In recent years, amorphous silica (rice husk ash or RHA) has been widely used as an economical raw material in various industrial fields, such as rubbers, paint, polymer composites, cement, construction materials, and sewage treatment[1-4]. Rice husk contains a large amount of natural organic silicon, which is an abundant resource in China. Chemical precipitation and combustion syntheses are the main methods for preparing high-purity silica from rice husk[5]. The traditional precipitation method requires the production of RHA by roasting rice husk in the first place, and then the silica is purified from the RHA by dissolution and precipitation. Although the process has the advantage of high quality, it can cause chemical pollution and energy dissipation. In contrast, combustion process can produce amorphous silica while generating power or heat, which makes it an efficient method for resource utilization[6].
RHA exhibits various colors and activities under different combustion conditions, depending on combustion temperature and equipment. Current common rice husk combustion equipment primarily includes rotary kiln, inclined grate furnace, cyclonic furnace, and fluidized bed[7]. Rice husk combustion using a rotary kiln has various disadvantages, such as a poor mixing effect of air and fuel, and a long combustion retention time. The inclined grate furnace is currently the main domestic rice husk industrial combustion equipment, however, incomplete combustion of rice husk leads to high residual carbon content in the RHA[8]. The cyclonic furnace can improve the gas-solid mixing effect and urge the rotary combustion of rice husk, while short combustion retention time greatly reduces the quality of the RHA[9]. Compared with the aforementioned types of combustion equipment, fluidized bed, especially circulating fluidized bed (CFB), has become the most promising equipment for producing amorphous nano-silica by rice husk combustion due to its merits of flexibility, high combustion efficiency, and low environmental pollution[10].
Various scales of combustion experiments have shown that the combustion efficiency of rice husk in the fluidized beds can generally exceed 90%[11-14]. To date, dozens of rice-husk-fueled bubbling fluidized bed and circulating fluidized bed boilers (2-75 t/h) are in operation in China. However, all of them generate RHA with an excessively high unburned carbon content (5-20 wt.%). Huang et al.[15] conducted rice husk combustion experiments in a bench-scale electric heating fluidized bed. Results showed that RHA obtained from fluidized bed combustion contains more than 10 wt.% of unburned carbon, and was then purified with HCl solution and roasted again to obtain white silica with higher quality. High residual carbon content in RHA, whether in a lab or an industrial operation, is a key issue to be solved urgently for the fluidized bed combustion of rice husk. However, few studies have reported the production of RHA with ultra-low carbon (< 1%) by rice husk combustion in the fluidized bed on a large scale.
Another key issue for the fluidized bed combustion of rice husk is the agglomeration, even the defluidization caused by bed agglomeration and the corrosion of the heating surfaces[10]. These disadvantages have been attributed to the alkali metals and chlorines which are rich in rice husk[16]. When the temperature exceeds 760 ℃, silica and potassium oxides melt on the surface of rice husk forming a glass-like coating which prevents the further oxidation of the internal organic carbon, finally resulting in the crystalline RHA with high residual carbon. Besides, if rice husk is burned in a fluidized bed with quartz sand as the bed material, bed agglomeration forms when the temperature approaches 800 ℃ and severe defluidization occurs once the temperature exceeds 800-820 ℃[17]. To solve such combustion problems and weaken the limiting effect of combustion temperature on the performance of RHA, impurities such as alkali metals and chlorines in rice husk must be removed beforehand. Leaching pretreatment with dilute acid can effectively remove a large amount of metal impurities in rice husk[18]. So far, acid pretreatment of rice husk has only been performed under lab conditions and always been used in the degradation of lignocellulose, while no study has reported on the combination of acid pretreatment and CFB combustion of rice husk for the production of nano-silica on an industrial scale.
Therefore, a new method is proposed in this paper to continuously produce nano-silica particles by rice husk combustion in a megawatt double-circulating fluidized bed (DCFB) based on acid pretreatment. This study investigated the combustion characteristics of pretreated rice husk in a specially-designed 0.7 MW DCFB and the performance of the obtained RHA. The specific objectives are: (a) to evaluate the chemical components and fuel properties of rice husk after acid pretreatment, (b) to investigate the actual combustion process of pretreated rice husk in the DCFB, and (c) to discuss the effects of experimental and collecting conditions on the performance of the obtained RHA. The results of this study are valuable for the industrial production of nano-silica particles.
2 Materials and Methods 2.1 MaterialsThe raw material rice husk used in this study was from the rice processing plant in Harbin, China. The moisture content of the original rice husk sample is approximately 7.11 wt.%. The physical parameters of the rice husk are given in Table 1.
| Table 1 Physical parameters of rice husk |
Because of its low bulk density (125 kg/m3) and non-granular shape (0-10 mm), rice husk is difficult to fluidize alone in the fluidized bed[19]. To ensure the normal fluidization of CFB in this experiment and to investigate the influence of bed material on the SiO2 purity in RHA, quartz sand with a silica purity of 99% was selected as the bed material. Since acid pretreatment removes most of the alkali metals in advance, agglomeration does not occur when rice husk is burned in the fluidized bed with quartz sand as the bed material. The size distribution of quartz sand is shown in Fig. 1. The median particle diameter D50 of the sand is 0.58 mm and the D90 is 0.66 mm.
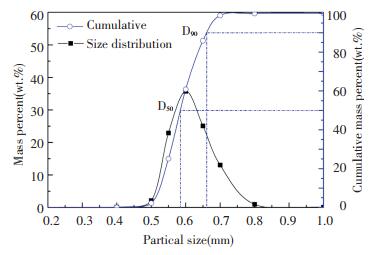
|
Fig.1 Particle size distribution and cumulative percent of the bed material |
2.2 Acid Pretreatment Tests
In this experiment, the acid pretreatment process before combustion was based on the steps for rice husk pretreatment, which was reported in a previous study[20]. Sulfuric acid and citric acid with initial concentrations of 0.5 M were chosen as the pretreatment agents. The pretreatment devices contained one soaking pool and three cleaning pools made of PVC. Thermal insulation was applied to the soaking pool to improve the acid treatment effect. Acid solution was firstly placed in the soaking pool, and the rice husk was then added into the soaking pool at a liquid/solid ratio of 14:1 (L/kg), which was leached for 2 h and stirred with a powerful stirrer (NZJ200S-200, China) at a speed of 200 r/min. The leached rice husk was transferred to the cleaning pools, washed with water, and finally dried to a moisture content of 10-12 wt.%. Untreated, sulfuric acid, and citric acid-pretreated rice husks were identified by URH, SRH, and CRH, respectively. The acid solutions in the soaking pool were reused, whereas the cleaning solutions were repeatedly used to supplement the soaking pool. The concentrations of the acid solutions were controlled by a densitometer and a pH meter.
2.3 Rice Husk Combustion in a 0.7 MW DCFBTo solve the problems of short combustion retention time and the incomplete combustion of rice husk, a 0.7 MW water-cooled CFB with dual circulating separation system was specially designed. Fig. 2 shows a schematic of the DCFB system. The combustion system consisted of a fluidized bed furnace, an under-bed ignition system, a screw feeder, three cyclone separators, a heat exchanger, and a baghouse. The inner diameter of the fluidized bed is 650 mm, the diameter of the air distributor is 400 mm, the height of the furnace is 12 m, and the height of the static bed is 0.5 m. The excess air coefficient is 1.6-1.8 and the ratio of primary air to secondary air is 6:4. The primary air was fed into the bed from the air chamber through the air distributor, while the secondary air was fed at the height of approximately 1.5 m away from the air distributor. Rice husk was fed into the dense phase zone at a height of 1 m from the air distributor by a speed-adjustable screw feeder, and the rated feeding rate was 200 kg/h. The resistance of the air distributor was 1.5 kPa, and the pressure of the air chamber reached about 6 kPa. The first (1st) and the second (2nd) cyclone separators were used to capture char, ash, and fine bed material that escape with the flue gas, which were then fed into the furnace through the return device for efficient circulation combustion. The third (3rd) cyclone separator was employed as a collection point for the residual RHA in the flue gas. The membrane water walls surrounding the furnace and the heat exchanger in the tail flue were used to absorb heat in the furnace and from the flue gas, and the inlet and outlet water temperatures were 20 ℃ and 80 ℃. The system consisted of four points at the bottoms of the 1st, 2nd, 3rd cyclones and the baghouse for collecting ash during the combustion experiments. The operating temperatures were recorded in real-time by thermocouples (type K) at 0.8, 3, 6, 9, and 12 m (exit) along the height of the furnace and at the outlets of the 1st and 2nd cyclones.

|
1—igniter; 2—primary air; 3—secondary air; 4—screw feeder; 5—fluidized bed; 6—1st cyclone separator; 7—2nd cyclone separator; 8—3rd cyclone separator; 9—heat exchanger; 10—baghouse Fig.2 Schematic of 1 MW DCFB system used for RHA production |
2.4 Analysis
According to the procedure described in our previous study[20], acid digestion was performed on the acid-pretreated and untreated rice husks. After digestion, the metal impurities in digestion solutions were measured using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Optima 5300DV, USA) to quantify the content of the residual metal impurities in the rice husk. During the pretreatment process, the concentration of the sulfuric acid solution was determined using an industrial acid-base densitometer (CLG-528BH, China), whereas the concentration of the citric acid was controlled using a pH meter (1.7-1.8). The ultimate analysis of the rice husk was obtained using an elemental analyzer (Vario EL cube, Germany). Micromorphology, chemical composition, and crystallinity of the obtained RHA were determined using a field-emission scanning electron microscope (Quanta 200FEG, USA), a transmission electron microscope (Tecnai G2 F30, USA), an X-ray fluorescence spectrometer (AXIOS-PW4400, Netherlands), and an X-ray diffractometer (D/max-rB, Japan), respectively. Pore characteristics were performed on a Micromeritics automatic physicochemical adsorption instrument (ASAP2020M, USA) using the Barrett-Joyner-Halenda (BJH) model.
3 Results and Discussion 3.1 Acid Pretreatment of Rice HuskIn order to confirm the acid pretreatment effect on metal impurities (particularly K) under industrial operating conditions, the contents of metal impurities in the untreated and acid-pretreated rice husks were studied (Table 2). Table 2 also presents the content of Si in the rice husk. After acid pretreatment, K in rice husks decreased sharply from 3.805 mg/g to less than 0.2 mg/g, and the content of other metal impurities also decreased to different extents. The removal rates of K, Mg, and Mn exceeded 90 wt.%, whereas those of Na, Ca, Al, and Fe were only 20-40 wt.%. As for lab results, sulfuric acid exerted a better leaching effect than citric acid on the metal impurities[20]. In addition, the content of Si in rice husk changed a little after acid pretreatment, which is consistent with the result that a small amount of Si was detected in the leachates. Table 3 shows the proximate and ultimate analyses results of the pretreated and untreated rice husks. Acid pretreatment process degraded a small amount of organic matrix in rice husk to a certain extent, resulting in a slight decrease in the carbon content[21]. The S content was also reduced. The leaching of those impurities led to a slight decrease in the ash content and the heat value[22-23], but had little effect on the fuel characteristics of the rice husk.
| Table 2 Metal component in the pretreated and untreated rice husks |
| Table 3 Proximate and ultimate analyses of pretreated and untreated rice husks |
3.2 Combustion of Rice Husk in a DCFB
Table 4 shows the operating parameters in the fluidized bed combustion experiment of rice husk. The mean particle diameter of the quartz sand is 580 μ m, which is suitable for fluidized bed combustion of rice husk[24]. The minimum fluidizing velocity (Umf) is a key parameter to ensure the normal fluidization of the fluidized bed boiler. In this experiment, the minimum fluidizing velocity Umf of quartz sand in a cold state was calculated as 0.553 m/s. The actual fluidizing velocity in dense phase zone of the DCFB reached above 5 m/s, which is larger than previously reported results in bubbling fluidized bed[25-27].
| Table 4 Operating parameters of rice husk combustion experiments in a DCFB |
After the acid pretreatment, the restriction of combustion temperature on the performance of RHA was weakened and no conversion of amorphous silica to crystalline form occurred at temperatures greater than 800 ℃[28]. However, the combustion temperature remained an important indicator for evaluating the combustion status of CFB. Fig. 3 shows the axial temperature curve along the height of the furnace. The temperature distribution along the height direction of the furnace was basically identical under the two combustion experiments. To be specific, the primary air, rice husk, and recycling materials were fed into the dense phase zone of the bed, and then the preliminary combustion of rice husk and the burnout of residual char in the recycling materials resulted in a temperature of approximately 770 ℃ in the dense phase zone. The supplement of the upper secondary air promoted the further combustion of rice husk char, which led to the temperature curve peaked at 3 m (about 830 ℃). Meanwhile, the flue gas velocity was close to 4 m/s, and then the temperature gradually decreased along the flue gas direction and finally reached 610-625 ℃ at the furnace outlet.
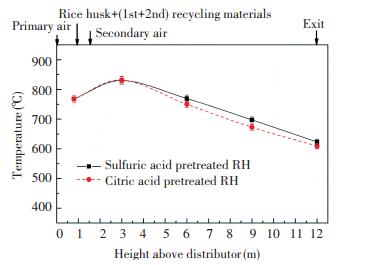
|
Fig.3 Axial temperature distribution along the height of the furnace under different pretreatment conditions |
Fig. 4 shows the temperature curves with the operation time in the DCFB system under stable operating conditions. The temperatures were recorded when the combustion operation was stable for 2 h after the ignition. T1, T2, T3, T4, and T5 are the temperatures in the dense-phase zone, the dilute-phase zone, at the heights of 6 m and 9 m, and at the furnace outlet, respectively, whereas T6, T7, and T8 are the temperatures at the outlets of the 1st, 2nd cyclones and at the baghouse inlet, respectively.
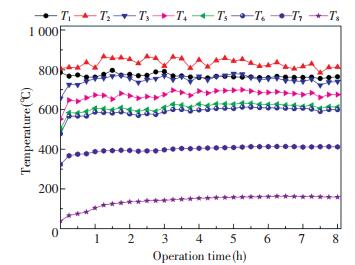
|
Fig.4 Temperature curves with operation time under stable operation condition |
It can be seen from Fig. 4 that all the temperatures of the combustion system remained stable throughout the operation time, indicating that the DCFB system operated well. As a result of the uniform heat transfer of the bed, temperature T1 in the dense-phase zone was always stable at approximately 770 ℃. However, temperature T2 in the dilute-phase zone fluctuated greatly due to the combustion pulsation. Temperatures T3, T4, and T5 of the freeboard zone remained stable within the range as shown in Fig. 3.
In the DCFB combustion process of rice husk, SO2 and NOx emissions in the flue gas were always lower than 200 mg/m3, which are in line with the latest emission standard of pollutants for boiler, the GB 13271-2014. In this experiment, the combustion efficiency and the heating rate intensity of the pretreated rice husk burned in DCFB were investigated, as shown in Table 5. The combustion efficiency ηc is expressed as[29]
| Table 5 Combustion efficiency for burning various rice husks |
| $ {\eta _c} = \left[ {\left( {{E_{\rm{f}}} - {E_{{\rm{ash}}}} - {E_{{\rm{fg}}}}} \right)/{E_{\rm{f}}}} \right] \times 100 $ | (1) |
where Ef is the higher heating value of rice husk (kJ/kg), Eash is the energy loss as unburned carbon in the ash (kJ/kg), and Efg is the energy loss as carbon monoxide in the flue gas (kJ/kg).
qc is the heat capacity per unit cross-sectional area of the boiler and is expressed as
| $ {q_{\rm{c}}} = \left[ {B \times {E_{\rm{f}}} \times \left( {{\eta _c} \times 100} \right)} \right]/A $ | (2) |
where B is the feeding rate of rice husk (kg/h), and A is the cross-sectional area of the bed (m2).
The unburned carbon content in Table 5 was the weighted average of the unburned carbon in the 3rd cyclone and the baghouse. CO emissions were measured online at the chimney inlet using a Testo-340 (Testo, Germany) flue gas analyzer and were converted into the value under standard oxygen (9%) according to the national standard GB 13271-2014. From Table 5, the combustion efficiency of rice husk reached 99% under both conditions, and the heating rate intensity was 2.1-2.3 MWth/m2, which is higher than those of other fluidized beds[13, 30]. Therefore, the pretreatment process eliminated the obstacle which may cause the incomplete combustion of rice husk at higher temperatures (>800 ℃), and the high circulation ratio of the DCFB further promoted combustion.
3.3 Characterization of Physicochemical Properties of RHAAfter the combustion experiments were completed, RHAs were collected from the bottoms of the 1st, 2nd, and 3rd cyclones and the bottom of the baghouse in the DCFB. The total yield of RHA (RHA/feed-RH mass ratio) could reach approximately 14.3-14.7 wt.%. The RHAs collected from the 1st cyclone and the 2nd cyclone accounted for about ~40 wt.% and ~38 wt.%, while those from the 3rd cyclone and the baghouse were ~13 wt.% and < 5 wt.%. Fig. 5 shows the appearance of the RHAs collected from the cyclones in the DCFB, where the pretreatment conditions were: a) untreated, b) sulfuric acid pretreatment, and c) citric acid pretreatment, with untreated RHA as a control. As shown in Fig. 5, the RHA presented a gray-black color after untreated rice husk was burned in the DCFB. Conversely, RHA under pretreatment condition appeared primarily white only with a small amount of gray particles.
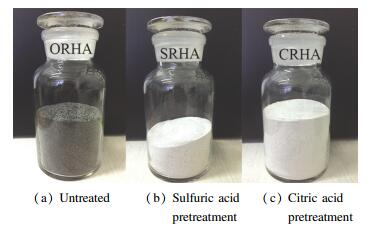
|
Fig.5 Appearance of RHA collected from the DCFB after combustion of various pretreated rice husks |
3.3.1 Influence of collecting points
The location of collection points in the DCFB has certain influences on the performance of RHA, among which the particle size is the first factor. The particle size distribution of RHA from different collection points was determined with sieves as shown in Table 6. The particle size of all RHAs was almost less than 300 μm without the original particle shape of the rice husk. As the collection point was moved toward the flue gas, the mean particle size of RHA gradually decreased especially after the 2nd cyclone. It is attributed to the strong attrition of quartz sand and the high circulation factor of materials which broke RHA into smaller fragments.
| Table 6 Fineness of RHA from different positions (sulfuric acid condition) |
Micrographs can directly reflect the micro-morphology of RHA and judge whether the RHA is in an amorphous or a crystalline state. Fig. 6 shows the SEM and TEM images of the RHA collected from the 1st and 3rd cyclones in the DCFB (sulfuric acid conditions). It can be seen from SEM images that the two RHAs that presented the porous layered or network structure were rich in about micron-sized macropores. However, the shape and the particle size of the RHA collected from the two cyclones were various (100 μ m SEM). The first RHA presented about 100 μ m fragments which retained the original morphology, while the RHA from the 3rd cyclone was mainly smaller-sized irregular shape, which is consistent with the variation of the particle size of the RHA as shown in Table 6. From TEM images, the porous structure of the RHA was composed of spherical silica particles, which had good dispersibility and no obvious agglomeration. However, the nanoparticle size in RHA from the 1st cyclone (25-40 nm) was slightly larger than that from the 3rd cyclone (20-30 nm). The nanoparticles also contained a large number of uniform nano-scale pores according to HRTEM, which indicates that the collection point has a certain influence on the micromorphology and pore structure of RHA. The pore structure of RHA obtained from the 3rd cyclone is more abundant, which results in a higher specific surface area.
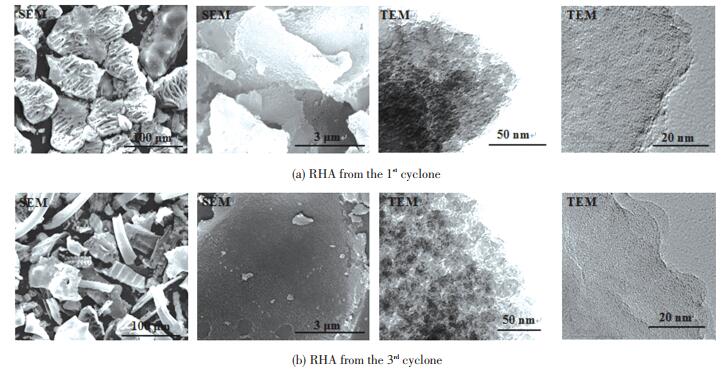
|
Fig.6 Micrographs of RHA from the 1st and 3rd cyclones in the DCFB (sulfuric acid condition) |
Information on the pore structure of amorphous RHA was obtained by measuring the N2 adsorption-desorption isotherm and is shown in Fig. 7 and Table 7. The adsorption-desorption isotherms of RHAs from different collection points in Fig. 7(a) exhibited the consistent characteristics of type Ⅳ adsorption isotherm. The adsorption-desorption isotherm displayed an inflection point at the relative pressure P/P0≈0.05 and then a hysteresis loop appeared at P/P0>0.4, indicating that capillary condensation occurred in the adsorption process[31]. This adsorption behavior suggests that RHA exhibits the characteristics of a mesoporous material with narrow-slit pores[32]. The volume adsorbed from RHA collected from different points increased according to the sequence of 3rd cyclone > 1st cyclone > 2nd cyclone. Fig. 7(b) shows the BJH pore size distribution curves with an intense peak at 2-10 nm for all RHAs, confirming that the mesoporous structure was dominant in RHA. Comparing the RHA from three collection points under sulfuric acid condition, the peak position slightly shifted to the right with the cyclone order, and the RHA separated from the 3rd cyclone contained more 10-100 nm mesopores, which is consistent with the rule of pore size in Table 7.
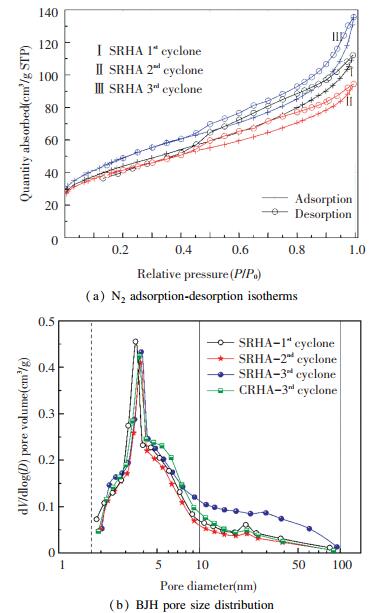
|
Fig.7 N2 adsorption-desorption isotherms and corresponding BJH pore size distribution of RHA obtained from DCFB system |
| Table 7 Surface area and pore characteristics of RHA (nano-silica) prepared under different conditions |
The order of the specific surface area and the pore volume of RHA from different collection points was 3rd cyclone > 1st cyclone > 2nd cyclone, and the largest values reached 178 m2/g and 0.188 cm3/g (at the 3rd cyclone). The RHA particles separated from the 3rd cyclone are smaller and easier to be fully burned in the furnace, resulting in a richer pore structure.
Fig. 8 shows the SiO2 purity and the residual carbon content of RHA collected from four collection points. The RHA obtained from all points had a very low overall carbon content (< 1 wt.%, except baghouse), but the residual carbon content increased as the collection point moved towards the flow of the flue gas. It suggests that the burnout effect of rice husk in the DCFB after acid pretreatment was good, corresponding to the high combustion efficiency in Table 5. The RHA from the 2nd cyclone had the highest SiO2 purity, i.e., 98.68 wt.% (SRHA) and 98.20 wt.% (CRHA), which are much higher than that obtained from the rice husk combustion in other fluidized beds[33-34]. Compared with the first three collection points, the SiO2 purity of RHA from the baghouse was as low as 95 wt.% and the impurity content increased substantially.
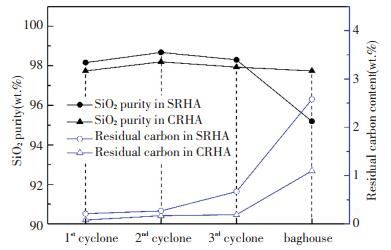
|
Fig.8 Residual carbon and SiO2 content of RHA collected from different points in the DCFB |
Taken together, in the DCFB combustion experiment, the location of the collection point has different effects on the physical and chemical properties of RHA. While for baghouse, the fineness, microstructure, and pore characteristics of RHA were gradually promoted as the collection point moved towards the flow direction of flue gas and reached the best at the 3rd cyclone. However, the purity of RHA reached the highest at the 2nd cyclone, and the residual carbon content in RHA increased with the flow direction of the flue gas.
3.3.2 Influence of acid typeAfter acid pretreatment, the properties of RHA obtained from the rice husk pilot-scale combustion test in the DCFB all improved greatly. It is due to the fact that the combination of acid pretreatment and CFB combustion increased the inner molecular pore gap of rice husk and substantially enhanced the pore structure of RHA, which resulted in a highly porous white RHA (silica). Comparing the two acid pretreatment effects, the overall performance of RHA under sulfuric acid condition was higher than that under citric acid condition, such as pore characteristics (Fig. 7(b) and Table 7) and SiO2 purity (Fig. 8). However, the residual carbon content was reversed, which was related to the rice husk combustion operation state in the DCFB.
3.3.3 Influence of bed materialsDerived from the micro-morphological characterization, the RHA obtained from the combustion of acid-pretreated rice husk in the DCFB was amorphous and no crystalline silica was evident. However, a crystalline phase might present in the RHA because of the elutriation of quartz sand and the high-rate material circulation in the fluidized bed. The composition and the structure of RHA collected from different cyclones were analyzed by FTIR and XRD as shown in Fig. 9 and Fig. 10.
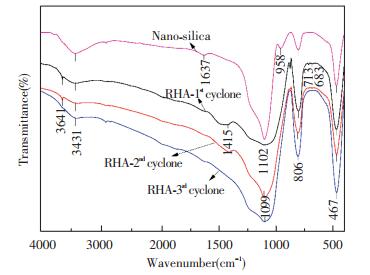
|
Fig.9 FTIR spectrum of RHA from different cyclones in the DCFB (under sulfuric acid pretreatment condition) |
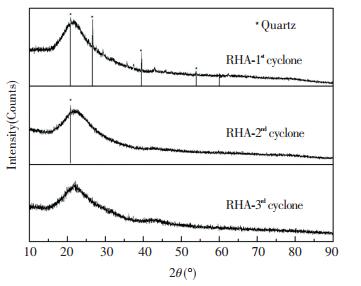
|
Fig.10 XRD patterns of RHA from different cyclones in the DCFB (under sulfuric acid pretreatment condition) |
The FTIR spectra of all RHAs were highly consistent with the typical infrared spectrum of nano-silica, and the bands of O-Si-O stretching (at 1099-1022 cm-1, 806 cm-1) and bending vibrations (at 467 cm-1). It clearly indicates that the composition of all RHAs is mainly silica. The bands at 3431 cm-1 and 1637 cm-1 corresponded to the O-H stretching and bending vibrations of adsorbed and bound water in the spectrum of nano-silica. However, the absorption broad band at 3431 cm-1 was significantly weakened and the band at 1637 cm-1 even disappeared in the RHAs. The peak changes are related to the disappearance of the adsorbed and the bound water in silica and meanwhile Si-OH condensed into a Si-O-Si bond. Weak peaks were found at 3641 and 1415 cm-1, which indicates that traces of organic carbon remained in the RHAs after combustion. The result corresponds well with the residual carbon content in RHA as reported in Fig. 8. It is noteworthy that weak peaks appeared in the infrared spectrum of the RHA collected from the 1st cyclone at 683-713 cm-1, which should exist in the infrared spectrum of the quartz sand. It means that trace amounts of quartz sand from the bed material may be mixed in this RHA.
For the clarification of quartz phase, the XRD results (Fig. 10) were examined. A broad peak at 2θ=22° occurred in all RHAs which were collected at the three cyclones, corresponding to the amorphous silica[35]. Acid pretreatment removed most of K in rice husk before combustion, thus leaving no carbon peaks in the XRD pattern of RHAs [36]. In particular, low-intensity crystalline diffraction peaks were observed at 2θ=20.86°, 26.64°, 39.46°, and 53.98° in the XRD pattern of RHA from the 1st cyclone, all of which corresponded to low-temperature quartz phase. Almost no quartz crystal diffraction peak appeared in the XRD pattern of RHA from the 2nd and 3rd cyclones. The result verified the results from the FTIR spectrum, and the good separation effect between RHA and bed material was attributed to the high-speed and multi-stage material circulation in the DCFB.
3.4 Comparison of RHA Prepared under Different ConditionsThe physicochemical performance of the RHA prepared in the DCFB and the RHA prepared under other conditions were systematically compared as shown in Table 8. Sample RHA0 was obtained from the untreated rice husk combustion at 800 ℃ in a lab electrical heating muffle furnace, RHA1 was obtained by burning the acid-pretreated rice husk at 800 ℃ in the same muffle furnace [21], RHA2 was obtained by the acid-pretreated rice husk combustion at 800 ℃ in a bench-scale fluidized bed [37], and RHA3 was obtained from the 1st cyclone in the DCFB in this industrial-scale combustion experiment.
| Table 8 Comparison of RHAs prepared under different conditions |
Under the same combustion conditions in a muffle furnace, the quality of RHA1 under acid pretreatment condition was substantially improved: the SiO2 content and the specific surface area increased rapidly, while the residual carbon content decreased. This is because acid pretreatment removed a large amount of metal impurities in rice husk, which reduced the inhibitory effect of combustion temperature on the performance of RHA and remarkably improved the quality of RHA1. However, the inhibition of combustion temperature was not completely eliminated as reported in our previous study [21]. Under the same pretreatment condition, RHA2 from the bench-scale fluidized bed showed a further improvement in physicochemical properties compared with RHA1 from the muffle furnace: the specific surface area and the SiO2 content further increased, whereas the content of metal impurities and the residual carbon decreased. The main reason for the performance improvement of RHA2 was that the fluidized bed combustion process further improved the combustion characteristics of rice husk on the basis of acid pretreatment. The performance of RHA3 from this combustion test of acid-pretreated rice husk in the DCFB was also excellent but slightly inferior to RHA2.
It has been verified that high-quality RHA (nano-silica) can be obtained from the acid-pretreated rice husk combustion in an industrial-scale CFB. First, acid pretreatment removes the major metal impurities in the rice husk and inhibits the dependence of RHA performance on the combustion temperature. Then, the fluidization and abrasion of bed material during fluidized bed combustion break rice husk into small fragments to promote the oxidation of carbon inside the husks, which eventually increases the carbon conversion rate of rice husk and reduces the residual carbon content of RHA. In addition, the short combustion retention time and the multi-stage high-rate material circulation retain the original amorphous structure of RHA, whose process is also a multi-value utilization process for rice husk resources. First, the problems of the defluidization of bed and the corrosion of heating surface during rice husk combustion in the fluidized bed are solved. Second, since the combustion of rice husk is a highly efficient energy conversion process, the heat energy can be used for heating or power generation. Finally, the production of amorphous RHA (nano-silica) is also an industrial process with high added value. In conclusion, the combining method of acid pretreatment and fluidized bed combustion for rice husk is proved to be a highly viable process for producing nano-silica.
4 ConclusionsIn this paper, a combined method of acid pretreatment and circulating fluidized bed combustion of rice husk is used to prepare nano-silica particles on a large scale for the first time. The combustion process of rice husk and the physicochemical properties of the obtained RHA were studied in detail. Results showed that the acid pretreatment process removed a large amount of metal impurities in rice husk, particularly K (removal rate ≥ 90 wt.%); meanwhile, the process had a very weak leaching effect on Si and did not destroy the fuel properties of rice husk. The combustion processes of two kinds of rice husks in the DCFB were very stable and the combustion efficiency reached 99.5%. The obtained RHA from the collection points (except the baghouse) were all white particles with a SiO2 purity > 97.7 wt.% and an unburned carbon content < 0.6 wt.%. Due to the different frequencies of material circulation, the location of collection points had different effects on the physicochemical properties of RHA. RHA collected from the 3rd cyclone had the richest microstructure and the highest specific surface area and pore volume, whereas that from the 2nd cyclone had the highest SiO2 purity. The high-speed and multi-stage material circulation process in the DCFB prevented bed material from mixing into RHA. Finally, the RHA prepared in this work and the RHA prepared under other conditions were compared. The results showed that CFB had obvious advantages in the combustion of rice husk for amorphous nano-silica production. The use of acid pretreatment process further improved the combustion performance of rice husk and the quality of RHA. The circulating fluidized bed combustion of rice husk based on acid pretreatment can prepare nano-silica while providing heat for industrial processes, which is a highly energy-rich process.
| [1] |
Sore S O, Messan A, Prud'homme E, et al. Synthesis and characterization of geopolymer binders based on local materials from Burkina Faso-Metakaolin and rice husk ash. Construction and Building Materials, 2016, 124: 301-311. DOI:10.1016/j.conbuildmat.2016.07.102 (  0) 0) |
| [2] |
Pode R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renewable and Sustainable Energy Reviews, 2016, 53: 1468-1485. DOI:10.1016/j.rser.2015.09.051 (  0) 0) |
| [3] |
Sensale G R D, Viacava I R. A study on blended Portland cements containing residual rice husk ash and limestone filler. Construction and Building Materials, 2018, 166: 873-888. DOI:10.1016/j.conbuildmat.2018.01.113 (  0) 0) |
| [4] |
Pongdong W, Kummerlöwe C, Vennemann N, et al. A comparative study of rice husk ash and siliceous earth as reinforcing fillers in epoxidized natural rubber composites. Polymer Composites, 2016, 39(2): 414-426. DOI:10.1002/pc.23951 (  0) 0) |
| [5] |
Liou T H, Wu S J. Kinetics study and characteristics of silica nanoparticles produced from biomass-based material. Industrial & Engineering Chemistry Research, 2010, 49(18): 8379-8387. DOI:10.1021/ie100050t (  0) 0) |
| [6] |
Gu S, Zhou J, Yu C, et al. A novel two-staged thermal synthesis method of generating nanosilica from rice husk via pre-pyrolysis combined with calcination. Industrial Crops and Products, 2015, 65: 1-6. DOI:10.1016/j.indcrop.2014.11.045 (  0) 0) |
| [7] |
Rozainee M, Ngo S P, Salema A A, et al. Fluidized bed combustion of rice husk to produce amorphous siliceous ash. Energy for Sustainable Development, 2008, 12(1): 33-42. DOI:10.1016/s0973-0826(08)60417-2 (  0) 0) |
| [8] |
Soltani N, Bahrami A, Pech-Canul M I, et al. Review on the physicochemical treatments of rice husk for production of advanced materials. Chemical Engineering Journal, 2015, 264: 899-935. DOI:10.1016/j.cej.2014.11.056 (  0) 0) |
| [9] |
Sun S, Zhao Y, Su F, et al. Gasification of rice husk in a cyclone gasifier. Korean Journal of Chemical Engineering, 2009, 26(2): 528-533. DOI:10.1007/s11814-009-0090-1 (  0) 0) |
| [10] |
Khan A A, Jong W D, Jansens P J, et al. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Processing Technology, 2009, 90(1): 21-50. DOI:10.1016/j.fuproc.2008.07.012 (  0) 0) |
| [11] |
Cai W, Liu R, He Y, et al. Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized bed reactor. Fuel Processing Technology, 2018, 171: 308-317. DOI:10.1016/j.fuproc.2017.12.001 (  0) 0) |
| [12] |
Fang M, Yang L, Chen G, et al. Experimental study on rice husk combustion in a circulating fluidized bed. Fuel Processing Technology, 2004, 85(11): 1273-1282. DOI:10.1016/j.fuproc.2003.08.002 (  0) 0) |
| [13] |
Madhiyanon T, Sathitruangsak P, Soponronnarit S. Combustion characteristics of rice-husk in a short-combustion-chamber fluidized-bed combustor (SFBC). Applied Thermal Engineering, 2010, 30(4): 347-353. DOI:10.1016/j.applthermaleng.2009.09.014 (  0) 0) |
| [14] |
Yoon S J, Son Y I, Kim Y K, et al. Gasification and power generation characteristics of rice husk and rice husk pellet using a downdraft fixed-bed gasifier. Renewable Energy, 2012, 42: 163-167. DOI:10.1016/j.renene.2011.08.028 (  0) 0) |
| [15] |
Huang S, Jing S, Wang J, et al. Silica white obtained from rice husk in a fluidized bed. Powder Technology, 2001, 117(3): 232-238. DOI:10.1016/S0032-5910(00)00372-7 (  0) 0) |
| [16] |
Singh R I, Mohapatra S K, Gangacharyulu D. Study of agglomeration in 3.5 MW AFBC using rice husk particles. International Journal of Renewable Energy Technology, 2011, 2(2): 120-142. DOI:10.1504/IJRET.2011.039289 (  0) 0) |
| [17] |
Madhiyanon T, Sathitruangsak P, Soponronnarit S. Co-combustion of rice husk with coal in a cyclonic fluidized-bed combustor (ψ -FBC). Fuel, 2009, 88(1): 132-138. DOI:10.1016/j.fuel.2008.08.008 (  0) 0) |
| [18] |
Ang T N, Ngoh G C, Chua A S M. Comparative study of various pretreatment reagents on rice husk and structural changes assessment of the optimized pretreated rice husk. Bioresource Technology, 2013, 135: 116-119. DOI:10.1016/j.biortech.2012.09.045 (  0) 0) |
| [19] |
Werther J, Saenger M, Hartge E U, et al. Combustion of agricultural residues. Progress in Energy and Combustion Science, 2000, 26(1): 1-27. DOI:10.1016/S0360-1285(99)00005-2 (  0) 0) |
| [20] |
Chen P, Bie H, Bie R. Leaching characteristics and kinetics of the metal impurities present in rice husk during pretreatment for the production of nanosilica particles. Korean Journal of Chemical Engineering, 2018, 35(9): 1911-1918. DOI:10.1007/s11814-018-0103-z (  0) 0) |
| [21] |
Chen P, Gu W, Fang W, et al. Removal of metal impurities in rice husk and characterization of rice husk ash under simplified acid pretreatment process. Environmental Progress & Sustainable Energy, 2017, 36(3): 830-837. DOI:10.1002/ep.12513 (  0) 0) |
| [22] |
Carmona V B, Oliveira R M, Silva W T L, et al. Nanosilica from rice husk: Extraction and characterization. Industrial Crops and Products, 2013, 43: 291-296. DOI:10.1016/j.indcrop.2012.06.050 (  0) 0) |
| [23] |
Deng L, Zhang T, Che D. Effect of water washing on fuel properties, pyrolysis and combustion characteristics, and ash fusibility of biomass. Fuel Processing Technology, 2013, 106: 712-720. DOI:10.1016/j.fuproc.2012.10.006 (  0) 0) |
| [24] |
Bhattacharya S C, Shah N, Alikhani Z. Some aspects of fluidized bed combustion of paddy husk. Applied Energy, 1984, 16(4): 307-316. DOI:10.1016/0306-2619(84)90005-9 (  0) 0) |
| [25] |
Chen G, Du G, Ma W, et al. Production of amorphous rice husk ash in a 500 kW fluidized bed combustor. Fuel, 2015, 144: 214-221. DOI:10.1016/j.fuel.2014.12.012 (  0) 0) |
| [26] |
Rao K V N S, Reddy G V. Comparison of the combustion characteristics of rice husk, sawdust, and groundnut shells in a bubbling fluidized bed. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2011, 33(23): 2181-2193. DOI:10.1080/15567030903515112 (  0) 0) |
| [27] |
Rozainee M, Ngo S P, Salema A A, et al. Effect of fluidising velocity on the combustion of rice husk in a bench-scale fluidised bed combustor for the production of amorphous rice husk ash. Bioresource Technology, 2008, 99(4): 703-713. DOI:10.1016/j.biortech.2007.01.049 (  0) 0) |
| [28] |
Sarangi M, Bhattacharyya S, Behera R C. Effect of temperature on morphology and phase transformation of nano-crystalline silica obtained from rice husk. Phase Transitions, 2009, 82(5): 377-386. DOI:10.1080/01411590902978502 (  0) 0) |
| [29] |
Gomes G M F, Philipssen C, Bard E K, et al. Rice husk bubbling fluidized bed combustion for amorphous silica synthesis. Journal of Environmental Chemical Engineering, 2016, 4(2): 2278-2290. DOI:10.1016/j.jece.2016.03.049 (  0) 0) |
| [30] |
Sathitruangsak P, Madhiyanon T. Effect of operating conditions on the combustion characteristics of coal, rice husk, and co-firing of coal and rice husk in a circulating fluidized bed combustor. Energy Fuel, 2017, 31(11): 12741-12755. DOI:10.1021/acs.energyfuels.7b01513 (  0) 0) |
| [31] |
Rehman M S U, Umer M A, Rashid N, et al. Sono-assisted sulfuric acid process for economical recovery of fermentable sugars and mesoporous pure silica from rice straw. Industrial Crops and Products, 2013, 49: 705-711. DOI:10.1016/j.indcrop.2013.06.034 (  0) 0) |
| [32] |
Liou T H, Yang C C. Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Materials Science and Engineering: B, 2011, 176(7): 521-529. DOI:10.1016/j.mseb.2011.01.007 (  0) 0) |
| [33] |
Singh R I, Mohapatra S K, Gangacharyulu D. Studies in an atmospheric bubbling fluidized-bed combustor of 10 MW power plant based on rice husk. Energy Conversion and Management, 2008, 49(11): 3086-3103. DOI:10.1016/j.enconman.2008.06.011 (  0) 0) |
| [34] |
Martínez J D, Pineda T, López J P, et al. Assessment of the rice husk lean-combustion in a bubbling fluidized bed for the production of amorphous silica-rich ash. Energy, 2011, 36(6): 3846-3854. DOI:10.1016/j.energy.2010.07.031 (  0) 0) |
| [35] |
Sarangi M, Nayak P, Tiwari T N. Effect of temperature on nano-crystalline silica and carbon composites obtained from rice-husk ash. Composites Part B: Engineering, 2011, 42(7): 1994-1998. DOI:10.1016/j.compositesb.2011.05.026 (  0) 0) |
| [36] |
Rafiee E, Shahebrahimi S, Feyzi M, et al. Optimization of synthesis and characterization of nanosilica produced from rice husk (a common waste material). International Nano Letters, 2012, 2: 29. DOI:10.1186/2228-5326-2-29 (  0) 0) |
| [37] |
Ji X, Bie H, Zhang Y, et al. Release of K and Cl and emissions of NOx and SO2 during reed black liquor combustion in fluidized bed. Energy & Fuel, 2017, 31(2): 1631-1637. DOI:10.1021/acs.energyfuels.6b02701 (  0) 0) |
 2020, Vol. 27
2020, Vol. 27


