2. Beijing Key Lab of Heating, Gas Supply, Ventilating and Air Conditioning Engineering, Beijing University of Civil Engineering and Architecture, Beijing 100044, China;
3. School of Civil Engineering, Henan Polytechnic University, Jiaozuo 454000, He′nan, China
The risk of formaldehyde in indoor air has received broadattention[1-3]. Although production limits on artificial materials containing formaldehyde have been strictly regulated, the indoor formaldehyde concentration still has the possibility of exceeding indoor air quality standards when artificial materials are used in great quantities. To meet the need of formaldehyde purification, degrading technologies of different principles have been attracting research interests in recent years.
Among degrading technologies, photo catalysis, plasma-driven catalysis and plasma are usually effective for various VOCs. However, reaction intermediate may be produced for VOCs containing high bond-energy chemicals, especially aromatic hydrocarbons.D'Hennezel[4] and Wang[5] reported that phenol was found in the products of benzene photocatalytic degradation. Besides phenol, He et al.[6]also detected 2-cyclohexanol, trans-2-Hexen-1-ol, acetylacetone, 1, 3-butanediol, 2-butanone and glycolic acid. In products of toluene, reaction intermediates from photocatalytic degradation included benzaldehyde, benzoic acid, benzyl alcohol[7-8]. Having a more complicated structure, xylene[9] could be degraded into multiple intermediates, such as toluene, benzaldehyde, 2, 5-furandione, methylbenzaldehyde, methylbenzoic acid, and 1, 3-1sobenzofurandione. Plasma technology also produces noxious intermediates during the degradation of indoor VOCs. Kim et al.[10] and Jiang et al.[11] found formic acid when they degraded benzene with a plasma-driven catalyst (PDC) reactor. Schmid et al.[12] guided cyclohexene, benzene, toluene, ethylbenzene and the xylene isomers through the plasma air purifier, and detected oxidized species such as alcohols, aldehydes, ketones and one epoxide. Durrme et al.[13] found reaction products in positive corona discharge non-thermal plasma research such as formic acid, benzaldehyde, benzyl alcohol, 3-methyl-4-nitrophenol, 4-methyl-2-nitrophenol, 4-methyl-2-propyl furan, 5-methyl-2-nitrophenol, 4-nitrophenol, and 2-methyl-4, 6-dinitrophenol.
In contrast to the above technologies, noble metal Au, Rh, Pd and Pt supported on TiO2 could degrade nothing but formaldehyde with additional thermal energy or even at ambient temperature[14-17]. The noble metal catalysts had high enough chemical activity to completely oxidize formaldehyde into CO2 and H2O. From the view of the harmless products, noble metal supported on TiO2 is ideal method to remove formaldehyde pollution.
Energy consumption is another drawback for air cleaners. The electric energy consumption of photocatalytic oxidation (PCO) purifiers is primarily due to the ultraviolet lamps. According to the experimental data conducted by Yang et al., a PCO purifier in a water-cooled air conditioning unit consumed 18 kJ energy to deal with per cubic meter air[18]. Plasma purifiers consume energy mainly for electricity discharging. Jung et al. investigated a non-thermal plasma reactor with multiple-wire-to-wire type electrodes. The energy consumption was about 30 kJ per cubic meter of air to reach considerable dust collection efficiency[19]. Thermal catalytic oxidation (TCO) needs heat energy for 60 kJ per cubic of meter air [17].
Noise from air cleaners was also a consideration in evaluating air cleaner performance. Yong et al. measured noise levels of 31 air cleaners, and found the average was 45.2 dB (A)[20]. The forced vibration and frequency was predicted with plate and shell theory[21].
In summary, from the view of intermediate production and noise, noble metal catalysts are ideal choice when formaldehyde is the dominate pollutant. This study proposed the method to coat noble metal catalyst on heating radiators to take the advantage of zero energy consumption and noise. The formaldehyde cleaning performance of a simulated catalyst-coated radiator was experimentally evaluated.
2 Experimental MethodA simulated radiator designed to degrade formaldehyde was assembled as shown in Fig. 1. Pt/TiO2 catalyst powder with Pt at 1% by weight was prepared with the method described in Ref.[17]. Next, the powder was mixed with organic binder and coated on a mica electric heater sealed in a stainless steel shell. The heater is a 7 cm square. Finally, the coated heater was dried in an oven at 260 ℃ for 3 hours to evaporate the binder and fix the catalyst.
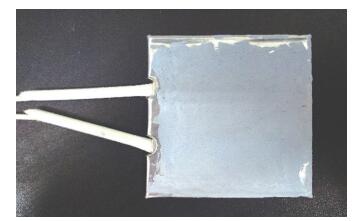
|
Fig.1 Simulated radiator coated with Pt/TiO2 |
A stainless steel chamber was built to simulate a single radiator-heated room (as shown in Fig. 2). The size of the room was calculated by the space-heating load and radiator temperature according to relevant Chinese standard. The calculated size was 64 cm long, 49 cm wide and 39 cm high. Two round openings were used for ventilating. The opening near a top corner was used for supplying air containing formaldehyde. The opening near bottom on the opposite side was used for exhaust. The diameter of the top opening was set as 2 cm, so that the core of supply air jet could reach the opposite wall at experimental condition.

|
Fig.2 Stainless steel chamber |
Catalyst powder was prepared to compare its performance with the coated catalyst. The performance test was performed with a quartz tube reactor settled in an electric thermostat.
The reactor and chamber shared the same air distribution system and GC detector as shown in Fig. 3. The devices denoted with K were valves, and the one denoted with M was mass flow controller. Formaldehyde was generated with gas-wash method from a solution bottle. The concentration of formaldehyde was controlled via valves K1 and K2. M1 was used to control the flow rate of the mixture of formaldehyde and compressed air.

|
Fig.3 Catalyst performance testing setup |
Formaldehyde concentration was measured with gas chromatograph (GC) Agilent 7820A. The gas entered the GC via a six-way valve with a sample loop so that the formaldehyde concentration could be quantified. The loop volume was 1 mL. Sampling interval was set in software OpenLAB for GC.
3 Results and Discussion 3.1 Performance Comparison of Catalyst Powder and Coated CatalystThe performances of catalyst powder and of coated catalyst were compared. The catalyst powder was tested in the flow-through reactor and the coated catalyst was tested in the stainless steel chamber.
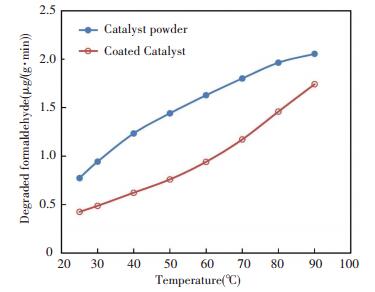
The air distribution system supplied air containing formaldehyde to the flow-through reactor or the chamber. The air velocity was set as 1 L/min for the flow-through reactor and 0.6 L/min for the chamber. For both materials, the concentration of formaldehyde was about 5 mg/m3, and the temperature range was 25-90 ℃. The outlet concentration was sampled every 10 min. The degradation rates were calculated based on mass balance and shown in Fig. 4.
|
Fig.4 Performance comparison of catalyst powder and coated catalyst |
The performance of catalyst powder was generally higher than that of the coated catalyst. In addition, the formaldehyde degradation performance for both materials was positively correlated withtemperature, but the performance tendency differed between the two materials. The performance of the catalyst powder improved rapidly at low temperature and then gradually became stable after 60 ℃. This result matched with the experiments reported in Ref.[18]. The slope of the coated catalyst performance curve kept increasing over temperature. It can be explained from the view of mass transfer. In the experiments for catalyst powder performance, the mass transfer caused by forced convection was steady. The mass transfer in the experiments for coated catalyst performance was caused by natural convection. At low temperature, the natural convection was weak and the air velocity through the catalyst was low. Higher temperature resulted in stronger transportation capability of formaldehyde to the surface of the catalyst.
3.2 Effect of Temperature and Concentration on the PerformanceThe influence of working temperature and original concentration on the degradation ability of the coated catalyst was tested. Although stainless steel was used for the chamber and the chamber was well sealed to reduce adsorption and leakage, natural decay effect was still tested carefully and deducted in the presented results.
The temperature range was established according to the normal working conditions of a radiator in a residence. The temperature of the hot water supplied for residential heating is usually less than 95 ℃, and the average temperature of radiators is less than 85 ℃. In seasons without heat being supplied, the temperature of radiators is the same as indoor air. Therefore, the temperature range was specified as 20-90 ℃, which covers almost all the possible working conditions of a radiator. During the experiment, the temperature of the radiator was controlled with a solid relay regulated by a signal generator.
The test was performed in the stainless chamber. The air distribution system supplied air containing formaldehyde to the chamber at the velocity of 0.6 L/min. The concentration of formaldehyde was set in the range of 1-6 mg/m3 to represent various indoor emission intensities.
The chamber air was assumed to be fully mixed, so that the outlet concentration was monitored and used for cleaning efficiency calculation. The outlet concentration was sampled every 20 min. The concentration decay process when the initial concentration was 6.1 mg/m3 was shown in Fig. 5. The concentration axis is in logarithmic coordinate. It can be seen that the outlet concentration at every temperature point decreased, and the working temperature influenced the degradation of formaldehyde positively. It means the catalyst will work better during heating period. Besides which, according to mass balance, the concentration curves should be linear in the case that the degradation rate is constant. While, we can see that the degradation rates became less when the concentration decreased significantly from the initial value. This result agreed with the experiments reported before[17]. To get a degradation rate at given initial concentration, the linear parts of the curves should be taken.
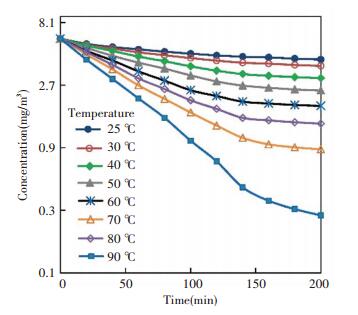
|
Fig.5 Concentration decay process in the chamber |
A series of degradation rates when the concentration varied in the range of 1-5 mg/m3 and the working temperature varied in the range of 25-90 ℃ were obtained from the concentration decay curves in Fig. 5. The result was plotted in Fig. 6.
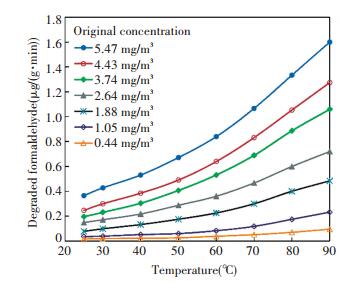
|
Fig.6 Degradation rate of formaldehyde |
The experimental results of degradation rate were fitted in polynomial form:
| $ r=\left(a_{1}+a_{2} C+a_{3} T+a_{4} C^{2}+a_{5} T^{2}+a_{6} C T\right) C $ | (1) |
where, r is the reaction rate (μg/(g·min)), C is original formaldehyde concentration before catalyst is employed (mg/m3), T is working temperature (℃). Coefficients a1 to a6 were determined from the data in Fig. 6 and shown in Table 1. The coefficient of multiple determination R2 was 0.996.
| Table 1 Determined coefficients |
3.3 Practical Cleaning Effect Analysis
The chamber system is not convenient to evaluate the practical formaldehyde cleaning effect for two reasons. Firstly, the air change rate of the chamber was decided by the need of formaldehyde dosing and differed from real residential air change rates. Besides, the real residential formaldehyde is in a state of equilibrium, which needs very long time to reach for the chamber. To illustrate the practical cleaning effect, theoretical prediction was conducted on the base of the experimental data and the following equations. The cleaning performance was evaluated with CADR (clean air delivery rate, RCAD), which is more suitable for air cleaning system design than degraded mass.
In the case that environmental formaldehyde concentration is zero, the indoor formaldehyde emission rate can be calculated as
| $ E=C_{0} V $ |
where E is the emission rate (mg/min), V is natural ventilation rate (m3/min), and C0 is the formaldehyde concentration without catalyst application (mg/m3). V was taken as 0.1 times/h in the practical cleaning effect analysis.
Assuming the indoor formaldehyde concentration is uniform, the formaldehyde concentration when passive thermal catalyst is employed will be
| $ C=\frac{E-m r}{V} $ | (2) |
According to Eq.(1), the degradation rate r is the function of formaldehyde concentration C and reaction temperature T. Therefore, the values of C and r need solving by iteration.
According to mass balance, RCAD can be calculated as
| $ R_{\mathrm{CAD}}=\frac{E}{C}-V $ |
Comparing with Eq.(2) and the fitted reaction rate r, RCAD per unit mass catalyst can also be evaluated as
| $ R_{\mathrm{CADm}}=a_{1}+a_{2} C+a_{3} T+a_{4} C^{2}+a_{5} T^{2}+a_{6} C T $ |
Fig. 7 shows the indoor formaldehyde concentration in equilibrium condition when the given amount of coated catalysts were employed. When the original concentration was 1 mg/m3, that is, 10 times of ISO standard, the indoor formaldehyde concentration could be reduced close to the limit in the standard at 40 ℃ working temperature. When the original concentration was 5 mg/m3, the indoor formaldehyde concentration could be reduced close to the limit at 80 ℃ working temperature.
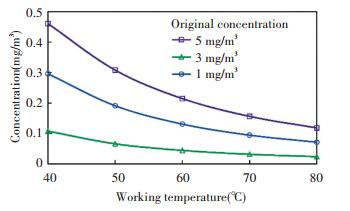
|
Fig.7 Equilibrium indoor formaldehyde concentration |
The RCADm corresponding to Fig. 7 were shown in Fig. 8. It can be seen that the RCADm was merely the function of temperature in the investigated parameter scopes. The RCADm can be fitted from the experimental data as
| $ R_{\mathrm{CADm}}=4.24 \times 10^{-2}-1.12 \times 10^{-4} T+3.10 \times 10^{-5} T^{2} $ | (3) |
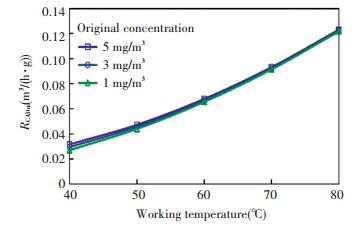
|
Fig.8 Degradation effect weighted in CADR |
The coefficient of multiple determination R2 in the fitting was 0.995. In most practical residential conditions, the formaldehyde concentration is far below the investigated parameter range, which means the concentration has less influence on RCADm and the fitted Eq. (3) is more reliable.
The room size and pollutant intensity varies in practice. With Eq.(3), the amount of catalyst for reducing the formaldehyde pollution can be estimated. It should be noticed that the degradation performance will be influenced by the practical surface processing technology, installation and dust.
3.4 Influence of Air Organization on Degradation Performance EvaluationIn the data processing, the chamber air was assumed as fullymixed, correspondingly the outlet concentration was monitored and used for calculating the degradation efficiency. The assumption should be reasonable when considering the practical situation. In a radiator-heated room, if the air can be well mixed in the view of temperature by natural ventilation, uniform mass concentration can be expected according to the analog of mass and heat transfer. While, in a room heated by catalyst-coated fan-coils, better degradation performance can be expected because of faster air recirculation rate and greater air velocity upon the catalyst.
4 ConclusionThis study investigated the feasibility of applying passive thermal catalysis technology to reduce indoor formaldehyde pollution without energy consumption, noise and by-products. It was found the coated catalyst performance was slightly inferior to that of the catalyst powder. The reason for the difference in results can be attributed to the mass transfer ability of the natural convection induced by radiators. In the analysis based on experimental data, formaldehyde concentration at 10 to 50 times of ISO standard could be reduced close to allowable limit. The RCAD value of per unit mass catalyst, which was found irrelevant with the formaldehyde concentration within investigated range, can be used for estimating the amount of the catalyst for specific room size and pollution intensity.It should be emphasized that in hot weather, the emission of formaldehyde increases and passive thermal catalysis works less effectively. Other technology should be used to purify formaldehyde when necessary.
| [1] |
Vaizoǧlu S A, Aycan S, Deveci M A, et al. Determining domestic formaldehyde levels in Ankara, Turkey. Indoor Built Environ, 2003, 12: 329-336. DOI:10.1177/142032603035546 (  0) 0) |
| [2] |
Salthammer T, Mentese S, Marutzky R. Formaldehyde in the indoor environment. Chemical Reviews, 2010, 110: 2536-2572. DOI:10.1021/cr800399g (  0) 0) |
| [3] |
Nielsen G D, Larsen S T, Wolkoff P. Recent trend in risk assessment of formaldehyde exposures from indoor air. Archives of Toxicology, 2013, 87: 73-98. DOI:10.1007/s00204-012-0975-3 (  0) 0) |
| [4] |
D'hennezel O, Pichat P, Ollis D F. Benzene and toluene gas-phase photocatalytic degradation over H2O and HCl pretreated TiO2: By-products and mechanisms. Journal of Photochemistry & Photobiology A Chemistry, 1998, 118: 197-204. (  0) 0) |
| [5] |
Wang W, Ku Y. Photocatalytic degradation of gaseous benzene in air streams by using an optical fiber photoreactor. Journal of Photochemistry & Photobiology A Chemistry, 2003, 159: 47-59. (  0) 0) |
| [6] |
He F, Li J, Li T, et al. Solvothermal synthesis of mesoporous TiO2 : The effect of morphology, size and calcination progress on photocatalytic activity in the degradation of gaseous benzene. Chemical Engineering Journal, 2014, 237: 312-321. DOI:10.1016/j.cej.2013.10.028 (  0) 0) |
| [7] |
Méndez-Román R, Cardona-MartíNez N. Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene. Catalysis Today, 1998, 40: 353-365. DOI:10.1016/S0920-5861(98)00064-9 (  0) 0) |
| [8] |
Marcì G, Addamo M, Augugliaro V, et al. Photocatalytic oxidation of toluene on irradiated TiO2 : Comparison of degradation performance in humidified air, in water and in water containing a zwitterionic surfactant. Journal of Photochemistry & Photobiology A Chemistry, 2003, 160: 105-114. (  0) 0) |
| [9] |
Blanco J, Avila P, Bahamonde A, et al. Photocatalytic destruction of toluene and xylene at gas phase on a titania based monolithic catalyst. Catalysis Today, 1996, 29: 437-442. DOI:10.1016/0920-5861(95)00317-7 (  0) 0) |
| [10] |
Kim H H, Oh S M, Ogata A, et al. Decomposition of gas-phase benzene using plasma-driven catalyst (PDC) reactor packed with Ag/TiO2 catalyst. Applied Catalysis B Environmental, 2005, 56: 213-220. DOI:10.1016/j.apcatb.2004.09.008 (  0) 0) |
| [11] |
Jiang N, Hu J, Li J, et al. Plasma-catalytic degradation of benzene over Ag-Ce bimetallic oxide catalysts using hybrid surface/packed-bed discharge plasmas. Applied Catalysis B: Environmental, 2016, 184: 355-363. DOI:10.1016/j.apcatb.2015.11.044 (  0) 0) |
| [12] |
Schmid S, Jecklin M C, Zenobi R. Degradation of volatile organic compounds in a non-thermal plasma air purifier. Chemosphere, 2010, 79: 124-130. DOI:10.1016/j.chemosphere.2010.01.049 (  0) 0) |
| [13] |
Van Durme J, Dewulf J, Sysmans W, et al. Abatement and degradation pathways of toluene in indoor air by positive corona discharge. Chemosphere, 2007, 68: 1821-1829. DOI:10.1016/j.chemosphere.2007.03.053 (  0) 0) |
| [14] |
Zhang C, He H, Tanaka K I. Perfect catalytic oxidation of formaldehyde over a Pt/TiO2 catalyst at room temperature. Catalysis Communications, 2005, 6: 211-214. DOI:10.1016/j.catcom.2004.12.012 (  0) 0) |
| [15] |
Zhang C, He H, Tanaka K. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Applied Catalysis B: Environmental, 2006, 65: 37-43. DOI:10.1016/j.apcatb.2005.12.010 (  0) 0) |
| [16] |
Wang L, Sakurai M, Kameyama H. Study of catalytic decomposition of formaldehyde on Pt/TiO2 alumite catalyst at ambient temperature. Journal of Hazardous Materials, 2009, 167: 399-405. DOI:10.1016/j.jhazmat.2008.12.129 (  0) 0) |
| [17] |
Zhang G, Hong Y, He W. Experimental study of factors affecting Pt-TiO2 thermal catalytic oxidation of formaldehyde. Indoor Built Environ, 2015, 24: 138-144. DOI:10.1177/1420326X14561131 (  0) 0) |
| [18] |
Yang L, Cai A, Luo C, et al. Performance analysis of a novel TiO2-coated foam-nickel PCO air purifier in HVAC systems. Separation and Purification Technology, 2009, 68: 232-237. DOI:10.1016/j.seppur.2009.05.008 (  0) 0) |
| [19] |
Jung J S, Kim J G. An indoor air purification technology using a non-thermal plasma reactor with multiple-wire-to-wire type electrodes and a fiber air filter. Journal of Electrostatics, 2017, 86: 12-17. DOI:10.1016/j.elstat.2016.12.011 (  0) 0) |
| [20] |
Yong H K, Ji H P. Noise Characteristics of Air Cleaners and Humidifiers. 2016, 26: 20-26. DOI: 10.5050/KSNVE.2016.26.1.020.
(  0) 0) |
| [21] |
Jr. White J A. Air Cleaner Shell Noise Analysis with Plate and Shell Theory. Warrendale, PA (United States): Society of Automotive Engineers, Inc., 1996.
(  0) 0) |
 2019, Vol. 26
2019, Vol. 26


