquid electrolytes. Therefore, cellulose has been used as reinforcement agent[73, 79-80] or supportive substrate[59, 72, 81] for enhancing the mechanical strength and flexibility of polymer electrolytes. For instance, Colò et al.[75] reported a hybrid SPE with good mechanical integrity by blending PEO with CMC. At the same time, more studies have focused on employing cellulose (e.g. cellulose paper) as substrates for fabricating highly flexible polymer electrolytes, as cellulose exhibits good affinity and permeability for liquid electrolytes. For example, Zhang et al.[81] explored that the cellulose nonwoven membrane was applied as the backbone to prepare poly(propylene carbonate) (PPC)-based SPEs for fabricating all-solid-state LIBs. The PPC-based SPEs showed outstanding electrochemical performances such as high ionic conductivity of 3×10-4 S/cm and wide operation window of 4.6 V as well as good mechanical properties. Their results facilitated the fabrication of all-solid-state LIBs working properly at room temperature. Nair et al.[73] modified surface property of cellulose by UV-grafting of poly(ethylene glycol) methyl ether methacrylate to enhance the compatibility of cellulose hand-sheets with the polymer matrix. They obtained hybrid GPEs displaying excellent mechanical properties such as flexibility and good overall electrochemical performance (see Fig. 3, The figures are adapted from Ref.[73](Reprinted with permission from Elsevier)).

|
Figure 3 Properties of high-performance cellulose-enhanced GPEs |
The cellulose-enhanced GPEs showed large uptake and good stability for liquid electrolyte as demonstrated in Fig. 3(a), as well as great flexibility (see Fig. 3(b)) with absorption of liquid electrolyte. The lithium half-cells also worked very well demonstrating typical LiFePO4 charge-discharge profiles by employing the GPEs (Fig. 3(c)) and exhibiting excellent rate performance with very slight capacity decay as shown in Fig. 3(d). In addition to improving mechanical properties, Liu et al.[80] found that the addition of cellulose acetate butyrate (CAB) to PVDF enhanced the compatibility between the electrolyte and electrode and thus delivered a highest ionic conductivity of 2.48×10-3 S/cm at room temperature. Moreover, they also found that CAB played a critical role in affecting the porous structure of the composite polymer electrolyte host, and therefore further impacted the uptake of the liquid electrolyte.
The functionality of cellulose for electrolyte application is not limited as reinforcement additive.In fact, the simple and facile processing to generate cellulose membranes as well as large uptake of liquid electrolytes also make cellulose a promising polymer host for electrolytes. Zhu et al.[56] employed highly porous carboxymethyl cellulose, CMC, membrane as the polymer matrix for producing a GPE with high uptake of the liquid electrolyte to deliver a highest ionic conductivity of 4.8×10-4 S/cm at room temperature and greater transference number of 0.46 than that of liquid electrolyte (0.2-0.3). Interestingly, they found that CMC was a poly anionic cellulose that could impede the passing through of the anions of the lithium salts, and more pores could provide greater resistance for the movement of anions. They also reported a facile method to finely control the porous structure by simply adjusting the ratio of the solvent and non-solvent mixture. As a matter of fact that for GPEs, the uptake of liquid electrolyte is significant as it determines many properties of the GPEs such as ionic conductivity, mechanical properties, stability and so forth. It is therefore accepted that porous structure is usually desired for greater uptake amount and lower ion transport resistance. However, Li et al.[82] reported a dense cellulose-based membrane with non-porous structure as the host for GPE. They demonstrated a simple fabrication method to obtain the solid membrane by dissolving hydroxyethyl cellulose, HEC, in water and casting/drying the solution at room temperature. Such HEC-based GPE showed an ionic conductivity of 1.8×10-4 S/cm at room temperature and high transference number of 0.48. They found that the uptake amount for the liquid electrolyte of the non-porous HEC membrane was up to 78.3 wt% (a little lower than that of the Celgard separator of 90.9 wt%) due to a strong interaction between the hydroxyl groups of HEC and carbonate-based electrolyte (see Scheme 1, the figure is adapted from Ref. [82] with permission from Elsevier.). Such interaction did not only produce uptake of the liquid electrolyte, but also gave rise to a better stability of the interface film between lithium metal and the GPE.
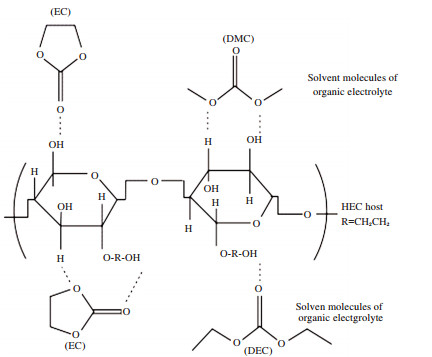
|
Scheme 1 Schematic illustration of the interactions between carbonate-based organic electrolytes and hydroxyl groups in HEC |
2.2 Chitosan
Chitosan has attracted extensive attention on account of its specific properties including biocompatibility, bioactivity and also because of its great potential in biomedical and industrial applications[83-84]. Chitosan also constitutes a polymer host in a polymer electrolyte for conducting protons, on which most research has been focusing on. As chitosan is also with the ability to dissolve lithium salts, it can be an alternative electrolyte material for conducting lithium ions. Chitosan-based SPEs, however, show a relatively low ionic conductivity resulting from the high crystallinity of it. However, because of its free amine and carboxyl groups, appropriate modifications can be made to generate a variety of chitosan derivatives. Therefore, modifications of native chitosan or blending it with other polymers are the primary approach to decreasing crystallinity and improving the ionic conductivity of chitosan electrolytes. For example, it was revealed that chemically modified chitosan, for instance, carboxymethyl chitosan, reported by Mobarak et al.[40], showed a two order of magnitude greater ionic conductivity of 3.6 × 10-6 S/cm at room temperature by increasing the oxygen content than that of native chitosan. Idiris et al.[85] found that the chitosan-PEO blend SPE exhibits an ambient ionic conductivity of ca.10-6 S/cm and good mechanical properties, but the ionic conductivity is still far from satisfactory. Further improvement was reported by Fuentes et al.[70] who fabricated composites of chitosan/poly(aminopropyltriethoxysilane)/poly(ethylene oxide) (CHI/pAPS/PEO) to deliver an optimal ionic conductivity of ca.10-5 S/cm at room temperature.
Despite tremendous efforts have been made to enhance the ionic conductivity of chitosan, in order to achieve further improvement towards practical applications, most research has focused on plasticizing chitosan by liquid electrolytes to form chitosan-based GPEs. For this purpose, carbonate based liquid electrolytes such as EC and PC[86] were frequently used to plasticize chitosan. The plasticized chitosan was usually prepared by dissolving chitosan and EC/PC in acetic acid solvent instead of plasticizing it after obtaining chitosan solid film. Osman et al.[87] studied through FTIR and the results indicated that EC did not interact with chitosan but reduced Tg for increasing segment motion; instead, EC strongly interacted with lithium salt due to solvation effect. At the same time, the ionic conductivity of chitosan was improved to 4×10-5 S/cm by using EC as a plasticizer. A deep understanding on chitosan-based polymer electrolytes was achieved by Navaratnam et al.[88] via studying the transport mechanism of chitosan. They introduced two types of lithium salts with different anion sizes, lithium acetate (LiCH3COO) and lithium triflate (LiCF3SO3). They found that the room temperature ionic conductivity of the LiCF3SO3 electrolyte was one order of magnitude higher than that of LiCH3COO electrolyte. This ionic conductivity enhancement was considered from the different dissociation capability and anion size of the two lithium salts. This study implied that larger anion size (e.g. triflate anion) gave rise to lower lattice energy and slow diffusion rate, which hindered the movement of lithium ions resulting in low ionic conductivity.
2.3 StarchStarch has been increasingly studied for various applications such as drug delivery, food packaging and medicine owing to its widely availability, low cost, and biodegradability[43, 63, 89-91]. Starch is a semi-crystalline polymer composed of a mixture of linear amylase and branched amylopectin polysaccharidechain[92]. Similar to the structure of PEO, starch also obtains -C-O-C-functional groups and a stable helix structure composed of repeating glucose monomers, which allows the reversible transportation of lithium ions[93]. Starch has excellent solubility for various lithium salts (e.g. 40% for LiClO4[94]) and at the same time, as a polymer host, starch also demonstrates good mechanical properties. Therefore, the ionic conductivity of starch-based electrolytes can be significantly improved by increasing loading of lithium salts. For example, Teoh et al.[94] reported a SPE based on corn starch, which showed a high ionic conductivity of 1.28×10-4 S/cm with a high lithium salt loading of 40 wt% at room temperature. Researchers have also been developing various strategies to enhance the ionic conductivity. Most studies have been focusing on plasticizing starch by glycerol to enhance the ionic conductivity. Glycerol is a widely used plasticizer due to its good compatibility with starch. Marcondes et al.[91] reported a GPE based on amylopectin-rich starch, which was plasticized by 30-35 wt% glycerol, showing a highest ionic conductivity of 1.1×10-4 S/cm at 30 ℃. In addition to glycerol, ionic liquid is another attractive candidate to plasticize starch. Liew et al.[95] found that the ionic conductivity of their corn starch-based electrolyte loaded with 80 wt% of ionic liquid (BmImTf) was increased by three orders of magnitude to 3.2×10-4 S/cm. They also found that N-O bonding was formed between imidazolium cations of the ionic liquid and starch polymer backbone, which increased the amorphous region to give rise to high ionic conductivity. Compared with glycerol, which is unstable and may react with lithium metal, ionic liquids, as good liquid electrolyte candidates, demonstrate overwhelming advantages including good electrochemical stability. Therefore, despite of its high cost, ionic liquids are a proper plasticizer for delivering desired ionic conductivity for fabricating starch based GPEs.
Plasticizing starch to promote chain motion is effective route to improving the ionic conductivity; however, the mechanical properties deteriorate simultaneously. Therefore, it is still a critical challenge to maintain a balance between ionic conductivity and mechanical properties. In fact, similar to the situation of conventional polymer electrolytes, employing ceramic nanofillers into the polymer hosts to fabricate composite electrolytes is believed to be one of the effective methods to improve the ionic conductivity while maintain good mechanical properties. Teoh et al.[96] investigated the effect of SiO2 on the corn-starch-based SPEs. They found that by applying a small loading of SiO2(4 wt%), the crystallinity of starch was significantly decreased and the ionic conductivity was increased to 1.24×10-4 S/cm. The complexations of starch, lithium salt and SiO2 could promote a new ion migration pathway and thus enhanced the lithium ion transport in the starch electrolyte.
In addition to above strategies, cross-linking the starch host is another effective solution to improve mechanical performance. Recently, an exceptional corn starch SPE with a high ionic conductivity of 3.39 × 10-4 S/cm at room temperature and high lithium ion transference number of 0.80 was reported by Lin et al.[93]. In their study, they cross-linked the starch by KH560 (see Fig. 4(a)) to improve the flexibility of the starch electrolyte film as shown in Fig. 4(b). Moreover, the starch SPE demonstrated good electrochemical properties and all-solid-state Li-S batteries were fabricated based on it. The Li-S batteries containing the starch SPE delivered a high initial discharge capacity of 1 442 mAh/g at a small current rate of 0.1 C (see Fig. 4(c)) at room temperature. At the same time, the SPE also showed excellent cycle stability at a high current rate of 0.5 C for 1 000 cycles with an average discharge capacity of around 562 mAh/g with approximately 100% efficiency for each cycle (see Fig. 4(d)). This study has demonstrated a great potential of starch for advanced flexible electrolyte for battery applications. In Fig. 4 the figures are adapted from Ref.[93], reprinted with permission from Royal Society of Chemistry.
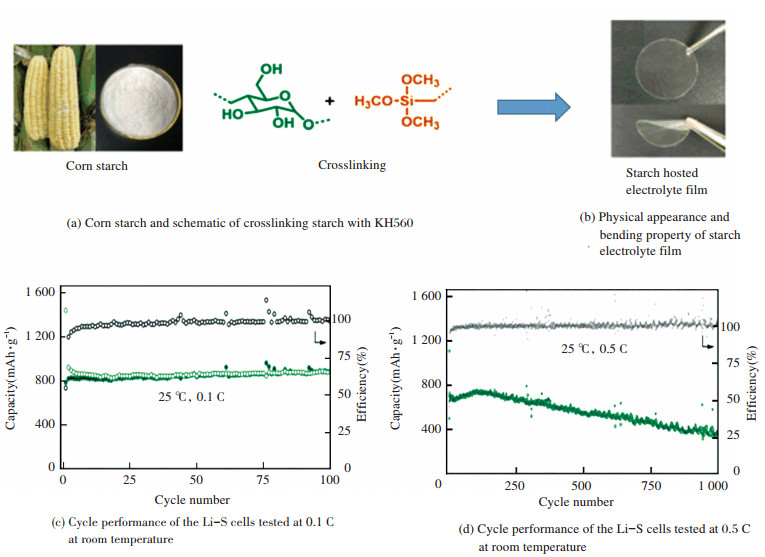
|
Figure 4 Properties and battery performance of corn starch based electrolyte |
2.4 Protein
Proteins are natural polymers composed of a series of amino acids covalently linked through peptide bonds. Compared with synthetic polymers, proteins in general are compositionally and structurally much more complex. Basically, proteins contain such four levels of structures as primary, secondary, tertiary and quaternary structures[97-98], and compactly enclose various functional groups, e.g. polar, nonpolar, charged groups, etc. The complex structures and compositions of proteins actually determine their multi-functionalities. It is well known that proteins possess good solubility for lithium salts and high modulus; thus, they can be utilized as polymer hosts for polymer electrolytes. Thus far, gelatin is the most widely studied protein for electrolyte applications.
Gelatin is able to be dissolved in water-based solvents and can easily form films via simple casting. Therefore, there are a number of studies reported on gelatin hosted polymer electrolytes. However, due to the fact that the ionic conductivity of liquid-free gelatin-based electrolytes is extremely low, most of the research has concentrated on preparation of gelatin-based GPEs. For instance, Ramadan et al.[64] investigated gelatin-based GPE by using glycerol as the plasticizer and cross-linked the gelatin by formaldehyde to enhance the mechanical properties of the resulting electrolyte. A highest ionic conductivity of ca. 10-4 S/cm at room temperature was achieved from the gelatin-based GPE. It was also found that the ionic conductivity was further enhanced by addition of an ionic liquid. The work presented by Leones et al.[99] demonstrated an ionic liquid doped gelatin electrolyte with a highest ionic conductivity of 1.18 × 10-4 S/cm at 30 ℃. However, the electrochemical stability window versus Li/Li+ is only 2.0 V, which was possibly led by the decomposition of glycerol. The researchers believed that the presence of glycerol significantly limited the practical applications for gelatin based electrolytes.
In addition to gelatin, Fu et al.[65] recently found that soy protein was potentially a high-performance lithium ion conductor. The ionic conductivity reached ca. 10-5 S/cm from their soy protein electrolyte optimized by controlling the procedures for sample preparation. In their studies, it was also found that soy protein had very strong interactions with the lithium salts due to the charged functional groups of the protein. The structures of the protein were found to be very sensitive to the lithium salt loading levels and the formation temperature as shown in Fig. 5(a). Therefore, the researchers optimized the protein-based electrolyte materials by adjusting the salt loading and formation temperature (see Fig. 5(b)), and then they found the ionic conductivity was increased by several orders of magnitude. At the same time, as indicated in Fig. 5(c), such lithium salt loaded protein electrolyte displayed good mechanical properties. In specific, its modulus was almost two orders of magnitude higher than that of PEO electrolyte. More importantly, they reported an extremely high transference number of 0.93 for the soy protein electrolyte (Fig. 5(c)), which indicated that the protein strongly attracted the anions and immobilize them. The possible ion transport behavior "decoupled ion transportation" is illustrated in Fig. 5(d).
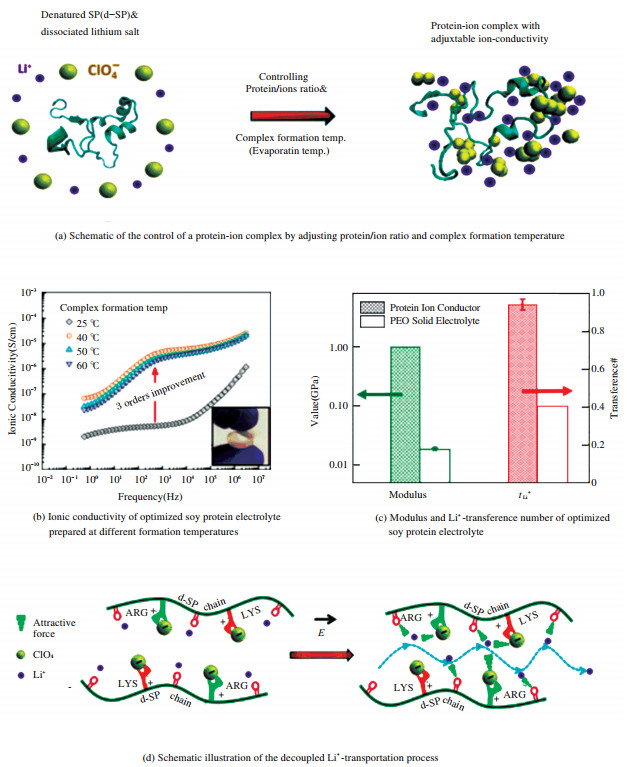
|
Figure 5 Ionic conductivity and mechanical properties of protein-ion-conductor |
It indicated that this mechanism completely differed from that of the conventional ion transport mechanism for polymer electrolytes. In Fig. 5, The figures are adapted from Ref.[65], reprinted with permission from American Chemical Society. The researchers believed that the lithium ions were able to transport via anion clusters formed by interaction between the anions and protein's positive charge groups, and the attraction from the negative oxygen atoms in the backbones of the protein. Therefore, the ionic conductivity was improved without deteriorating the mechanical properties, which resolved one of the main issues for polymer electrolytes. This study provides a new avenue to investigation and development of biopolymer-based electrolytes via taking advantages of their unique structures and functionalities from nature.
In the reported studies on electrolytes, proteins have been applied beyond as polymer hosts. Due to their abundant functional groups that are able to interact with polar polymers, proteins can also be used as multi-functional additives for enhancing the mechanical properties of conventional polymer electrolytes. Ji et al.[66] reported an ultra-elastic blend of PEO and soy protein with a fully amorphous structure. They found that there might be strong interactions among the three components including soy protein, PEO and lithium salt, to eventually result in the fully amorphous structure of PEO as well as great flexibility. In addition, the blend of PEO/soy-protein electrolyte film exhibited excellent elasticity and an average ultimate tensile strength of 0.98 MPa as illustrated in Figs. 6(a)-(b). The nanoindentation results (Fig. 6(c)) also indicated that the PEO/soy-protein film was more elastic than PEO film. At the same time, the PEO/soy-protein showed significantly reduced bulk resistance (Fig. 6(d)) and a highest ionic conductivity of 2.63 × 10-6 S/cm at room temperature (Fig. 6(e)).
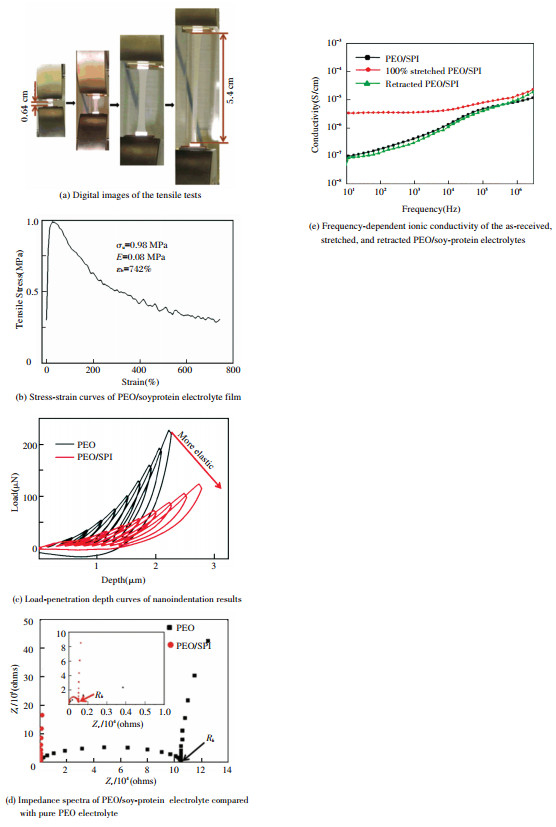
|
Figure 6 Mechanical and electrochemical properties of soy protein reinforced electrolytes |
In Fig. 6, The figures are adapted from Ref.[66], reprinted with permission from American Chemical Society. The highly stretchable and elastic PEO/soy-protein electrolyte film was of great potential for fabrication of fully flexible LIBs. Proteins can also reinforce other properties of the polymer electrolytes besides mechanical properties. For instance, Wang et al.[100] investigated protein reinforced adhesive composite electrolytes with high ionic conductivity ~ 10-3 S/cm at room temperature. The researchers believed that flexible adhesive electrolytes were one of the effective solutions to achieving good contact between the electrolyte and electrodes, especially when the battery was experiencing deformations such as volume change of the electrodes generated by charging/discharging. They investigated two types of proteins, gelatin and soy protein, and found that they both were effective multi-functional fillers for enhancing not only the mechanical properties but also adhesive property of the PEO-based composite electrolytes. The protein-reinforced electrolytes possessed good adhesive property to the electrode and current collector surfaces due to protein's rich functional groups providing various interactions to the substrates. In specific, the gelatin reinforced electrolytes exhibited greater storage modulus and adhesive strength compared with soy protein reinforced ones. The studies indicated that the compatibility between the fillers and polymer matrix as well as particle size of the fillers were critical factors for the mechanical properties and adhesive property of the electrolytes.
3 Conclusions and OutlookThis review summarizes the primary and representative progress in the development of natural polymer-based electrolytes for application of LIBs. All-solid-state or flexible LIBs have been pursued for many years and offer many significant advantages over commercial LIBs with liquid electrolytes, including enhanced safety, higher energy density and wider operation temperature. Polymer electrolytes are the most promising candidates to replace liquid electrolytes in order to achieve lightweight all-solid-state LIBs; however, polymer electrolytes suffer from unsatisfactory ionic conductivity (10-8-10-4 S/cm at room temperature). At present, the development of polymer electrolytes seems to meet a bottleneck in spite of tremendous efforts having been made. For polymer electrolytes, due to their coupled ion transport mechanism, the ionic conductivity is seriously limited and a trade-off effect exists between ionic conductivity and mechanical properties. Recent studies implied that some of the limitations of conventional polymer electrolytes can be overcome by natural polymers. As compared with simple synthetic polymers, natural polymers are more complex in their composition, structure as well as functionality. An in-depth understanding and utilization of their unique attributes can promote the efforts of developing advanced polymer electrolytes.
Motivated by specific properties of the natural polymers, people can design new materials with advanced performance for different application purposes. For instance, for those natural polymers such as starch and protein, which can greatly dissociate lithium salts, they are good candidates as polymer hosts for SPEs with high ionic conductivity. For crystalline or semi-crystalline biopolymers including chitosan and starch, in order to achieve high ionic conductivity, basically one of the most useful routes is to reduce crystallinity via chemical modifications, addition of nanoparticles, cross-linking or addition of liquid electrolytes to result in GPEs. Some natural polymers (e.g. cellulose derivatives) with good affinity with liquid electrolytes can be used as scaffold to adsorb a large amount of liquid electrolytes for advanced flexible GPEs. In addition, natural polymers are also effective fillers for enhancing conventional polymer electrolytes. Thus far, it has been found that addition of cellulose and protein can significantly reinforce the mechanical performance of the electrolytes; in specific, protein as a multifunctional filler has been found to significantly enhance the adhesive property.
Based on the significant progress having been achieved thus far, the following research directions for further development of advanced high-performance natural polymer electrolytes are recommended. First, because a complete replacement of conventional synthetic polymer electrolytes by natural polymers remains a big challenge, a combination of synthetic polymers and natural polymers would be an attractive solution to achieve good comprehensive properties. Exploration on various natural polymers with good compatibility with developed synthetic polymer electrolytes, good mechanical properties or even unique affinity with electrolyte ions should help identify a class of effective enhancement additive. Second, to obtain thorough natural polymer electrolytes with high ionic conductivity for practical applications, the best strategy lies in using liquid electrolytes to plasticize the natural polymer hosts. In this case, there is a strong need for exploration of natural polymers that can be well compatible with liquid electrolytes. Third, in-depth studies and understanding of the ion transport mechanism in the natural polymers are critically demanded for further improvement in the electrolyte performance. It is believed that the ion transport mechanism of natural biopolymers should be more complicated than that in simple synthetic polymer electrolytes. Via learning the mechanisms, the electrolyte properties can be improved by designing desired architecture or composition conducive for ion transportation process. In brief, nature is a rich "warehouse" of inspirations and provides us a wise route to explore and fabricate more efficient electrolytes for advanced LIBs.
| [1] |
Wang H, Yang Y, Guo L. Nature-inspired electrochemical energy-storage materials and devices. Advanced Energy Materials, 2016, 7(5): 1601709. DOI:10.1002/aenm.201601709 ( 0) 0)
|
| [2] |
Palacin M R, de Guibert A. Why do batteries fail?. Science, 2016, 351: 1253292. DOI:10.1126/science.1253292 ( 0) 0)
|
| [3] |
Scrosati B, Hassoun J, Sun Y K. Lithium-ion batteries. A Look into the Future. Energy Environ. Sci., 2011, 4(9): 3287. DOI:10.1039/C1EE01388B ( 0) 0)
|
| [4] |
Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chemical Reviews, 2004, 104(10): 4303-4417. DOI:10.1002/chin.200450271 ( 0) 0)
|
| [5] |
Xue Z, He D, Xie X. Poly(ethylene Oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A, 2015, 3(38): 19218-19253. DOI:10.1039/C5TA03471J ( 0) 0)
|
| [6] |
Agrawal R C, Pandey G P. Solid polymer electrolytes: Materials designing and all-solid-state battery applications: An overview. Journal of Physics D: Applied Physics, 2008, 41(22): 223001. DOI:10.1088/0022-3727/41/22/223001 ( 0) 0)
|
| [7] |
Wang Y, Zhong W H. Development of electrolytes towards achieving safe and high-performance energy-storage devices: A review. ChemElectroChem, 2015, 2(1): 22-36. DOI:10.1002/celc.201402277 ( 0) 0)
|
| [8] |
Lee Y H, Kim J S, Noh J, et al. Wearable textile battery rechargeable by solar energy. Nano Lett, 2013, 13: 5753-5761. DOI:10.1021/nl403860k ( 0) 0)
|
| [9] |
Liang J, Zhao Y, Guo L, et al. Flexible free-standing graphene/SnO2 nanocomposites paper for Li-Ion battery. ACS Applied Materials and Interfaces, 2012, 4(11): 5742-5748. DOI:10.1021/am301962d ( 0) 0)
|
| [10] |
Liang B, Tang S, Jiang Q, et al. Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochimica Acta, 2015, 169: 334-341. DOI:10.1016/j.electacta.2015.04.03 ( 0) 0)
|
| [11] |
Stephan A M. Review on gel polymer electrolytes for lithium batteries. European Polymer Journal, 2006, 42(1): 21-42. DOI:10.1016/j.eurpolymj.2005.09.017 ( 0) 0)
|
| [12] |
Cao C, Li Z B, Wang X L, et al. Recent advances in inorganic solid electrolytes for lithium batteries. Frontiers in Energy Research, 2014, 2: 25. DOI:10.3389/fenrg.2014.00025 ( 0) 0)
|
| [13] |
Bachman J C, Muy S, Grimaud A, et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chemical Reviews, 2016, 116(1): 140-162. DOI:10.1021/acs.chemrev.5b00563 ( 0) 0)
|
| [14] |
Chun-Guey W, Chiung-Hui W, Ming-I L, et al. New solid polymer electrolytes based on PEO/PAN hybrids. Journal of Applied Polymer Science, 2006, 99(4): 1530-1540. DOI:10.1002/app.22250 ( 0) 0)
|
| [15] |
Zhao Y, Huang Z, Chen S, et al. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries. Solid State Ionics, 2016, 295: 65-71. DOI:10.1016/j.ssi.2016.07.013 ( 0) 0)
|
| [16] |
Shin W K, Cho J, Kannan A G, et al. Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Scientific Reports, 2016, 6(1): 26332. DOI:10.1038/srep26332 ( 0) 0)
|
| [17] |
Sil A, Sharma R, Ray S. Carbon nanofibers reinforced P (VdF-HFP) based gel polymer electrolyte for lithium-ion battery application. Materials and Metallurgical Engineering, 2015, 9(10): 1124-1127. ( 0) 0)
|
| [18] |
Fu J, Zhang J, Song X, et al. A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc-air batteries. Energy Environ. Sci., 2016, 9(2): 663-670. DOI:10.1039/C5EE03404C ( 0) 0)
|
| [19] |
Wang X, Lu X, Liu B, et al. Flexible energy-storage devices: Design consideration and recent progress. Advanced Materials, 2014, 26(28): 4763-4782. DOI:10.1002/adma.201400910 ( 0) 0)
|
| [20] |
Khurana R, Schaefer J L, Archer L A. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: A new approach for practical lithium-metal polymer batteries. Journal of the American Chemical Society, 2014, 136(20): 7395-7402. DOI:10.1021/ja502133j ( 0) 0)
|
| [21] |
Kim S H, Choi K H, Cho S J, et al. Mechanically compliant and lithium dendrite growth-suppressing composite polymer electrolytes for flexible lithium-ion batteries. Journal of Materials Chemistry A, 2013, 1(16): 4949. DOI:10.1039/C3TA10612H ( 0) 0)
|
| [22] |
Mohd Noor S A B, Ahmad A, Rahman M Y, et al. Solid polymeric electrolyte of poly(ethylene)oxide-50% epoxidized natural rubber-lithium triflate (PEO-ENR50-LiCF3SO3). Nat. Sci., 2010, 2: 190-196. DOI:10.4236/ns.2010.23029 ( 0) 0)
|
| [23] |
Kumar B, Rodrigues S J, Koka S. The crystalline to amorphous transition in PEO-based composite electrolytes: Role of lithium salts. Electrochimica Acta, 2002, 47(25): 4125-4131. DOI:10.1016/S0013-4686(02)00442-5 ( 0) 0)
|
| [24] |
Zhang H, Kulkarni, S, Wunder S L. Blends of POSS-PEO(n=4)8 and high molecular weight poly(ethylene oxide) as solid polymer electrolytes for lithium batteries. J. Phys. Chem B, 2007, 111(14): 3582-3590. DOI:10.1021/jp064585g ( 0) 0)
|
| [25] |
Watanabe M, Nagaoka K, Kanba M, et al. Ionic conductivity of polymeric solid electrolytes based on poly(propylene oxide) or poly(tetramethylene oxide). Polymer Journal, 1982, 14(11): 877-886. DOI:10.1295/polymj.14.877 ( 0) 0)
|
| [26] |
Choi Y S, Bae Y C, Sun Y K. Phase behaviors of polymer blend(PEO-PPO) electrolyte/LiCF3SO3 systems in lithium battery. Journal of Applied Polymer Science, 2005, 98: 2314-2319. DOI:10.1002/app.22418 ( 0) 0)
|
| [27] |
Wang S H, Hou S S, Kuo P L, et al. Poly(ethylene oxide)-co-poly(propylene oxide)-based gel electrolyte with high ionic conductivity and mechanical integrity for lithium-ion batteries. ACS Applied Materials and Interfaces, 2013, 5(17): 8477-8485. DOI:10.1021/am4019115 ( 0) 0)
|
| [28] |
Helan Flora X, Ulaganathan M, Rajendran S. Influence of lithium salt concentration on PAN-PMMA blend polymer electrolytes. International Journal of Electrochemical Science, 2012, 7: 7451-7462. ( 0) 0)
|
| [29] |
Kuo P L, Wu C A, Lu C Y, et al. High performance of transferring lithium ion for polyacrylonitrile-interpenetrating crosslinked polyoxyethylene network as gel polymer electrolyte. ACS Applied Materials & Interfaces, 2014, 6(5): 3156-3162. DOI:10.1021/am404248b ( 0) 0)
|
| [30] |
Agapov A L, Sokolov A P. Decoupling ionic conductivity from structural relaxation: A way to solid polymer electrolytes?. Macromolecules, 2011, 44(11): 4410-4414. DOI:10.1021/ma2001096 ( 0) 0)
|
| [31] |
Diddens D, Heuer A, Borodin O. Understanding the lithium transport within a rouse-based model for a PEO/LiTFSI polymer electrolyte. Macromolecules, 2010, 43(4): 2028-2036. DOI:10.1021/ma901893h ( 0) 0)
|
| [32] |
Teran A A, Tang M H, Mullin S A. Effect of molecular weight on conductivity of polymer electrolytes. Solid State Ionics, 2011, 203(1): 18-21. DOI:10.1016/j.ssi.2011.09.021 ( 0) 0)
|
| [33] |
Qian X, Gu N, Cheng Z, et al. Plasticizer effect on the ionic conductivity of PEO-based polymer electrolyte. Materials Chemistry and Physics, 2002, 74(1): 98-103. DOI:10.1016/S0254-0584(01)00408-4 ( 0) 0)
|
| [34] |
Suthanthiraraj S A, Vadivel M K. Effect of propylene carbonate as a plasticizer on (PEO)50AgCF3SO3:SnO2 nanocomposite polymer electrolyte. Applied Nanoscience, 2012, 2(3): 239-246. DOI:10.1007/s13204-012-0099-3 ( 0) 0)
|
| [35] |
Lim Y J, An Y H, Jo N J. Polystyrene-Al2O3 composite solid polymer electrolyte for lithium secondary battery. Nanoscale Research Letters, 2012, 7(1): 19. DOI:10.1186/1556-276X-7-19 ( 0) 0)
|
| [36] |
Tambelli C C, Bloise A C, Rosário A V, et al. Characterisation of PEO-Al2O3 composite polymer electrolytes. Electrochimica Acta, 2002, 47(11): 1677-1682. DOI:10.1016/S0013-4686(01)00900-8 ( 0) 0)
|
| [37] |
Ni Mah Y L, Cheng M Y, Cheng J H, et al. Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. Journal of Power Sources, 2015, 278: 375-381. DOI:10.1016/j.jpowsour.2014.11.047 ( 0) 0)
|
| [38] |
Singh P K, Bhattacharya B, Nagarale R K. Effect of nano-TiO2 dispersion on PEO polymer electrolyte property. Journal of Applied Polymer Science, 2010, 118: 2976-2980. DOI:10.1002/app.32726 ( 0) 0)
|
| [39] |
Ren Z, Liu Y, Sun K, et al. A microporous gel electrolyte based on poly(vinylidene fluoride-co-hexafluoropropylene)/fully cyanoethylated cellulose derivative blend for lithium-ion battery. Electrochimica Acta, 2009, 54(6): 1888-1892. DOI:10.1016/j.electacta.2008.10.011 ( 0) 0)
|
| [40] |
Mobarak N N, Ahmad A, Abdullah M P, et al. Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochimica Acta, 2013, 92: 161-167. DOI:10.1016/j.electacta.2012.12.126 ( 0) 0)
|
| [41] |
Liu W, Lin D, Sun J, et al. Improved lithium ionic conductivity in composite polymer electrolytes with oxide-ion conducting nanowires. ACS Nano, 2016, 10(12): 11407-11413. DOI:10.1021/acsnano.6b06797 ( 0) 0)
|
| [42] |
Kumar B, Scanlon L, Marsh R, et al. Structural evolution and conductivity of PEO:LiBF4-MgO composite electrolytes. Electrochimica Acta, 2001, 46(10-11): 1515-1521. DOI:10.1016/S0013-4686(00)00747-7 ( 0) 0)
|
| [43] |
Varshney P K, Gupta S. Natural polymer-based electrolytes for electrochemical devices: A review. Ionics, 2011, 17(6): 479-483. DOI:10.1007/s11581-011-0563-1 ( 0) 0)
|
| [44] |
Zhao X G, Jin E M, Park J Y, et al. Hybrid polymer electrolyte composite with SiO2 nanofiber filler for solid-state dye-sensitized solar cells. Composites Science and Technology, 2014, 103: 100-105. DOI:10.1016/j.compscitech.2014.08.020 ( 0) 0)
|
| [45] |
Liu W, Liu N, Sun J, et al. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Letters, 2015, 15(4): 2740-2745. DOI:10.1021/acs.nanolett.5b00600 ( 0) 0)
|
| [46] |
Shim J, Kim D G, Kim H J, et al. Polymer composite electrolytes having core-shell silica fillers with anion-trapping boron moiety in the shell layer for all-solid-state lithium-ion batteries. ACS Applied Materials and Interfaces, 2015, 7(14): 7690-7701. DOI:10.1021/acsami.5b00618 ( 0) 0)
|
| [47] |
Bouchet R, Maria S, Meziane R, et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nature Materials, 2013, 12(5): 452-457. DOI:10.1038/nmat3602 ( 0) 0)
|
| [48] |
Tran B, Oladeji I O, Zou J, et al. Adhesive poly(PEGMA-Co-MMA-Co-IBVE) copolymer electrolyte. Solid State Ionics, 2013, 232: 37-43. DOI:10.1016/j.ssi.2012.11.007 ( 0) 0)
|
| [49] |
Ghosh A, Wang C, Kofinas P. Block copolymer solid battery electrolyte with high Li-Ion transference number. Journal of The Electrochemical Society, 2010, 157(7): A846-A849. DOI:10.1149/1.3428710 ( 0) 0)
|
| [50] |
Rao M M, Liu J S, Li W S, et al. Preparation and performance analysis of PE-supported P(AN-co-MMA) gel polymer electrolyte for lithium ion battery application. Journal of Membrane Science, 2008, 322(2): 314-319. DOI:10.1016/j.memsci.2008.06.004 ( 0) 0)
|
| [51] |
Devaux D, Gle D, Phan T N T, et al. Optimization of block copolymer electrolytes for lithium metal batteries. Chemistry of Materials, 2015, 27(13): 4682-4692. DOI:10.1021/acs.chemmater.5b01273 ( 0) 0)
|
| [52] |
Wang Y, Li B, Ji J, et al. A gum-like electrolyte: Safety of a solid, performance of a liquid. Advanced Energy Materials, 2013, 3(12): 1557-1562. DOI:10.1002/aenm.201300495 ( 0) 0)
|
| [53] |
Zhang H, Ma X, Lin C, et al. Gel polymer electrolyte-based on PVDF/fluorinated amphiphilic copolymer blends for high performance lithium-ion batteries. RSC Advances, 2014, 4(64): 33713. DOI:10.1039/C4RA04443F ( 0) 0)
|
| [54] |
Miao R, Liu B, Zhu Z, et al. PVDF-HFP-based porous polymer electrolyte membranes for lithium-ion batteries. Journal of Power Sources, 2008, 184(2): 420-426. DOI:10.1016/j.jpowsour.2008.03.045 ( 0) 0)
|
| [55] |
Choi N S, Park J K. New polymer electrolytes based on PVC/PMMA blend for plastic lithium-ion batteries. Electrochimica Acta, 2001, 46(10-11): 1453-1459. DOI:10.1016/S0013-4686(00)00739-8 ( 0) 0)
|
| [56] |
Zhu Y S, Xiao S Y, Li M X, et al. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries. Journal of Power Sources, 2015, 288: 368-375. DOI:10.1016/j.jpowsour.2015.04.117 ( 0) 0)
|
| [57] |
Li P, Zhang Y, Fa W, et al. Synthesis of a grafted cellulose gel electrolyte in an ionic liquid ([Bmim]I) for dye-sensitized solar cells. Carbohydrate Polymers, 2011, 86(3): 1216-1220. DOI:10.1016/j.carbpol.2011.06.017 ( 0) 0)
|
| [58] |
Regiani A M, de Oliveira-Machado G, LeNest J F, et al. Cellulose derivatives as solid electrolyte matrixes. Macromolecular Symposia, 2001, 175: 45-53. DOI:10.1002/1521-3900(200110)175:1<45::AID-MASY45>3.0.CO;2-M ( 0) 0)
|
| [59] |
Zhao J, Zhang J, Hu P, et al. A sustainable and rigid-flexible coupling cellulose-supported poly(propylene carbonate) polymer electrolyte towards 5 V high voltage lithium batteries. Electrochimica Acta, 2016, 188: 23-30. DOI:10.1016/j.electacta.2015.11.088 ( 0) 0)
|
| [60] |
Sudhakar Y N, Selvakumar M. Ionic conductivity studies and dielectric studies of Poly(styrene sulphonic acid)/starch blend polymer electrolyte containing LiClO4. Journal of Applied Electrochemistry, 2013, 43(1): 21-29. DOI:10.1007/s10800-012-0493-2 ( 0) 0)
|
| [61] |
Ramesh S, Shanti R, Morris E, et al. Utilisation of corn starch in production of "green" polymer electrolytes. Mater. Res. Innov., 2011, 15(sup2): s13-s18. DOI:10.1179/143307511X13031890747291 ( 0) 0)
|
| [62] |
Kumar M, Tiwari T, Srivastava N. Electrical transport behaviour of bio-polymer electrolyte system: Potato starch+ ammonium iodide. Carbohydrate Polymers, 2012, 88(1): 54-60. DOI:10.1016/j.carbpol.2011.11.059 ( 0) 0)
|
| [63] |
Khanmirzaei M H, Ramesh S. Ionic transport and FTIR properties of lithium iodide doped biodegradable rice starch based polymer electrolytes. International Journal of Electrochemical Science, 2013, 8(7): 9977-9991. ( 0) 0)
|
| [64] |
Ramadan R, Kamal H, Hashem H M, et al. Gelatin-based solid electrolyte releasing Li+ for smart window applications. Solar Energy Materials and Solar Cells, 2014, 127: 147-156. DOI:10.1016/j.solmat.2014.04.016 ( 0) 0)
|
| [65] |
Fu X, Jewel Y, Wang Y, et al. Decoupled Ion transport in a protein-based solid Ion conductor. Journal of Physical Chemistry Letters, 2016, 7(21): 4304-4310. DOI:10.1021/acs.jpclett.6b02071 ( 0) 0)
|
| [66] |
Ji J, Li B, Zhong W H. An ultraelastic poly(ethylene oxide)/soy protein film with fully amorphous structure. Macromolecules, 2012, 45(1): 602-606. DOI:10.1021/ma202347v ( 0) 0)
|
| [67] |
Aziz N A, Majid S R, Yahya R, et al. Conductivity, structure, and thermal properties of chitosan-based polymer electrolytes with nanofillers. Polymers for Advanced Technologies, 2011, 22(9): 1345-1348. DOI:10.1002/pat.1619 ( 0) 0)
|
| [68] |
Khiar A S A, Puteh R, Arof A K. Conductivity studies of a chitosan-based polymer electrolyte. Physica B: Condensed Matter, 2006, 373(1): 23-27. DOI:10.1016/j.physb.2005.10.104 ( 0) 0)
|
| [69] |
Osman Z, Arof A K. FTIR studies of chitosan acetate based polymer electrolytes. Electrochimica Acta, 2003, 48(8): 993-999. DOI:10.1016/S0013-4686(02)00812-5 ( 0) 0)
|
| [70] |
Fuentes S, Retuert P J, González G. Lithium ion conductivity of molecularly compatibilized chitosan-poly(aminopropyltriethoxysilane)-poly(ethylene oxide) nanocomposites. Electrochimica Acta, 2007, 53(4): 1417-1421. DOI:10.1016/j.electacta.2007.05.057 ( 0) 0)
|
| [71] |
Cherian B M, Leão A L, de Souza S F, et al. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydrate Polymers, 2011, 86(4): 1790-1798. DOI:10.1016/j.carbpol.2011.07.009 ( 0) 0)
|
| [72] |
Zhang J, Yue L, Hu P, et al. Taichi-inspired rigid-flexible coupling cellulose-supported solid polymer electrolyte for high-performance lithium batteries. Scientific Reports, 2014, 4(1): 6272. DOI:10.1038/srep06272 ( 0) 0)
|
| [73] |
Nair J R, Chiappone A, Gerbaldi C, et al. Novel cellulose reinforcement for polymer electrolyte membranes with outstanding mechanical properties. Electrochimica Acta, 2011, 57(1): 104-111. DOI:10.1016/j.electacta.2011.03.124 ( 0) 0)
|
| [74] |
Reddy N, Yang Y. Properties and potential applications of natural cellulose fibers from the bark of cotton stalks. Bioresource Technology, 2009, 100(14): 3563-3569. DOI:10.1016/j.biortech.2009.02.047 ( 0) 0)
|
| [75] |
Colò F, Bella F, Nair J R, et al. Cellulose-based novel hybrid polymer electrolytes for green and efficient Na-ion batteries. Electrochimica Acta, 2015, 174: 185-190. DOI:10.1016/j.electacta.2015.05.178 ( 0) 0)
|
| [76] |
Bella F, Nair J R, Gerbaldi C. Towards green, efficient and durable quasi-solid dye-sensitized solar cells integrated with a cellulose-based gel-polymer electrolyte optimized by a chemometric DoE approach. RSC Advances, 2013, 3(36): 15993-16001. DOI:10.1039/c3ra41267a ( 0) 0)
|
| [77] |
MacHado G O, Ferreira H C A, Pawlicka A. Influence of plasticizer contents on the properties of HEC-based solid polymeric electrolytes. Electrochimica Acta, 2005, 50(19): 3827-3831. DOI:10.1016/j.electacta.2005.02.041 ( 0) 0)
|
| [78] |
Tambelli C E, Donoso J P, Regiani A M, et al. Nuclear magnetic resonance and conductivity study of HEC/polyether-based polymer electrolytes. Electrochimica Acta, 2001, 46(10-11): 1665-1672. DOI:10.1016/S0013-4686(00)00768-4 ( 0) 0)
|
| [79] |
Nair J R, Gerbaldi C, Chiappone A, et al. UV-cured polymer electrolyte membranes for Li-cells: Improved mechanical properties by a novel cellulose reinforcement. Electrochemistry Communications, 2009, 11(9): 1796-1798. DOI:10.1016/j.elecom.2009.07.021 ( 0) 0)
|
| [80] |
Liu J, Li W, Zuo X, et al. Polyethylene-supported polyvinylidene fluoride-cellulose acetate butyrate blended polymer electrolyte for lithium ion battery. Journal of Power Sources, 2013, 226: 101-106. DOI:10.1016/j.jpowsour.2012.10.078 ( 0) 0)
|
| [81] |
Zhang J, Zhao J, Yue L, et al. Safety-reinforced poly(propylene carbonate)-based all-solid-state polymer electrolyte for ambient-temperature solid polymer lithium batteries. Advanced Energy Materials, 2015, 5(24): 1-10. DOI:10.1002/aenm.201501082 ( 0) 0)
|
| [82] |
Li M X, Wang X W, Yang Y Q, et al. A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. Journal of Membrane Science, 2015, 476: 112-118. DOI:10.1016/j.memsci.2014.10.056 ( 0) 0)
|
| [83] |
Arof A, Osman Z, Morni N, et al. Chitosan-based electrolyte for secondary lithium cells. Journal of Materials Science, 2001, 36(3): 791-793. DOI:10.1023/A:1004869815261 ( 0) 0)
|
| [84] |
Yamagata M, Soeda K, Ikebe S, et al. Chitosan-based gel electrolyte containing an ionic liquid for high-performance nonaqueous supercapacitors. Electrochimica Acta, 2013, 100: 275-280. DOI:10.1016/j.electacta.2012.05.073 ( 0) 0)
|
| [85] |
Idris N H, Maji S R, Khiar A S A, et al. Conductivity Studies on chitosan/PEO blends with LiTFSI salt. Ionics, 2005, 11(5-6): 375-377. DOI:10.1007/BF02430249 ( 0) 0)
|
| [86] |
Yulianti E, Karo A K, Susita L, et al. Synthesis of electrolyte polymer based on natural polymer chitosan by ion implantation technique. Procedia Chemistry, 2012, 4: 202-207. DOI:10.1016/j.proche.2012.06.028 ( 0) 0)
|
| [87] |
Osman Z, Ibrahim Z A, Arof A K. Conductivity enhancement due to ion dissociation in plasticized chitosan based polymer electrolytes. Carbohydrate Polymers, 2001, 44(2): 167-173. DOI:10.1016/S0144-8617(00)00236-8 ( 0) 0)
|
| [88] |
Navaratnam S, Ramesh K, Ramesh S, et al. Transport mechanism studies of chitosan electrolyte systems. Electrochimica Acta, 2015, 175: 68-73. DOI:10.1016/j.electacta.2015.01.087 ( 0) 0)
|
| [89] |
Ramesh S, Liew C W, Arof A K. Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. Journal of Non-Crystalline Solids, 2011, 357(21): 3654-3660. DOI:10.1016/j.jnoncrysol.2011.06.030 ( 0) 0)
|
| [90] |
Shukur M F, Ibrahim F M, Majid N A, et al. Electrical analysis of amorphous corn starch-based polymer electrolyte membranes doped with LiI. Phys. Scr., 2013, 88: 25601. DOI:10.1088/0031-8949/88/02/025601 ( 0) 0)
|
| [91] |
Marcondes R F M S, D'Agostini P S, Ferreira J, et al. Amylopectin-rich starch plasticized with glycerol for polymer electrolyte application. Solid State Ionics, 2010, 181(13-14): 586-591. DOI:10.1016/j.ssi.2010.03.016 ( 0) 0)
|
| [92] |
Mattos R I, Tambelli C E, Donoso J P, et al. NMR study of starch based polymer gel electrolytes: Humidity effects. Electrochimica Acta, 2007, 53(4): 1461-1465. DOI:10.1016/j.electacta.2007.05.061 ( 0) 0)
|
| [93] |
Lin Y, Li J, Liu K, et al. Unique starch polymer electrolyte for high capacity all-solid-state lithium sulfur battery. Green Chem., 2016, 18(13): 3796-3803. DOI:10.1039/C6GC00444J ( 0) 0)
|
| [94] |
Teoh K H, Lim C S, Ramesh S. Lithium ion conduction in corn starch based solid polymer electrolytes. Measurement, 2013, 48: 87-95. DOI:10.1016/j.measurement,2013.10.040 ( 0) 0)
|
| [95] |
Liew C W, Ramesh S. Studies on ionic liquid-based corn starch biopolymer electrolytes coupling with high ionic transport number. Cellulose, 2013, 20(6): 3227-3237. DOI:10.1007/s10570-013-0079-0 ( 0) 0)
|
| [96] |
Teoh K H, Ramesh S, Arof A K. Investigation on the effect of nanosilica towards corn starch-lithium perchlorate-based polymer electrolytes. Journal of Solid State Electrochemistry, 2012, 16(10): 3165-3170. DOI:10.1007/s10008-012-1741-4 ( 0) 0)
|
| [97] |
Adachi M, Kanamori J, Masuda T, et al. Crystal structure of soybean 11S globulin: glycinin A3B4 homohexamer. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(12): 7395-7400. DOI:10.1073/pnas.0832158100 ( 0) 0)
|
| [98] |
Eyler A, Wang Y, Liu T, et al. Ion-induced effective control of morphologies of soy protein biocomposites. Journal of Materials Science, 2015, 50(7): 2691-2699. DOI:10.1007/s10853-015-8816-4 ( 0) 0)
|
| [99] |
Leones R, Sentanin F, Rodrigues L C, et al. Novel polymer electrolytes based on gelatin and ionic liquids. Optical Materials, 2012, 35(2): 187-195. DOI:10.1016/j.optmat.2012.07.027 ( 0) 0)
|
| [100] |
Wang X, Fu X, Wang Y, et al. A protein-reinforced adhesive composite electrolyte. Polymer (United Kingdom), 2016, 106: 43-52. DOI:10.1016/j.polymer.2016.10.052 ( 0) 0)
|
 2018, Vol. 25
2018, Vol. 25


