2. Shandong Institute for Product Quality Inspection, Jinan 250100, China;
3. Shandong Quality and Technical Examination Assess Center, Jinan 250014, China
Boronizing is an effective surface modification treatment widely used in industry. The boride layer is obtained by the thermodiffusion of active boron atoms followed by chemical reaction into various metal surfaces. There are various studies in the literature on the successfully boriding of a wide range of metals at high temperature. AISI 1045 steel was paste boronized at temperatures range of 1 193-1 273 K[1-4], and the variation of the growth of Fe2B layer with the thickness of boron paste during the boriding process was studied. Some mechanical properties of AISI W4 steel boronized, such as boronizing thickness, hardness, the structure and fracture toughness, were studied by Ozbek at 850, 950 and 1 050 ℃[5]. Boronizing of CoCrMo alloy was performed by a powder-pack method at 850, 900 and 950 ℃ for 8 h, and the results showed that the oxidation resistance of CoCrMo alloy was improved by boronizing process[6]. Yoon et al.[7]prepared the boride layer by plasma paste boronizing on the AISI 304 stainless steel substrate with different temperatures between 1 023 and 1 223 K, and various surface properties of the boride layer were examined. The results indicated that the boride layer consisted of FeB, Ni3B, and CrB. And the microhardness of boride layer was in the range of 180-2 000. A boride coating was fabricated by thermo-reactive diffusion (TRD) at 1 220 K for 6 h[8].The corrosion resistance of boride coatings was investigated, and the results showed that it can be improved by the boronizing process. Evaluation of the corrosion resistance of iron boride layers on the AISI steel boronized at temperatures of 1 173, 1 223, and 1 273 K was carried out by Campos et al.[9] It was obtained that the corrosion resistance was maximized with a boron paste thickness of 4 mm and a treatment time of 4 h. Series of experiments were performed by Genel et al. at temperatures of 1 073, 1 173 and 1 273 K, and kinetics and the case properties of borided AISI H13 steel were investigated[10]. The kinetics of boriding showed a parabolic relationship between layer thickness and process time. Sert et al.[11] studied the wear resistance of boride layer on 45 steel surfaces when treated at 900 ℃. The high-temperature behavior of boronized layer of steel 45 was studied in detail by Yan et al.[12]They found that the transformation temperature from FeB phase to Fe2B phase was about 860 ℃ and the hardness of boride layer decreased with the increase of temperature. Jain et al.[13] examined the thickness, microstructure, microhardness and abrasion resistance of low carbon steel samples boronized at 940 ℃ with different pack thickness in the range 2-25 mm. Results showed that the boride coating obtained by a pack thickness of 10 mm had adequate thickness and optimum properties. Campos et al. studied the growth kinetics of Fe2B and FeB phases forming on AISI M2 steel by paste boriding under different values of treating temperature, paste thickness and exposure time[14]. And the results showed that the boron mobility and growth kinetics of iron borides increased considerably with the increase of paste thickness. Boride layers were fabricated on low alloy steel substrates by pack boriding process at 900 ℃[15]. In this study, the estimation of the yield strength of FeB layer was conducted by combining the experimental indentation works and finite element modeling.
The disadvantages of the high-temperature boronizing process are that it is high cost and high brittleness of boride layer, and the properties and structure of the base materials are influenced significantly by the high temperature. Researches have been carried out to resolve those problems. Post boronizing N+ ion implantation of C45 steel was firstly used by Yan et al.[16] to reduce the embrittlement of the boride layer and improve its properties. The C45 steel was boronized by gaseous boronizing with solid boron-yielding agents at 920 ℃ for 4 h. Morimoto et al.[17] revealed that the laser boronizing processes had no influence on the structure and properties of base material which avoid the drawbacks of pack boronizing and laser-assisted boronizing. An improved process, two-step plasma-assisted boriding technology, was carried out to reduce the pore density by Nam et al.[18] Direct current field (DCF) was employed in pack boriding, which favors boron's diffusion in the substrate at low and moderate temperatures[19-20]. A nanostructured layer in the top surface of low carbon steel was fabricated by FMRR technique[21]. We can conclude that the surface nanocrystallization was effective to improve the penetrating speed. In this paper, a boride layer was fabricated on steel Q235 by using low-temperature Cr-Rare earth-boronizing technique induced by surface nanocrystallization. The process has been thoroughly studied, and the mechanism was also investigated.
2 Experimental 2.1 Experimental MaterialsSteel Q235 with the dimension of 10 mm×10 mm×10 mm was used as substrate in this paper. The Cr-Rare earth-boriding process was performed at 843, 873 and 923 K for 6 h. Table 1 shows the compositions of the Cr-Rare earth-boronizing agent.
| Table 1 Compositions of the agents |
2.2 Experimental Methods
FMRR device (Fig. 1) was used to perform the surface nanocrystallization on steel Q235[21]. The tow parameters of pressure (P) and rotational speed (ω) for the FMRR treatment were 4 MPa, and 1 600 r/min, respectively. Fig. 2 shows the penetrating tank used in the experiment[22]. The Cr-Rare earth-boriding process was introduced detailed in previous research[22]. Digital low-vacuum scanning electron microscopy (JSM-6380LA) was used to characterize the morphology of the deformation layer induced by surface nanocrystallization and the boride layer, and the thickness of the boride layer was also measured. Three values were measured at each condition. The thickness of boride layers before and after FMRR treatment was the average values of these tests for each condition.Local high-density dislocations and nanostructures on the specimen surface were characterized by Transmission electron microscopy (TEM).
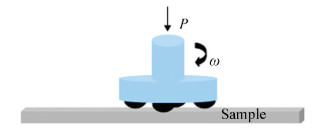
|
Figure 1 Schematic diagram of FMRR device |
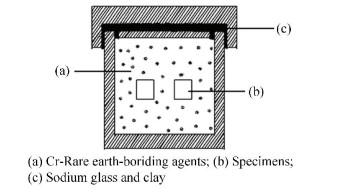
|
Figure 2 Schematic diagram of the penetrating tank |
3 Results 3.1 Microstructure of Boride Layers
The original samples and FMRR specimens were Cr-Rare earth-borided at different temperatures. The thickness of boride layers is shown in Table 2. It can be observed that the surface nanocrystalization has significant effect on the thickness of boride layers. The penetrating rate for FMRR specimens increased by 1.9, 1.7 and 1.5 times. The high-density dislocation caused by the FMRR treatment is expected to be able to reduce the diffusion activation energy of boron atoms, which is beneficial for the diffusion of boron atoms into steels. It can be also seen that the thickness of boride layers increases when the temperature increases.
| Table 2 Thickness of boride layers at different temperatures |
SEM images of the boride layers Cr-Rare-boronized at different temperatures before and after FMRR treatment are shown in Fig. 3. It is observed from Fig. 3 that a boride layer with saw-toothed structure was obtained. The zigzag teeth structure of the boride layer is attributed to the growth crystals along a direction of minimum resistance, perpendicular to the external surface. The boride layer obtained for the FMRR sample is denser, more uniform and adheres better to the metallic substrate than the original sample.

|
Figure 3 SEM observations of the boride layer at different temperatures for 6 h before and after FMRR treatment |
Significant surface deformation was also observed on steel Q235 surface after FMRR treatment. In the deformation layer the size of grains gets smaller, and the grain boundaries, dislocations and vacancies increase. The dispersion of boron atoms was promoted by the finer grains on steel Q235 surface with the increased boundaries. The activation energy of dispersion for boron atoms was decreased by the high-density dislocations and vacancies, which is beneficial for the dispersion of boron atoms. Meanwhile, it is easier for the aggregation of active boron atoms and the nucleation and growth of boride when it reaches a certain concentration, resulting in the prohibition of dislocation slip and the delay of recovery and recrystallization process which is favorable to the dispersion of boron atoms into substrate.
3.2 Microstructure of the Surface LayerThe microhardness of deformation layer on steel Q235 surface induced by surface nanocrystallization is shown in Fig. 4. It illustrated that the microhardness of steel Q235 surface is improved by surface nanocrystallization. Meanwhile, the micro-hardness gradient between the boride layer and the substrate was decreased as a result of the increase of the microhardness of the top surface induced by surface nanocrystallization. The adherence of boride to the substrate was then improved. In Fig. 5, the SEM micrograph of the upper-layer of FMRR specimen, is shown. A severe plastic deformation layer was observed.
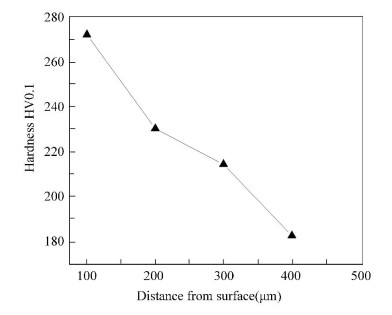
|
Figure 4 Influence of the distance from surface on the micro-hardness of deformation layer |
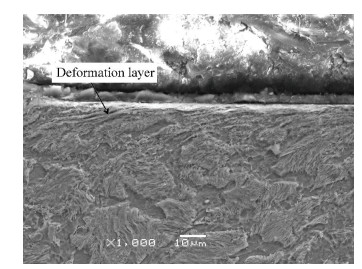
|
Figure 5 SEM micrograph of the upper layer of FMRR specimen |
Fig. 6 illustrated the TEM images of FMRR sample.Ultrafine equiaxed grains, which grain size was approximately 100 nm, were found on top surface. Fig. 6(b) reveals that the crystallographic orientations of nanocrystalline grains are randomized arrangement.

|
Figure 6 TEM micrographs of FMRR specimen |
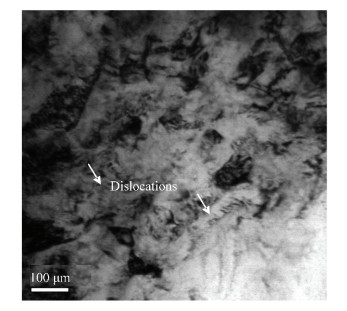
In Fig. 7, TEM micrograph of the top surface layer of FMRR sample, is shown. It can be observed that there are high-density dislocations on FMRR specimen surface. This is probably because of the high stacking fault energy induced by the severe plastic deformation on FMRR specimen surface. However, the mechanism of surface nanocrystallization induced by the FMRR technique is still needed to investigate in detail[22-23].
|
Figure 7 TEM micrograph of the top surface layer of FMRR sample |
3.3 Mechanism of Cr-Rare Earth-Boriding
In the Cr-Rare earth-boriding procedure, Ferro-boron is used as boron source, LaCl3 and KBF4 are used as activator, HC FeCr is used as chrome source, and SiC is used as diluent. The reaction in the Cr-Rare earth-boriding is expressed as:
| $ {\rm{2HCl + Cr}} \to {\rm{CrC}}{{\rm{l}}_{\rm{2}}} $ | (1) |
| $ {\rm{CrC}}{{\rm{l}}_{\rm{2}}}{\rm{ + Ca}} \to \left[{{\rm{Cr}}} \right]{\rm{ + CaC}}{{\rm{l}}_{\rm{2}}} $ | (2) |
| $ {\rm{C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}\left( {\rm{s}} \right){\rm{ + 3Ca}} \to {\rm{2}}\left[{{\rm{Cr}}} \right]{\rm{ + 3CaO}} $ | (3) |
| $ {\rm{2C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}\left( {\rm{s}} \right){\rm{ + 3Si}} \to {\rm{4}}\left[{{\rm{Cr}}} \right]{\rm{ + 3Si}}{{\rm{O}}_{\rm{2}}} $ | (4) |
| $ {\rm{C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}\left( {\rm{s}} \right){\rm{ + 6HCl}} \to {\rm{2}}\left[{{\rm{Cr}}} \right]{\rm{ + 3}}{{\rm{H}}_{\rm{2}}}{\rm{O + 3C}}{{\rm{l}}_{\rm{2}}} $ | (5) |
| $ {\rm{2LaC}}{{\rm{l}}_{\rm{3}}}{\rm{}}\left( {\rm{s}} \right){\rm{ + 3Ca}} \to {\rm{2}}\left[{{\rm{La}}} \right]{\rm{ + 3CaC}}{{\rm{l}}_{\rm{2}}} $ | (6) |
| $ {\rm{2CeC}}{{\rm{l}}_{\rm{3}}}\left( {\rm{s}} \right){\rm{ + 3Ca}} \to {\rm{2}}\left[{{\rm{Ce}}} \right]{\rm{ + 3CaC}}{{\rm{l}}_{\rm{2}}} $ | (7) |
Active chrome atoms are obtained by reactions (1)-(5). Active RE atoms are obtained by reactions (6)-(7). The high stacking fault energy induced by FMRR treatment promotes the diffusion of RE atom carrying active boron atoms and chrome atoms into substrate through vacancies, dislocations and boundaries. Then active boron atoms take up vacancies leading to the aggregation of boron atoms and the nucleation and growth of the boride.
4 Conclusions1) A nanostructured layer was fabricated by FMRR technique in the top surface of steel Q235. The Cr-Rare earth-boriding process was carried out followed at 843, 873 and 923 K for 6 h.
2) The thickness of the boride layer was significantly increased by surface nanocrystallization. The penetrating speed of FMRR specimens was enhanced by 1.9, 1.7 and 1.5 times when the Cr-Rare earth-boriding temperature was 843, 873 and 923 K.The morphology of the layer was saw-toothed. The boride layer was continuous, dense and uniform, and adhered well to the substrate.
3) The microhardness of steel Q235 surface was improved by surface nanocrystallization. Severe plastic deformation with the grain size of approximately 100 nm on steel Q235 surface was observed.
| [1] |
Keddam M. Computer simulation of monolayer growth kinetics of Fe2B phase during the paste-boriding process: Influence of the paste thickness. Applied Surface Science, 2006, 253(2): 757-761. DOI:10.1016/j.apsusc.2006.01.011 ( 0) 0)
|
| [2] |
Campos I, Bautista O, Raml'rez G, et al. Effect of boron paste thickness on the growth kinetics of Fe2B boride layers during the boriding process. Applied Surface Science, 2005, 243(1/2/3/4): 429-436. ( 0) 0)
|
| [3] |
Campos I, Islas M, Raml'rez G, et al. Growth kinetics of borided layers: Artificial neural network and least square approaches. Applied Surface Science, 2007, 253(14): 6226-6231. DOI:10.1016/j.apsusc.2007.01.070 ( 0) 0)
|
| [4] |
Campos I, Islas M, Gonza'lez E, et al. Use of fuzzy logic for modeling the growth of Fe2B boride layers during boronizing. Surface & Coatings Technology, 2006, 201(6): 2717-2723. ( 0) 0)
|
| [5] |
Ozbek I, Bindal C. Mechanical properties of boronized AISI W4 steel. Surface and Coatings Technology, 2002, 154(1): 14-20. DOI:10.1016/S0257-8972(01)01409-8 ( 0) 0)
|
| [6] |
Mu Dong, Shen Baoluo. Oxidation resistance of boronized CoCrMo alloy. Int Journal of Refractory Metals & Hard Materials, 2010, 28(3): 424-428. ( 0) 0)
|
| [7] |
Yoon J H, Jee Y K, Lee S Y. Plasma paste boronizing treatment of the stainless steel AISI 304. Surface and Coatings Technology, 1999, 112(1/2/3): 71-75. ( 0) 0)
|
| [8] |
Tavakoli H, Khoie S M. An electrochemical study of the corrosion resistance of boride coating obtained by thermo-reactive diffusion. Materials Chemistry and Physics, 2010, 124(2/3): 1134-1138. ( 0) 0)
|
| [9] |
Campos I, Palomar M, Amador A, et al. Evaluation of the corrosion resistance of iron boride coatings obtained by paste boriding process. Surface & Coatings Technology, 2006, 201(6): 2438-2442. ( 0) 0)
|
| [10] |
Genel K. Boriding kinetics of H13 steel. Vacuum, 2006, 80(5): 451-457. DOI:10.1016/j.vacuum.2005.07.013 ( 0) 0)
|
| [11] |
Sert H, Can A, Arikan H, et al. Wear behavior of different surface treated cam spindles. Wear, 2006, 260(9/10): 1013-1019. ( 0) 0)
|
| [12] |
Yan P X, Zhang X M, Xu J W, et al. High-temperature behavior of the boride layer of 45#carbon steel. Materials Chemistry and Physics, 2001, 71(1): 107-110. DOI:10.1016/S0254-0584(01)00270-X ( 0) 0)
|
| [13] |
Jain Vipin, Sundararajan G. Influence of the pack thickness of the boronizing mixture on the boriding of steel. Surface and Coatings Technology, 2002, 149(1): 21-26. DOI:10.1016/S0257-8972(01)01385-8 ( 0) 0)
|
| [14] |
Campos I, Torres R, Bautista O, et al. Effect of boron paste thickness on the growth kinetics of polyphase boride coatings during the boriding process. Applied Surface Science, 2006, 252(6): 2396-2403. DOI:10.1016/j.apsusc.2005.04.022 ( 0) 0)
|
| [15] |
Culha O, Toparli M, Aksoy T. Estimation of FeB layer's yield strength by comparison of finite element modeling with experimental data. Advances in Engineering Software, 2009, 40(11): 1140-1147. DOI:10.1016/j.advengsoft.2009.05.005 ( 0) 0)
|
| [16] |
Yan P X, Wei Z Q, Wen X L, et al. Post boronizing ion implantation of C45 steel. Applied Surface Science, 2002, 195(1/2/3/4): 74-79. ( 0) 0)
|
| [17] |
Morimoto J, Ozaki T, Kubohori T, et al. Some properties of boronized layers on steels with direct diode laser. Vacuum, 2009(83): 185-189. ( 0) 0)
|
| [18] |
Nam K S, Lee K H, Lee D Y, et al. Metal surface modification by plasma boronizing in a two-temperature-stage process. Surface & Coatings Technology, 2005, 197(1): 51-55. ( 0) 0)
|
| [19] |
Zhou Zhenghua, Xie Fei, Hu Jing. A novel powder aluminizing technology assisted by direct current field at low temperatures. Surface & Coatings Technology, 2008, 203(1/2): 23-27. ( 0) 0)
|
| [20] |
Xie Fei, Sun Li, Pan Jianwei. Characteristics and mechanisms of accelerating pack boriding by direct current field at low and mo-derate temperatures. Surface & Coatings Technology, 2012, 206(11/12): 2839-2844. ( 0) 0)
|
| [21] |
Chui Pengfei, Sun Kangning, Sun Chang, et al. Effect of surface nanocrystallization induced by fast multiple rotation rolling on hardness and corrosion behavior of 316L stainless steel. Applied Surface Science, 2011, 257(15): 6787-6791. DOI:10.1016/j.apsusc.2011.02.127 ( 0) 0)
|
| [22] |
Yuan Xingdong, Xu Bin, Cai Yucheng. Effect of surface nanocrystallization induced by fast multiple rotation rolling on RE boron-chromizing for steel 45 under low-temperature. Journal of Harbin Institute of Technology (New Series), 2015, 22(2): 118-122. ( 0) 0)
|
| [23] |
Yuan Xingdong, Xu Bin, Cai Yucheng. Surface nanocrystallization of steel 20 induced by fast multiple rotation rolling. Journal of Harbin Institute of Technology (New Series), 2015, 22(5): 38-41. ( 0) 0)
|
 2018, Vol. 25
2018, Vol. 25


