2. Key Laboratory of Advanced Structural-Functional Integration Materials & Green Manufacturing Technology, Harbin Institute of Technology, Harbin 150001, China
Geopolymer, with a heat-resistant temperature up to 1 300-1 600 ℃, is a high-temperature material developed on the basis of cement, ceramic, and refractory materials. In addition to its intrinsic characteristics such as heat resistance, flame resistance, high specific strength, and low density, geopolymer also exhibits advantages like facile processing, low cost, low energy consumption, and environmental friendliness. These unique features make geopolymer promising in the fields of transportation, national defense, aerospace, and nuclear energy, for instance in the fire-resistant components of aeroengine, aerofoil, aerospace vehicle, and geopolymer cement for nuclear waste treatment[1-2]. However, in order to overcome its disadvantages of intrinsic brittleness, large shrinkage stress, and low reliability, it is necessary to strengthen and toughen the geopolymer matrix by means of efficient reinforcing phases. Owing to its low-temperature (30-150 ℃) moulding nature, a wide range of materials can in principle serve as reinforcing phases to ameliorate mechanical properties of phosphate matrix, including metal and ceramic particles, short fibers, and continuous fibers. Among them, fibers reinforced geopolymer composites exhibit good mechanical properties, high-temperature resistance, and stability, which thus have gained extensive concerns and attentions in recent studies[3-5].
In recent years, carbon fiber (Cf) has been widely recognized as a promising reinforcing phase in the field of composite materials due to its high specific modulus and specific strength, especially for the development of low-cost and environmental friendly refractory composites. It not only meets the requirements of strength, rigidity, light weight, fatigue resistance, high temperature resistance, and corrosion resistance, but also possesses unique features such as high conductivity, high thermal conductivity, and low expansion coefficient[6-8]. However, the surface of pure carbon fiber is chemically inert and shows a weak interaction with matrix, which results in poor interlayer shear strength of the composites[9]. To realize better interfacial bonding between carbon fiber and matrix, it is of great importance to treat carbon fibers in advance by using appropriate methods for surface modification[10-11].Another problem regarding to the refractory applications of Cf/geopolymer composites lies in their thermal evolution. A fundamental understanding of the temperature dependence of phase composition and mechanical properties of Cf/geopolymer composites remains elusive.
In this work, two-dimensional continuous Cf was used as the reinforcing phase to fabricate Cf reinforced geopolymer composites (Cf/geopolymer). Acetone treatment process was performed to improve the interfacial bonding between Cf and phosphate matrix. Effects of acetone treatment, high-temperature treatment, and Sol-SiO2 re-impregnation treatment on the properties of Cf/geopolymer composites were systematically investigated.
2 Materials and Experiment 2.1 MaterialsThe information of raw materials and the properties of Cf used in this study are listed in Table 1 and Table 2, respectively.
| Table 1 Information of raw materials used in this study |
| Table 2 Properties of woven carbon fiber cloth used in this study |
2.2 Preparation of Cf/Geopolymer Composites
To achieve a better interfacial bonding between fiber and geopolymer matrix, carbon fibers were treated in acetone by immersing for 24 h. H3PO4 and Al(OH)3 were employed as raw materials to prepare the phosphate solution. Meanwhile, a small amount of CrO3 and CH3OH were introduced into the solution. After that, curing agents and fillers (Al2O3) were added and the mixture was milled by mechanical ball milling for a certain time to get geopolymer matrix. Two-dimensional woven carbon fiber cloth was cut into squares (10 cm×10 cm), which were then placed into the geopolymer matrix under the ultrasonic field for impregnation (5 min). The impregnated carbon fiber cloth was then fished out and stacked together layer by layer. Finally, the stacked carbon fiber cloth with a layer number of 25-40 was placed in the mold, pressurized and cured at 60 ℃ for 7 d for geopolymerization. The composites were further high-temperature treated in Ar atomosphere, and the high-temperature treated samples were impregnated by Sol-SiO2 solution for 3 times.
2.3 CharacterizationsMechanical properties of the obtaiend composites were tested by electronic universal tester (AGX-plus 20 kN, Japan). Chemical state of carbon was investigated by X-ray photoelectron spectroscopy (ESCALAB 250Xi, ThermoFisher, America). Surface wetting angles of carbon fibers before and after acetone treatments were determined by wetting angle tester (VAF-30, China). Simultaneous thermogravimetry and differential thermal analysis were performed by thermal analyzer (TG/DTA, Netzsch STA 449 C, Germany). Phase compositions of the composites were characterized by X-ray diffractometer (XRD, Rigaku-D/max-γB, Japan). Surface morphologies of Cf and fractographies of the composites and their high-temperature products were studied by scanning electron microscope (SEM, FEI HELIOS NanoLab 600i, America). Flexural strength and Young's modulus of the composites were measured on specimens (4 mm×3 mm×36 mm) using a three-point bending fixture on an Instron-500 tester (America) with a span length of 30 mm at a cross-head speed of 0.5 mm/min. Results from two loading directions, i.e., perpendicular (X) to the laying-up direction and parallel (Y) to the laying-up direction, were measured.
3 Results and Discussion 3.1 Effects of Acetone Treatments on Properties of Cf/Geopolymer CompositesIn this section, acetone was used to treat Cf and the properties of Cf before and after acetone treatments were compared. The role of acetone treatments in modifying the properties of Cf was discussed. Fig. 1 shows the XPS spectra of Cf before and after acetone treatments. In both cases, the main characteristic peaks (C1), presenting (-C-C-) functional group, were found to be at 284.8 eV. However, C4 characteristic peak appearred after acetone treatments, indicating the emergence of acidic functional groups such as carboxyl (-COOH) and ester (-COOR). C2 and C3 characteristic peaks also became more pronounced in acetone-treated Cf, corresponding to increasing contents of -C-O- and >C=O, respectively. Table 3 lists the binding energy and weight of different characteristic peaks before and after acetone treatments. It can be seen that after acetone treatments, the weight of C1 characteristic peak (-C-C-) decreased from 83.8% to 70%, whereas those of oxygen-containing functional groups (i.e., C2, C3, and C4) increased to varying degrees. In particular, the weight of acidic functional groups (i.e., C4) increased to 4.5% after acetone treatments.
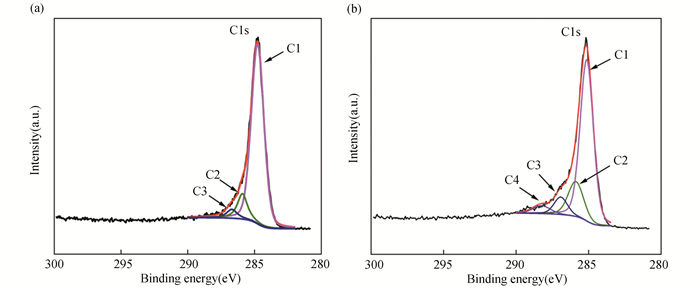
|
Fig.1 XPS spectra of Cf(a) before and (b) after acetone treatments |
| Table 3 Binding energy and weight of different characteristic peaks before and after acetone treatments |
Fig. 2 presents the surface morphology of Cf before and after acetone treatments. It is apparent that the surface of the untreated Cf is covered by a gel-like substance (Fig. 2(a)), which can be removed by using acetone treatments, as shown in Fig. 2(b). The acetone-treated Cf exhibits a relatively smooth surface and the intrinsic patterns of Cf can be observed more clearly.

|
Fig.2 Surface morphologies of Cf(a) before and (b) after acetone treatments |
To further understand how acetone treatments affect the surface properties of Cf, wetting angles were tested for acetone-treated and non-treated Cf, respectively. The wettability of a solid surface is mainly determined by the nature of the atoms and/or the atomic groups on the interface. As the surface of the untreated Cf is dominated by the hydrophobic gel-like substance (Fig. 2(a)), the non-treated Cf shows a relatively big wetting angle around 90° (Fig. 3(a)). During the acetone treatments, such hydrophobic gel-like substance gradually dissolves into the medium, and the intrinsically undulating patterns of Cf govern the surface (as shown in Fig. 2(b)). Owing to its greater surface roughness and bigger wetting force, acetone-treated Cf shows a much smaller wetting angle (~40°), and thus has better wettability.

|
Fig.3 Surface wetting angles of Cf(a) before and (b) after acetone treatments |
To further understand how acetone treatments affect the surface properties of Cf, wetting angles were tested for acetone-treated and non-treated Cf, respectively. The wettability of a solid surface is mainly determined by the nature of the atoms and/or the atomic groups on the interface. As the surface of the untreated Cf is dominated by the hydrophobic gel-like substance (Fig. 2(a)), the non-treated Cf shows a relatively big wetting angle around 90° (Fig. 3(a)). During the acetone treatments, such hydrophobic gel-like substance gradually dissolves into the medium, and the intrinsically undulating patterns of Cf govern the surface (as shown in Fig. 2(b)). Owing to its greater surface roughness and bigger wetting force, acetone-treated Cf shows a much smaller wetting angle (~40°), and thus has better wettability.
Both acetone-treated and non-treated Cf were used to prepare Cf/geopolymer composites. Fig. 4 displays the fracture surfaces of Cf/geopolymer composites without (Fig. 4(a)) and with (Fig. 4(b)) acetone treatments. It is obvious that the composites reinforced by acetone-treated Cf have more continuous and homogeneous surfaces, which further reflects the fact that acetone treatments help to improve the wettability of Cf surfaces, leading to a stronger interaction between Cf and phosphate matrix.
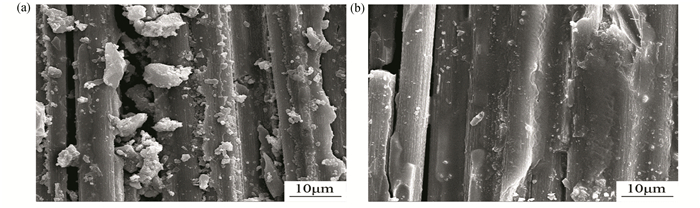
|
Fig.4 Fracture surfaces of Cf/geopolymer composites (a) without and (b) with acetone treatment |
Fig. 5 shows the comparison of the mechanical properties of Cf/geopolymer composites prepared by using Cf with and without acetone treatments. The obtained Cf/geopolymer composites had anisotropic mechanical properties, mainly due to the preferred distribution of Cf. Obviously, the Cf/geopolymer composites prepared by acetone-treated Cf have higher flexural strength (Fig. 5(a)) and elastic modulus (Fig. 5(b)) in both X and Y directions than those prepared by non-treated Cf, leading to a stronger interaction between Cf and phosphate matrix as discussed above. It is noteworthy that a more compact structure may also contribute to the enhanced mechanical properties of the acetone-treated Cf/geopolymer composites due to the better matrix filling. It can also be found that the Cf/geopolymer composites prepared by acetone-treated Cf showed a flexural strength of 156.1 MPa and an elastic modulus of 39.7 GPa in the Y direction. The mechanical properties of different fiber reinforced geopolymer composites are summarized in Table 4. Apparently, the mechanical properties of the as-prepared Cf/geopolymer composites are much higher than those reinforced by polypropylene fiber and micro steel fiber[12], cotton fiber[13], and short SiC fiber[14], and are comparable to those fabricated using unidirectional carbon fiber[15] and continuous SiC fiber[16].

|
Fig.5 Mechanical properties of Cf/geopolymer composites using Cf without and with acetone treatment: (a) flexural strength; (b) elastic modulus |
| Table 4 Comparison of mechanical properties of different fiber reinforced geopolymer composites |
Therefore, we attributed the enhanced mechanical properties achieved by acetone-treated Cf/geopolymer composites to the following reasons: 1) after the mild acetone treatments, the removal of hydrophobic gel-like substance on the surface of Cf allows a stronger interaction between Cf and phosphate matrix; 2) the acetone treatments may regulate the fiber to matrix ratio in the obtained composites to a more reasonable value, therefore, optimizing the interfacial bonding in the composites.
3.2 Effects of High-Temperature Treatments on Properties of Cf/Geopolymer CompositesIn this section, the thermal evolution of phosphate matrix and effects of high-temperature treatments on the properties of Cf /geopolymer composites were investigated. Fig. 6 shows the TG/DTA curves of the phosphate matrix when being treated from room temperature to 1 200 ℃ in the air. An about 10% weight loss was observed for the matrix upon heating with a rapid weight loss process within 400 ℃ and a slow one in the temperature range of 400 ℃-600 ℃. Meanwhile, two endothermic peaks at 87 ℃ and 166 ℃ were noticed, corresponding to the evaporation of free water and organic solvents in the system and the cross-linking reaction of phosphate matrix, respectively. In addition, another endothermic peak at 1 125 ℃ was assigned to the phase transition of phosphate matrix and accompanied by no obvious weight loss. It can be concluded that the polymerization of geopolymer was almost accomplished within 400 ℃, indicating that the proposed synthetic method can serve as a low-temperature route for preparing geopolymer and phosphate-based composites.

|
Fig.6 TG/DTA curves of Cf/geopolymer composites when being treated from room temperature to 1 200 ℃ |
Fig. 7 shows the XRD patterns of Cf/geopolymer composites before and after being treated at different temperatures. It can be seen that Al2O3 and AlPO4 are the main crystalline phases of Cf/geopolymer composites, regardless of the treatment temperature in the employed temperature range (from room temperature to 900 ℃). With increasing treatment temperature, AlPO4 becomes more dominant, accompanied by the formation of amorphous phase (supported by the emergence of the diffuse scattering peak around 28° 2θ), which suggests a more complete geopolymerization process at high temperatures.
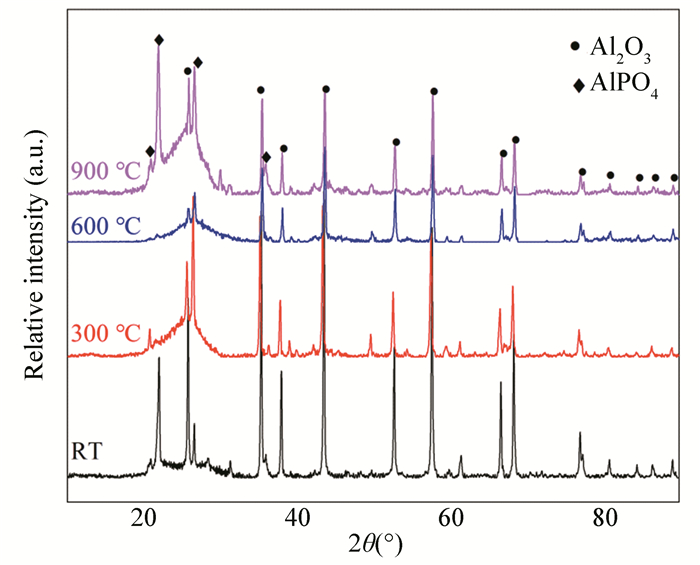
|
Fig.7 XRD patterns of Cf/geopolymer composites at different temperatures before and after treatments |
Fig. 8 shows the microstructure of non-treated Cf/geopolymer composites after being treated at different temperatures. Matrix between neighboring fibers exhibited shrinkage and desorption behaviors to varying degrees, depending on the treatment temperature. With the increase of the treatment temperature from 300 ℃ (Fig. 8(a)) to 600 ℃ (Fig. 8(b)), along with more noticeable cracks and pores in the composites, more obvious desorption behaviors of Cf were observed. In particular, after being treated at 900 ℃ (Fig. 8(c)), the matrix which bridges surrounding fibers is almost removed, and only a small amount of matrix debris was observed. The gap between fibers became quite large, which broke the continuity of the composites. Such observation is attributed to the cross-linking reaction of phosphate matrix during high-temperature treatments. The dehydration condensation of phosphate matrix results in the desorption behaviors of Cf and the formation of cracks and pores in the composites.

|
Fig.8 Fracture surfaces of non-treated Cf/geopolymer composites after being treated at different temperatures: (a) 300 ℃; (b) 600 ℃; (c) 900 ℃ |
Fig. 9 shows the microstructure of acetone-treated Cf/geopolymer composites after being treated at different temperatures. The composites also experienced temperature-dependent shrinkage and desorption behaviors upon heating, accompanied by the emergence of cracks and pores among Cf. However, compared with those prepared by non-treated Cf, the composites made by acetone-treated Cf showed less shrinkage and defects under same conditions. This further supports the conclusion that acetone-treated Cf possesses better interfacial interaction with phosphate matrix than non-treated one.

|
Fig.9 Fracture surfaces of acetone-treated Cf/geopolymer composites after being treated at different temperatures: (a) 300 ℃; (b) 600 ℃; (c) 900 ℃ |
Fig. 10 plots the mechanical properties of Cf/geopolymer composites prepared by acetone-treated and non-treated Cf before and after high-temperature treatments. Both groups exhibited decreasing flexural strength and elastic modulus with increasing treatment temperature. However, the composites prepared by acetone-treated Cf always had higher flexural strength and elastic modulus than those prepared by non-treated Cf after the same high-temperature treatment. After being treated at 900 ℃, the Cf/geopolymer composites prepared by acetone-treated Cf maintained about 60% of flexural strength, whereas those prepared by non-treated Cf only had about 32% of flexural strength. Moreover, the elastic modulus of Cf/geopolymer composites with and without acetone treatments after 900 ℃ treatment was 23.3 GPa and 16.0 GPa, respectively. It further emphasizes that the acetone treatments are beneficial for improving the mechanical properties of Cf/geopolymer composites as well as their high-temperature products.
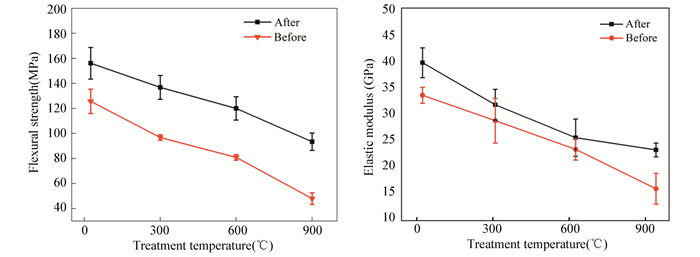
|
Fig.10 Mechanical properties of Cf/geopolymer composites after being treated at different temperatures (Y direction): (a) flexural strength; (b) elastic modulus |
3.3 Effects of Sol-SiO2 Re-impregnation Treatments on Properties of Heated Cf/Geopolymer Composites
In this section, Sol-SiO2 re-impregnation treatments were performed for high-temperature products (obtained at 900 ℃) of Cf/geopolymer composites, aiming for optimizing their mechanical properties.
Fig. 11 presents the microstructure of the composites treated with different Sol-SiO2 concentrations. In the untreated composites (Fig. 11(a)), lots of fibers were pulled out of the matrix, indicating that the bonding strength between Cf and matrix is relatively weak.

|
Fig.11 Fracture surfaces of composites (obtained at 900 ℃) treated with different Sol-SiO2 concentrations: (a) 0; (b) 20%; (c) 30%; (d) 40% |
However, for the composites that were treated with 20%, 30%, and 40% Sol-SiO2, the pull-out of Cf was gradually suppressed. It can be inferred that after Sol-SiO2 re-impregnation treatments, the surface of the resulting composites is covered by sol-gel coatings, and a dense substance forms between Cf, which bridges them effectively. This suggests that Sol-SiO2 re-impregnation treatments can repair the cracks and fill the pores inside the resulting composites. In the employed Sol-SiO2 concentration range (0-40 wt %), it was noticed that the sol-gel coating becomes denser and more homogeneous at higher Sol-SiO2 concentration, leading to better continuity between Cf and matrix.
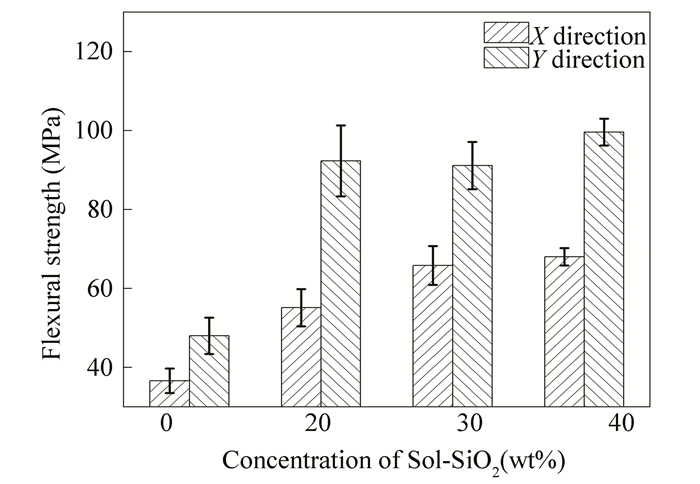
To quantitatively describe and further support our observation, the flexural strength of the resulting composites treated with different Sol-SiO2 concentrations was tested in both X and Y directions, as shown in Fig. 12. As expected, by using 40 wt% Sol-SiO2 as the re-impregnation agent, the flexural strength of the composites (obtained at 900 ℃) exceeded 99 MPa and was 107% higher than those without Sol-SiO2 re-impregnation treatments.
|
Fig.12 Flexural strength of the composites (obtained at 900 ℃) treated with different Sol-SiO2 |
4 Conclusions
1) Acetone treatments had significant effects on tailoring the surface properties of Cf, which can ameliorate the interfacial interaction between Cf and phosphate matrix and improve the mechanical properties of Cf/geopolymer composites. The flexural strength and elastic modulus (in Y direction) of the acetone-treated Cf/geopolymer composites were 156.1 MPa and 39.7 GPa, respectively.
2) Upon post high-temperature treatment, Cf/geopolymer composites underwent cross-linking reaction and dehydration condensation, leading to the shrinkage of matrix. This was accompanied by the introduction of cracks and pores in the composites and the reduction in mechanical properties. However, the composites prepared by acetone-treated Cf always showed better mechanical properties than their non-treated counterparts under the same conditions.
3) A further Sol-SiO2 re-impregnation treatment on the high temperature treated Cf/geopolymer composites led to the formation of dense sol-gel coatings on the surface of the heated composites, enhancing their mechanical properties through repairing the cracks and filling the pores. By using 40 wt% Sol-SiO2 as the re-impregnation agent, the flexural strength of the heated composites was 107% higher than those without Sol-SiO2 re-impregnation treatments.
| [1] |
Li Q, Sun Z, Tao D, et al. Immobilization of simulated radionuclide 133Cs+ by fly ash-based geopolymer. Journal of Hazardous Materials, 2013, 262: 325-331. DOI:10.1016/j.jhazmat.2013.08.049 (  0) 0) |
| [2] |
Blackford M G, Hanna J V, Pike K J, et al. Transmission electron microscopy and nuclear magnetic resonance studies of geopolymers for radioactive waste immobilization. Journal of the American Ceramic Society, 2007, 90(4): 1193-1199. DOI:10.1111/j.1551-2916.2007.01532.x (  0) 0) |
| [3] |
He P, Jia L, Ma G, et al. Effects of fiber contents on the mechanical and microwave absorbent properties of carbon fiber felt reinforced geopolymer composites. Ceramics International, 2018, 44(9): 10726-10734. DOI:10.1016/j.ceramint.2018.03.107 (  0) 0) |
| [4] |
Krüger R, Seitz J M, Ewald A, et al. Strong and tough magnesium wire reinforced phosphate cement composites for load-bearing bone replacement. Journal of the Mechanical Behavior of Biomedical Materials, 2013, 20: 36-44. DOI:10.1016/j.jmbbm.2012.12.012 (  0) 0) |
| [5] |
Sakulich A R. Reinforced geopolymer composites for enhanced material greenness and durability. Sustainable Cities and Society, 2011, 1(4): 195-210. DOI:10.1016/j.scs.2011.07.009 (  0) 0) |
| [6] |
Shirvanimoghaddam K, Hamim S U, Akbari M K, et al. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Composites Part A: Applied Science and Manufacturing, 2017, 92: 70-96. DOI:10.1016/j.compositesa.2016.10.032 (  0) 0) |
| [7] |
Chung D. Carbon Fiber Composites. Amsterdam: Elsevier, 2012. (  0) 0) |
| [8] |
Thostenson E T, Li W Z, Wang D Z, et al. Carbon nanotube/carbon fiber hybrid multiscale composites. Journal of Applied Physics, 2002, 91(9): 6034-6037. DOI:10.1063/1.1466880 (  0) 0) |
| [9] |
Weitzsacker C L, Xie M, Drzal L T. Using XPS to investigate fiber/matrix chemical interactions in carbon-fiber-reinforced composites. Surface and Interface Analysis, 1997, 25(2): 53-63. DOI:10.1002/(SICI)1096-9918(199702)25:2<53::AID-SIA222>3.0.CO;2-E (  0) 0) |
| [10] |
Yuan H, Wang C, Zhang S, et al. Effect of surface modification on carbon fiber and its reinforced phenolic matrix composite. Applied Surface Science, 2012, 259: 288-293. DOI:10.1016/j.apsusc.2012.07.034 (  0) 0) |
| [11] |
Liu Y, Zhang X, Song C, et al. An effective surface modification of carbon fiber for improving the interfacial adhesion of polypropylene composites. Materials & Design, 2015, 88: 810-819. DOI:10.1016/j.matdes.2015.09.100 (  0) 0) |
| [12] |
Ranjbar N, Talebian S, Mehrali M, et al. Mechanisms of interfacial bond in steel and polypropylene fiber reinforced geopolymer composites. Composites Science and Technology, 2016, 122: 73-81. DOI:10.1016/j.compscitech.2015.11.009 (  0) 0) |
| [13] |
Alomayri T, Shaikh F U A, Low I M. Synthesis and mechanical properties of cotton fabric reinforced geopolymer composites. Composites Part B: Engineering, 2014, 60: 36-42. DOI:10.1016/j.compositesb.2013.12.036 (  0) 0) |
| [14] |
Yuan J, He P, Jia D, et al. SiC fiber reinforced geopolymer composites, part 1:Short SiC fiber. Ceramics International, 2016, 42(4): 5345-5352. DOI:10.1016/j.ceramint.2015.12.067 (  0) 0) |
| [15] |
He P, Jia D, Lin T, et al. Effects of high-temperature heat treatment on the mechanical properties of unidirectional carbon fiber reinforced geopolymer composites. Ceramics International, 2010, 36(4): 1447-1453. DOI:10.1016/j.ceramint.2010.02.012 (  0) 0) |
| [16] |
He P, Jia D, Zheng B, et al. SiC fiber reinforced geopolymer composites, part 2: Continuous SiC fiber. Ceramics International, 2016, 42(10): 12239-12245. DOI:10.1016/j.ceramint.2016.04.168 (  0) 0) |
 2020, Vol. 27
2020, Vol. 27


