Nowadays, benzothiazole derivative (BTH), a representative xenobiotic heterocyclic chemical, is widely used as medical intermediates and corrosion inhibitors in lots of fields such as drug preparations, rubber materials, herbicides, slimicides, algicides, fungicides, photosensitizers, azo dyes, de-icing/anti-icing fluids, and food flavors[1-4]. Because of their special chemical structure, BTHs are regarded as limited biodegradability chemicals which have potential toxicity toward microorganisms[5-6]. Their appearance in the environment has also aroused significant public concern due to their allergenicity and potential mutagenic effects[7-11].
The biodegradation mechanism of BTHs is rarely reported in published researches, except that 2-hydroxybenzothiazole (OHBT), benzothiazole-2-sulfonate, and BTH could be decomposed by Rhodococcus erythropolis[12]. As to the biodegradation pathways of BTHs, Rhodococcus pyridinovorans, the Rhodococcus rhodochrous, and R. rhodochrous were reported as capable bacteria which can decompose BTHs incompletely[6, 13-15]. Furthermore, a recent study[16] reported the degradation of BTH to OHBT by the Gram-negative bacterium Pseudomonas putida strain HKT554, which was caused by the oxidation of the thiazole-ring. However, because of the biodegradation resistance and the bio-accumulation of BTHs, there is no apparent removal after a conventional biological wastewater treatment process[17-19].
An efficient method to degrade BTH from the wastewater by an up-flow internal circulation microbial electrolysis reactor (UICMER) was provided in the last work of our laboratory[20], in which BTH is almost converted into less toxic intermediate products under 24 h reaction and the toxicity of wastewater is significantly reduced after the bioelectrochemical treatment process, but the degradation process and its mechanism still need to be investigated.
In this study, the degradation pathway in the UICMER was investigated, and the relationship between microbial community structure and the transformation of BTH was revealed. Compared with the open circuit reactor, the removal efficiency and reduction rate of BTH were significantly improved in the microbial electrolysis cell (MEC). Results of this study will provide theoretical basis for the MEC treatment of refractory pollutants such as BTH.
2 Materials and Methods 2.1 Experimental SetupThe fundamental structure of the UICMER is shown in Fig. 1, in which the inoculated sludge was taken from a pharmaceutical factory in Harbin, China. Glucose, NH4Cl, and KH2PO4 were used in the wastewater to provide the carbon, nitrogen, and phosphorus sources, and the COD:N:P proportion was controlled as 200:5:1, which was reported in our previous paper[20].
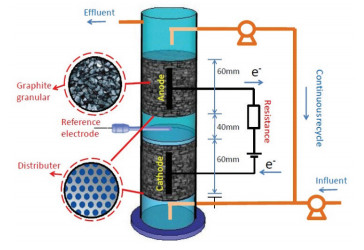
|
Fig.1 Schematic of the UICMER |
2.2 Operation
After sludge inoculation, three reactors were fed with artificial wastewater with a constant COD of 2 000 mg/L and a BTH of 60 mg/L with a hydraulic retention time (HRT) of 48 h under a batch mode of multiple cycles (5 times), and each time the reactors were provided with fresh wastewater. The voltage of MECs was controlled to 0.7 V and the temperature was controlled between 25 and 30 degrees Celsius. Details of R3 as a control experiment were reported in our previous paper[20].
2.3 AnalysisThe determinations of COD, TOC, NH4+, SO42-, and UV254 were carried out by using the standard methods[21]. The determination methods of benzothiazole and 2-hydroxyl benzothiazole concentrations were reported in our previous paper[20]. HPLC/MS was used to analyze the metabolic intermediates, and the conditions of HPLC and MS were described by El-Bassi et al.[16]
After the experiment, the biofilm on the electrode surface was used for microbial community analysis, which was consigned to Sangon Biotech Co., Ltd., Shanghai, China.
3 Results and Discussion 3.1 Kinetic Analysis of BTH in Three ReactorsTo investigate the BTH biodegradation products in the two reactors (R1 and R2), all experiments were carried out in batch with a voltage of 0.7 V by using an initial BTH and COD concentrations of 60 mg/L and 2 000 mg/L for multiple cycles (5 times). R3 was carried out under the same experimental condition with R1 as a control experiment to check up the expense.
In this study, three different reactors (R1, R2, and R3) were used for comparison and the kinetics of transformation was monitored by HPLC. As a control experiment (without microbes), the concentration of BTH in R3 decreased slightly without detectable intermediates, which was mainly caused by the adsorption of electrodes (granular-graphite and the graphite plate). Another control experiment (without current and microbes) showed the same phenomenon. Among the three operational modes (Fig. 2), the apparent first-order reaction model was used to fit the straight line for kinetic analysis.
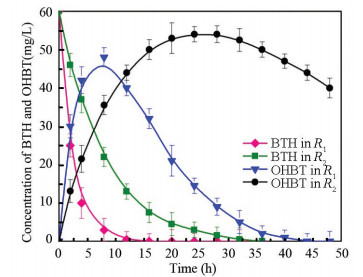
|
Fig.2 Degradation of BTH and OHBT in the MEC reactors |
The removal rate (k) and half-life time (t1/2) of BTH under different conditions were 0.7 V, R2 = 0.989 8, k = 0.484±0.013 3, and t1/2 = 2 h, whereas for the open circuit operation, R2 = 0.970 9, k=0.259±0.065 2, t1/2=6 h, and the initial concentration of BTH was 60 mg/L. Under a circuit condition (i.e., initial current density is 61 A/m3 based on the anode volumes), R1 showed the highest kBTH and ErBTH. In particular, efficiency of the BTH reduction during the first 8 h reached 95% within the existence of current, which was 1.52 times larger than that of the fermentation reactor (66.3%) of R2. Besides, the BTH degradation rate constant (k) and half time(t1/2) reached 0.484 and 2 h in the microbial electrolysis, while those were 0.259 and 6 h with microbial process in R2. Considering that electrode performance is reflected in the combination of anode and cathode potential and current density, it was assumed that the enhanced BTH reductive efficiency of microbial electrode was caused by its better electrochemical characteristics. During the biotransformation of BTH by reactors R1 and R2, as the reaction proceeded, the corresponding signal of BTH vanished gradually and a new peak occurred after BTH was completely transformed.
The BTH product was further detected by GC-MS, which proved that the product of bioelectrochemical degradation of BTH was OHBT. The hydroxylation process of BTH to OHBT was also proved, which was observed in some studies[6, 13]. Considering the possible keto-enol tautomerism of OHBT, benzothiazolinone may be the product of strain[16, 22], which was reported as the end product of BTH by Pseudomonas putida HKT554[16]. Another identi fied intermediate, acetylated BTH(BTH-acetyl), was found only in the beginning of the reaction (peaked at 2 h in R1 and at 4 h in R2), which implied that the acetylation of OHBT was accompanied by the generation of BTH-acetyl group during microbial metabolism. Certain bacteria have acetyl transferase that catalyzes the reaction of 2-hydroxyl acetylation. Since the BTH-acetyl group is extremely unstable, it was quickly deoxidized to amine form and then rapidly translated into other intermediate products within 48 h by MEC.
Under an open circuit condition, OHBT was depredated slowly in R2 and it maintained a high concentration (45 mg/L) until the end of the reaction, while it was almost exhausted at the end of the reaction (48 h) in R1. The results showed that the chemical process of OHBT removal in pure microorganism system was relatively weak. Several intermediate products were detected during the process and BTH degradation pathways appeared the same as those in R1.
3.2 BTH Degradation PathwayTo further study the degradation pathway of BTH, OHBT (66 mg/L) was used to serve as the only nutrient substrate of R1. The experiment setup process is shown in Table 1, which provides the difference between TOC, ammonia nitrogen, and sulfate concentration of the theoretical contribution of OHBT and the actual values. Various indicators were very close to the theoretical calculation values, which proved the accuracy of the experiment. Fig. 3 shows that OHBT participated as the sole electron donor and substrate in the reaction, which intuitively demonstrates the degradation process of OHBT in MEC. Through HPLC analysis, it was found that OHBT degraded completely within 56 h after the lag period of 4 h.
| Table 1 Biodegradation of OHBT in R1 after 60 h |

|
Fig.3 Reduction of OHBT in fed batch MEC (R1) |
However, the degradation pathway of OHBT was rarely elucidated in the literature. It was reported that the oxidation products of BTH in guinea pigs are mainly 2-(methy1mercapto) aniline[23]. Other oxidation products such as 2-methylsulfinylaniline and the corresponding hydroxylamine were considered degradable by most microorganisms through aerobic reaction[24]. The degradation of heterocyclic compounds usually begins with hydroxylation reaction, and is followed by the cleavage of heterocyclic rings. In addition, when connected to a six-membered ring, the five-membered ring usually breaks first[1, 9].
Based on the identification of reaction intermediates, the reaction pathway of BTH degradation by MEC is proposed in this paper. Samples were identified by liquid chromatography electrospray ionization tandem mass spectrometry (MS). The structure of some intermediate products was predicted by MS/MS combined with chemical derivation. In addition to a strong response to molecular anions, the molecular ion spectra of metabolites also conveyed a gratifying message, which was consistent with the predicted molecular formula of the product (m/z 124.183 7). Thereafter, fragment ions (m/z 109.169) produced by deamination during degradation process were detected, which clearly demonstrates the presence of amino groups in the metabolites of BTH and indicates that the thiazole-ring of OHBT may be cracked into catechol.
Oxidation by-products with masses of 2-sulfinylaniline (m/z 156.182 5, C6H7NO2S), 2-aminobenzenesulfonic acid (m/z 172.181 9, C6H7NO3S), and the corresponding hydroxylated product of 3-sulphocatechol (m/z 189.166, C6H6O5S) are expected to be degradable by bacteria. The enzyme presumably catalyzed the formation of the ring of a 2-amino-2, 3-diolmoiety, and the elimination in the amino group led to a rearomatization. 3-sulphocatechol was further degraded via meta ring cleavage, and 1 mol of 3-sulphocatechol and O2 release of 1 mol of sulphite and 2-hydroxymuconic acid were eventually mineralized[25].
To summarize, the degradation of BTH could be divided into the following steps (Fig. 4). First, the thiazole-ring was fractured and a series of oxidation and hydrolysis reactions were carried out to form aromatic intermediates (such as amines). Second, aromatic intermediates were degraded into various carboxylic acids (fumaric acid, maleic acid, oxalic acid, and formic acid) by deamination and hydroxylation. Finally, various carboxylic acids were completely oxidized into carbon dioxide and water under heterotrophic bacteria in the reactor.

|
Fig.4 Proposed biodegradation pathway of BTH in MEC |
3.3 Analysis of Microbial Community 3.3.1 Biodiversity of bacteria
By means of high-throughput sequencing, the effective sequence labels of samples from anode and cathode surfaces of R1 and R2 were 14 780, 12 995, and 14 170, respectively. The sparse curve shows that at the 3% abundance level (Fig. 5), the observed number of operational taxonomic units (OTUs) were 598 (anode biofilm), 496 (cathode biofilm), and 298 (R2), which indicates that the bacterial community structure in the anode biofilm and cathode biofilm of R1 was more abundant. Higher biodiversity means higher stability of the system, which shows that the electrochemical system of R1 is more inclined to have higher environmental impact resistance[26-27].
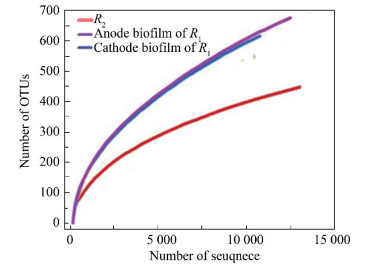
|
Fig.5 Rarefaction curves analysis chart of biofilm and cathode biofilm in R1 and R2 |
3.3.2 Comparison of microbial community
As shown in Fig. 6, the community structures of bacteria and archaea were compared. Cluster analysis of sample community structure was carried out by complete linkage method. The color of scales represents the number of OTUs detected and red to green indicates the abundance of bacteria from low to high. It can be seen that bacterial communities in the anode and cathode biomembrane of R1 belonged to the same cluster, which was quite different from that of R2, indicating that the stimulation in MEC had a dominant role in the enrichment of electrophilic microorganisms on the electrode surface.

|
Fig.6 Hierarchical cluster analysis of bacterial communities in biofilms of R1 and R2 |
The beta diversity index between the anode and cathode surface communities of R1 was 0.153 6, and that of R2 was 0.200 1 (Fig. 7). The number and response values of dominant population in anode biofilm were significantly higher than those in cathode biofilm and R2, which also indicates that the stimulus effect of the applied current significantly changed the structure of bacterial community. Besides, the oxidation of the anode played a dominant role in the degradation of BTH by MEC.
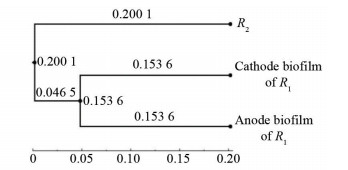
|
Fig.7 Comparison of beta diversity index among anode and cathode biofilms of R1 and R2 |
3.3.3 Analysis of bacterial taxonomy
As shown in Fig. 8, the abundance of relative bacterial communities was evaluated at the phylum level. In reactors R1 and R2, Proteobacteria, Bacteroidetes, and Firmicutes accounted for the most. The difference of bacterial community was mainly manifested in the relative quantity of three kinds of bacteria. For example, the relative abundance of the phylum Proteobacteria in the anode and cathode biofilm of R1 were 33% and 40%, which were significantly higher than that of R2 (25%).
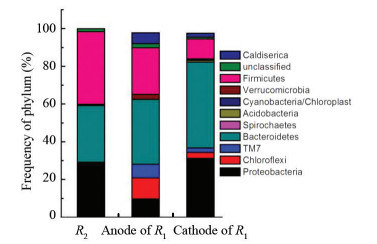
|
Fig.8 Taxonomic classification of the dominant phylogenetic groups at the phylum level |
From the above results, most populations existed in the two reactors at the same time. However, some populations such as Anaerophaga, Fibrobacteres/Acidobacteria group, Rhodocyclaceae, Syntrophobacterales, epsilon protein bacteria, and Gamma protein bacteria were in the anode biofilm, but not in the cathode biofilm, suggesting that electric field can be used to enrich certain species, which was consistent with the results in Ref. [18]. Besides, the stimulation of current to the microorganism led to high biological diversity of both anode and cathode in R1.
Through high-throughput sequencing, it was found that the diversity of microbial community structure in R1 was much higher than that in R2. Under the action of electric field, special bacteria and archaea were enriched on the surface of the anode selectively, which corresponded to the results of high bacterial abundance on the anode biofilm. At the same time, bacteria and archaea had a good synergistic effect, which could significantly reduce the concentration of volatile acid in the reactor, help to reduce the internal resistance of the reactor, increase the current density, and lead to more efficient degradation of pollutants such as BTH.
4 ConclusionsUICMER was proved to be efficient at the treatment of wastewater containing BTH, during which BTH and its intermediate products can be thoroughly mineralized to uncomplicated inorganic matters. In the meantime, about 85% of the COD was removed under the HRT of 24 h. The reaction pathways of BTH in the reactor were unveiled, all pathways were converted into OHBT and then mineralized within three steps: the fracture of thiazole ring through a series of oxidation and hydrolysis, the deamination and hydroxylation of 2-aminobenzenesulfonic acid, and the mineralization of various carboxylic acids to CO2 and H2O. With the presence of electric field, intermediate biodegradability of BTH decomposition increased significantly, which led to the improvement of the treatment performance of BTH wastewater as reflected in COD and BTH removal rate. Also, high-throughput sequencing analysis showed that the addition of electric field in MEC greatly improved the diversity of bacterial and archaeological communities on the anode and cathode biofilms, which led to the rapid improvement of the degradation efficiency of BTH.
Results show that this bioelectrochemistry system has excellent performance in dealing with wastewater containing high-concentration resistant organic contaminants like BTH. The mechanism research in this paper provides a reasonable interpretation for the BTH degradation process, which could be a powerful evidence to enlarge the scale of the UICMER reactor and advance its practical application.
| [1] |
De Wever H, Verachtert H. Biodegradation and toxicity of benzothiazoles. Water Research, 1997, 31(11): 2673-2684. DOI:10.1016/S0043-1354(97)00138-3 (  0) 0) |
| [2] |
Richardson S D. Environmental mass spectrometry: Emerging contaminants and current issues. Analytical Chemistry, 2010, 82(12): 4742-4774. DOI:10.1021/ac060682u (  0) 0) |
| [3] |
Asimakopoulos A G, Bletsou A, Thomaidis N S. Emerging contaminants: A tutorial mini-review. Global Nest Journal, 2012, 14(1): 72-79. (  0) 0) |
| [4] |
Asimakopoulos A G, Wang L, Thomaidis N S, et al. Benzotriazoles and benzothiazoles in human urine from several countries: A perspective on occurrence, biotransformation, and human exposure. Environment International, 2013, 59: 274-281. DOI:10.1016/j.envint.2013.06.007 (  0) 0) |
| [5] |
De Wever H, De Moor K, Verachtert H. Toxicity of 2-mercaptobenzothiazole towards bacterial growth and respiration. Applied Microbiology and Biotechnology, 1994, 42(4): 631-635. DOI:10.1007/BF00173931 (  0) 0) |
| [6] |
Haroune N, Combourieu B, Besse P, et al. Benzothiazole degradation by Rhodococcus pyridinovorans strain PA: Evidence of a catechol 1, 2-dioxygenase activity. Applied and Environmental Microbiology, 2002, 68(12): 6114-6120. DOI:10.1128/AEM.68.12.6114-6120.2002 (  0) 0) |
| [7] |
Chipinda I, Hettick J M, Simoyi R H, et al. Oxidation of 2-mercaptobenzothiazole in latex gloves and its possible haptenation pathway. Chemical Research in Toxicology, 2007, 20(8): 1084-1092. DOI:10.1021/tx700139g (  0) 0) |
| [8] |
Gold L S, Slone T H, Stern B R, et al. Comparison of target organs of carcinogenicity for mutagenic and non-mutagenic chemicals. Mutation Research/ Fundamental and Molecular Mechanisms of Mutagenesis, 1993, 286(1): 75-100. DOI:10.1016/0027-5107(93)90004-Y (  0) 0) |
| [9] |
Zhang X X, Zhang T, Fang H H P. Antibiotic resistance genes in water environment. Applied Microbiology and Biotechnology, 2009, 82(3): 397-414. DOI:10.1007/s00253-008-1829-z (  0) 0) |
| [10] |
Zhang Y, Angelidaki I. Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges. Water Research, 2014, 56: 11-25. DOI:10.1016/j.watres.2014.02.031 (  0) 0) |
| [11] |
Zhang Y, Angelidaki I. Bioelectrochemical recovery of waste-derived volatile fatty acids and production of hydrogen and alkali. Water Research, 2015, 81: 188-195. DOI:10.1016/j.watres.2015.05.058 (  0) 0) |
| [12] |
De Wever H, Vereecken K, Stolz A, et al. Initial transformations in the biodegradation of benzothiazoles by Rhodococcus isolates. Applied and Environmental Microbiology, 1998, 64(9): 3270-3274. DOI:10.1128/AEM.64.9.3270-3274.1998 (  0) 0) |
| [13] |
Besse-Hoggan P, Haroune N, Combourieu B, et al. Liquid and solid state NMR study of benzothiazole degradation by Rhodococcus isolates. European Symposium on Environmental Biotechnology, 2004, 705-708. (  0) 0) |
| [14] |
Bunescu A, Besse-Hoggan P, Sancelme M, et al. Comparison of microbial and photochemical processes and their combination for degradation of 2-aminobenzothiazole. Applied and Environmental Microbiology, 2008, 74(10): 2976-2984. DOI:10.1128/AEM.01696-07 (  0) 0) |
| [15] |
Chorao C, Charmantray F, Besse-Hoggan P, et al. 2-Aminobenzothiazole degradation by free and Ca-alginate immobilized cells of Rhodococcus rhodochrous. Chemosphere, 2009, 75(1): 121-128. DOI:10.1016/j.chemosphere.2008.11.021 (  0) 0) |
| [16] |
El-Bassi L, Iwasaki H, Oku H, et al. Biotransformation of benzothiazole derivatives by the Pseudomonas putida strain HKT554. Chemosphere, 2010, 81(1): 109-113. DOI:10.1016/j.chemosphere.2010.07.024 (  0) 0) |
| [17] |
Gaja M A, Knapp J S. Removal of 2-mercaptobenzothiazole by activated sludge: A cautionary note. Water Research, 1998, 32(12): 3786-3789. DOI:10.1016/S0043-1354(98)00146-8 (  0) 0) |
| [18] |
Zhang J, Zhang Y, Quan X, et al. Effects of ferric iron on the anaerobic treatment and microbial biodiversity in a coupled microbial electrolysis cell (MEC) - Anaerobic reactor. Water Research, 2013, 47(15): 5719-5728. DOI:10.1016/j.watres.2013.06.056 (  0) 0) |
| [19] |
Zhang J, Zhang Y, Quan X, et al. An anaerobic reactor packed with a pair of Fe-graphite plate electrodes for bioaugmentation of azo dye wastewater treatment. Biochemical Engineering Journal, 2012, 63: 31-37. DOI:10.1016/j.bej.2012.01.008 (  0) 0) |
| [20] |
Liu X, Ding J, Ren N, et al. The detoxification and degradation of benzothiazole from the wastewater in microbial electrolysis cells. International Journal of Environmental Research and Public Health, 2016, 13(12): 1259-1271. DOI:10.3390/ijerph13121259 (  0) 0) |
| [21] |
Clesceri L S, Greenberg A E, Eaton A D. Standard methods for the examination of water and wastewater, 20th edition. Washington, D. C.: American Public Health Association, 1998.
(  0) 0) |
| [22] |
Harb V, Kocjan D, Hadži D. A comparative semiempirical and ab initio study of tautomerization energies. 7-Methyl-2, 3, 4, 7-tetrahydroisothiazolo [5, 4b] pyridine-3, 4-dione and other models of antibacterial quinolone analogues. Journal of Molecular Structure: THEOCHEM, 1992, 257(3-4): 475-483. DOI:10.1016/0166-1280(92)85056-Q (  0) 0) |
| [23] |
Fowler A M, Chissick H H, Frearson M J, et al. The role of aldehyde oxidase in the in vivo metabolism of benzothiazole. Biochemical Society Transactions, 1995, 23(4): 604S. DOI:10.1042/bst023604s (  0) 0) |
| [24] |
Reemtsma T, Fiehn O, Kalnowski G, et al. Microbial transformations and biological effects of fungicide-derived benzothiazoles determined in industrial wastewater. Environmental Science & Technology, 1995, 29(2): 478-485. DOI:10.1021/es00002a025 (  0) 0) |
| [25] |
Junker F, Field J A, Bangerter F, et al. Oxygenation and spontaneous deamination of 2-aminobenzenesulphonic acid in Alcaligenes sp. strain O-1 with subsequent meta ring cleavage and spontaneous desulphonation to 2-hydroxymuconic acid. Biochemical Journal, 1994, 300(2): 429-436. DOI:10.1042/bj3000429 (  0) 0) |
| [26] |
Stabnikov V P, Tay S T L, Tay D K, et al. Effect of iron hydroxide on phosphate removal during anaerobic digestion of activated sludge. Applied Biochemistry and Microbiology, 2004, 40(4): 376-380. DOI:10.1023/B:ABIM.0000033914.52026.e5 (  0) 0) |
| [27] |
Tilman D, Reich P B, Knops J M H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature, 2006, 441: 629-632. DOI:10.1038/nature04742 (  0) 0) |
 2019, Vol. 26
2019, Vol. 26


