2. State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China;
3. Department of Chemical and Materials Engineering, I-Lan University, I-Lan 26047, Taiwan, China
Waste activated sludge (WAS) generated increasingly from wastewater treatment plants (WWTPs) has led to plenty of environmental contamination threats and risks to soil, air and underground water environment, with an average annual growth of 13 % from 2007 to 2013 in China[1]. However, WAS can be also regarded as a suitable resource for bioenergy/resource recovery due to the high enrichment of organic matters in WAS biosolids phase. Anaerobic fermentation is a vital option for WAS treatment and reduction with important intermediate products production (such as volatile fatty acids, VFAs). In recent years, researchers have realized that more valuable end-products via VFAs platform can be achieved by the comparison of direct methane generation, such as biopolymers synthesis[2], a kind of external carbon source for enhancing biological nutrients removal (BNR) efficiency[3], medium chain fatty acids formation[4] or electrosynthesis via bioelectrochemical systems[5]. Thus considerable research has been concentrated on prompting VFA production via WAS anaerobic fermentation. Moreover, higher fraction of fermentable organics can result in more value-added products through fermentation[4]. However, the poor biodegradability of WAS due to its complicated composition constrains the efficiency of biological fermentation processes[6]. Based on aforesaid background, the chemical[7], physical[8], and biological[9-10] methods were utilized widely to improve WAS solubilization and hydrolysis. Among them, enzymes catalysis method displays incomparable superiorities: efficient soluble organics dissolve out in a brief reaction time (just in hours)[11], and no risk of secondary environment contaminations[12].
Indeed, the stability of an anaerobic fermentation reactor relies considerably on microbial population structure, which is usually influenced by operational parameters, including temperature, organic loading rate (OLR), pH, and oxidation-reduction potential (ORP) changes[13]. Among these factors, the temperature was thoroughly investigated due to its vital impacts on fermentation performance, which is closely related to fermentation reaction rate, reaction pathway, microorganism community structure, microbial activity, the degree of acidification, and substrate conversion rate[14]. Thus, performance of mesophilic anaerobic fermentation (MAF, 35 ℃) and thermophilic anaerobic fermentation (TAF, 55 ℃) has been compared with focuses on advantages and drawbacks, but no distinct conclusions have been drawn so far. Studies reported that the optimum temperature range is around 35-37 ℃ (MAF) for VFA accumulation with a higher biodiversity than that under 55 ℃ (TAF)[15-17]. Nevertheless, other researchers pointed out that low VFA generation is found in mesophilic anaerobic treatment process (30-40 ℃) and increasing temperature (to thermophilic condition) is a simple and effective approach to improve VFA production[18-19]. In contrast, TAF brings about two main drawbacks: (1) high energy consumption to maintain the thermophilic condition; (2) free ammonia (FA) released at high temperature impairing the anaerobic fermentation performance inevitably[3, 20]. It is highly possible that the difference of pre-treatment or adding promoters could be responsible for the contradictory conclusion above. However, scarce research has been investigated the optimized fermentation temperature related to VFA accumulation from the enzymolysis-pretreated WAS so far. Thus it is of great necessity to optimize the fermentation temperature appropriately to reduce the cost of fermentation process with more VFA production.
In view of the complexity of intricate biochemical processes and interactions by heterogeneous players (microbes), the microbial ecology in anaerobic fermentation process still has not been elucidated sufficiently. A thorough manifestation of microbial ecology is required to better grasp the roles of impact factors (such as temperature) on microbiome evolutionary trajectories and performance stability. Previous studies have verified that temperature changes play an important role in microbial community evolutions within anaerobic fermentation process[21-22], which affects the fermentation performance intimately. Moreover, community evenness is a crucial indicator for describing community distribution traits and closely associated with the microbial ecology functionality. Microbial community distributive equitability (initial distributive evenness) is associated with preserving the functionality within an ecosystem, but with less resistant to environmental stress[23], which can be described by Pareto-Lorenz evenness curve (PL curve) figuratively[24]. Tao et al.[25] indicates that the ANAMMOX start-up process can be shortened by using seeding sludge with high evenness community, which supports the conclusion that initial community evenness plays a critical role on ecosystem function. Balvanera et al.[26] also verified that community evenness associates with the function stability of an ecosystem positively. Thus, it is vital to disclose the microbial consortia evolutions from MAF to TAF fed with pretreated WAS, and reveal the relationship of VFA production with community evenness changes during fermentation process. However, dominant previous studies focused on VFA production from WAS with different pretreatments or adding promoters, like oxidation regents or co-substrates[17, 27], but scarce of them paid enough attention to microbial community evolutional trajectories from MAF to TAF associated VFA accumulation from WAS pretreated by enzymolysis.
Thus main purpose of the present study is to (ⅰ) investigate the effect of fermentative temperature on VFA accumulation and microbiome evolutionary trajectories in WAS fermentation pretreated by enzymolysis; (ⅱ) demonstrate the relationship of microbial population evenness with VFA production in MAF and TAF process; (ⅲ) a conceptual illustration of bio-energy recovery from WAS organics via anaerobic fermentation by enzymes catalysis associated with MFC process is proposed for energy-sufficient in future wastewater treatment.
2 Methods and Materials 2.1 WAS, Hydrolytic Enzymes, and WAS PretreatmentThe WAS was taken from the secondary sedimentation tank of a full-scale municipal wastewater treatment plant in Harbin, China, with the detailed traits listed in Table 1. The hydrolytic enzymes mixture comprised lysozyme, α -amylases, protease, and cellulase, which were extracted and purified from natural sources. The enzymatic activity of each single enzyme in mixture (lysozyme, α -amylases, protease, and cellulase) exceeded 20000 U/mg, 6000 U/mg, 60 U/mg, and 30 U/mg, respectively.
| Table 1 Changes of WAS characteristics pretreated by hydrolytic enzymes catalysis |
The WAS pretreatment was implemented by the catalyzing effect of the above compound enzymes mixture to boost WAS solubilization. The proportion of single enzyme in the mixture was kept at 1:1:1:1 (mass ratio) with the adding dosage of 10% (enzymes weight/TSS, w/w). The mass ratio of 1:1:1:1 and adding ratio of 10% (enzymes /TSS) were selected based on the operational protocols of some previous studies. In a previous study, the mixed ratio of 1:1 in enzymes blend (α -amylase: protease) was used to disintegrate WAS and resulted in a remarkable pre-hydrolysis performance. Meanwhile, the adding dosages of 3%, 6%, 12% and 18% (w/w) were investigated comprehensively, the result of which indicated that the dosage ratio of 6% to 12% was the optimal dosage range[11]. In Yu et al.'s study, the identical enzyme adding ratio (protease solution with identical volume of amylase solution) was used to disintegrate WAS[10]. In addition, the WAS pre-hydrolysis performance resulted from the mass ratio of 1:1:1:1 (α -amylases, protease, lysozyme, and cellulase), 10 % adding dosage (w/w) and 3 h water-bath incubation have been investigated in detail[12]. The selection of these parameters in this study ensured its consistency. The WAS enzymolysis pretreatment was conducted at 37 ± 1 ℃ for 3 h by water-bath and shaken in a shaking bed at a proper speed. The pre-hydrolyzed WAS was collected and used for fermentation experiments.
2.2 Sludge Anaerobic Fermentation Device and Operational ProtocolsFour identical cylindrical anaerobic reactors (made of the polymethyl methacrylate) were used to conduct the sequencing batch WAS anaerobic fermentation assays under various temperatures in this study. Each reactor has the effective volume of 2 L with 20-centimeter height (height to diameter ratio was 2:1). The fermentation mixture was stirred properly (60 r /min) by a blender installed at the top of the fermentation reactors. A water seal unit on the device was used to block the interface tightly to keep the anaerobic state (less than 0.1 mg/L of dissolved oxygen). Meanwhile, N2 was injected into each reactor to expel oxygen. The sampling port on the reactor was used to collect the fermentative WAS samples regularly for further analysis. In the MAF tests, the fermentation temperature was kept at 35 ±1 ℃ by the water circulation around the anaerobic bioreactor. The TAF tests were conducted subsequently according to the identical operations with MAF tests at the temperature of 55 ± 1 ℃. The seeding sludge (about 50 mL) for sludge fermentation tests were obtained from a stable lab-scale anaerobic reactor with feeding domestic wastewater. Detailed operations of MAF and TAF tests are listed in Table 2.
| Table 2 Detailed operations of pretreated-WAS MAF and TAF process |
Fermentation time was set as 10 d in all tests in order to form non-methanogenic fermentation for VFA accumulation. The 10-d fermentation was adopted by referring to some previous publications[19, 28-29]. A batch of reduplicate fermentation tests was conducted subsequently to reduce experimental errors.
2.3 Analytical Methods 2.3.1 General analysisThedetection of soluble chemical oxygen demand (COD), total suspended solids (TSS), and volatile suspended solids (VSS) were implemented according to Standard Methods[30]. Soluble protein was measured by a Modified BCA kit (Sangon, China) with BSA as the standard. The phenol-sulfuric acid method was adopted for soluble carbohydrate detection with glucose as the standard[31]. The concentration and composition of VFA were detected by gas chromatography (GC) by an Agilent 6890 GC with flame ionization detector. The VFA detection started based on a previous literature[32]. The biogas composition was detected by a gas chromatograph (GC) (HJAT, SP2100, China)[33]. The detections above were implemented in triplicate, and their average values were adopted. The pH value was assessed by the pH measuring instrument (Hanna, Italy). The experimental results were reported as the format of average value ± standard deviation.
2.3.2 Bacterial community analysisTerminal restriction fragment length polymorphism (T-RFLP) wasadopted to observe bacterial community dynamics during the WAS AF process. Detailed steps contained: (1) Genomic DNA was extracted from 10 mL sludge sample (mixed with the sludge sample from the corresponding duplicate fermentation test); (2) Polymerase chain reaction (PCR) and products recovery by common primers of former primer 8F (5'-AGAGTTTGATCCTGCCTCA G-3'), labeled by FAM fluorescent sign and reverse primer 1492R (5'-GGTTACCTTGTTACGACTT-3') (Sangon, China), were used to amplify the bacterial 16S rDNA sequence[34]; (3) Restriction endonuclease digestion: FAM-labeled terminal restriction fragments (T-RFs) were generated by Rsa I and Msp I restriction endonuclease (TransGen Biotech, China); (4) Capillary electrophoresis were used to gain the detailed information of T-RFs, phylogenetic assignment was conducted through a default database produced from MiCA (http://mica.ibest.uidaho.edu/).
2.4 Data AnalysisThe SCOD increase was attributed to the VSS reduction in WAS biosolids phase approximately:
| $ {\rm COD_{increase \;insolution}=COD (VSS_{0 }- VSS_{pretreated}) } $ | (1) |
where CODincreaseinsolution is the COD increase by the enzymatic pretreatment. COD (VSS0 -VSSpretreated) stands for COD production caused by VSS reduction. VSS0 is the initial VSS of fresh WAS. The VSSpretreated is the VSS of the pretreated WAS.
The empirical relationship of COD' with VSS (represented by C5H7NO2) can be evaluated by the stoichiometry formulas below[35]:
| $ \begin{array}{c} {\rm C}_{n}{\rm H}_{a}{\rm O}_{b}{\rm N}_{c}+((2n+0.5a-1.5c-b)/2){\rm O}_{2}→\\ n{\rm CO}_{2}+ c{\rm NH}_{3}+ ((a-3c)/2){\rm H}_{2}{\rm O} \end{array} $ | (2) |
| $ {\rm COD}'= \frac{{ (2n+0.5a-15c-b)16}}{{ 12n+a+16b+14c }} $ | (3) |
The COD' represented the oxygen required for full oxidation of the cellular carbon per unit weight of cells.
The potential recoverable electric energy from the WAS fermentation is assessed as follows[36]:
| $ -ΔQ=M·(-ΔU)·A·B $ | (4) |
where -ΔQ is the potential electric energy by methane combustion at constant volume (kW·h), M is the total CH4 generation (m3), -ΔU is the energy of combustion at constant volume for methane, which equals to 40 MJ/m3[37], A is the conversion coefficient of methane chemical energy to electricity through combustion, which equals to 35%[38], B is the conversion coefficient of energy (MJ) to electric energy (kW·h), which equals to 0.28[36]. The conversation relationship of VFA (expressed as COD) with CH4 volume (m3) is 0.35 m3 per kg COD (VFA)[39].
The Pareto-Lorenz curve (PL curve) was adopted in this study to assess the microbial population evenness[40]. The vertical axis within it stands for the cumulative proportion of T-RFs abundance, while the abscissa axis represents the cumulative proportion of T-RFs. The 45° diagonal is the perfect evenness (100% evenness) within a community.
2.5 Statistical AnalysisRedundancy analysis (RDA) used for assessing the correlation between bacterial community composition and single VFA production were established by CANOCO 4.5 software. The Monte Carlo test was implemented with 500 permutations of forward selection in the RDA analysis[41].
3 Results and Discussion 3.1 WAS Pretreatment and Soluble Organic Substance Variations in WAS Anaerobic FermentationThe low fraction of fermentable organic substance was a palpable property of WAS due to its refractory substance and firm microbial cell wall in biosolids phase. Enzymolysis of hydrolytic enzymes (lysozyme, α -amylases, protease, and cellulase blend) prompted WAS solubilization dramatically, which could be seen from the result in Table 1. Soluble COD rose sharply from initial 254 ± 31 mg/L to 8725 ± 452 mg/L just in 3 h with the enzymes dosage of 10 % (enzymes dosage/TSS, w/w). Meanwhile, the soluble protein and carbohydrate increased obviously (from initial 152 ±34 mg/L to 2560 ± 323 mg/L for protein and 86 ± 15 mg/L to 345 ± 54 mg/L for carbohydrate, respectively). It was noticeable that the enzymolysis of compound enzymes improved WAS disintegration degree dramatically, which was attributed to VSS decrease in WAS biosolids phase. The WAS quantity reduced clearly with VSS decrease from 11630 to 5945 mg/L (equal to 5685 mg/L loss) by the enzymolysis. The VSS reduction of 5685 mg/L led to more than 8000 mg/L COD increase approximately according to the stoichiometry formulas of (2) and (3). The attacking effect from hydrolytic enzymes' active site on digesting extracellular polymeric substance (EPS) in biosolids contributed to sludge flocs structure partial collapse[11]. Teo and Wong's work[42] supported this study by using a hydrolytic enzyme blend of proteases, amylases and lipases to digest organic particulates with 22% VSS reduction. Additionally, the detailed cost and performance between enzymatic pretreatment and thermal pre-hydrolysis for sludge solubilization were compared and summarized in Table 3. Results showed that the enzymatic pretreatment for prompting organics bioconversion efficiency exhibited obvious merits: (1) broad enzymes source with slight cost; (2) no extra energy or regents expenditure and environmentally sound; and (3) rapid degradation of enzymes without causing extra environmental burden[29].
| Table 3 Comparison of sludge enzymatic pretreatment with thermal pre-hydrolysis |
VFA generation in WAS anaerobic fermentation was related to bioconversion of soluble organic substances by microorganisms[15]. Soluble organic substances contents (soluble COD, protein, and carbohydrate in Fig. 1 (a)-(c)) decreased gradually in MAF and TAF tests (Fig. 2). Compared with the control tests (fed with fresh WAS), soluble COD in MAF and TAF (fed with pretreated WAS) reduced obviously from 8700 mg/L to 4000 mg/L levels roughly (Fig. 1 (a)). Meanwhile, soluble protein decreased from 1100 mg/L to nearly 600 mg/L (Fig. 1 (b)) and soluble carbohydrate reduced from 300-350 mg/L to 150-200 mg/L levels (Fig. 1(c)). The soluble protein and carbohydrate presented higher levels in MAF compared with those in TAF, which implied that the WAS organics solubilization in MAF got to a higher degree and attributed to the higher key hydrolytic enzymes activities[15].
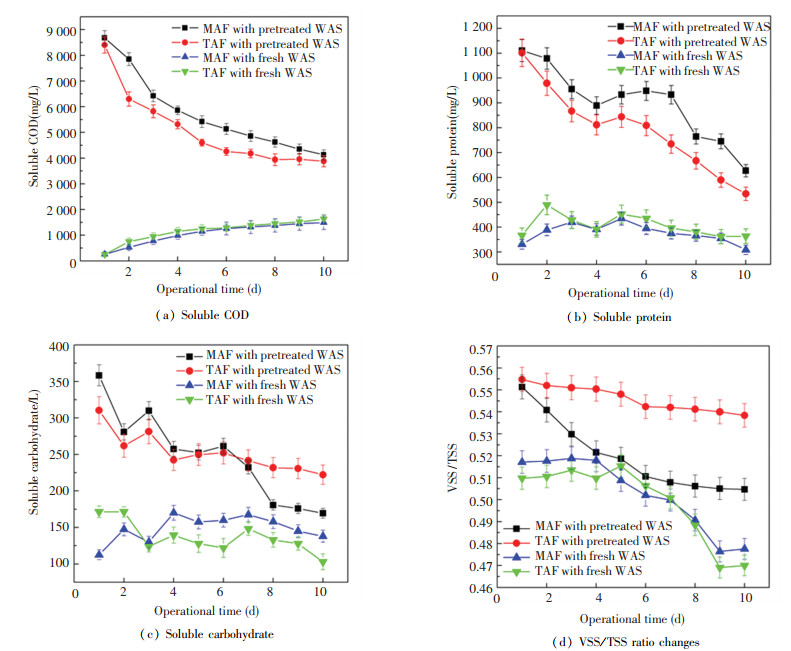
|
Fig.1 Changes of soluble organic substances in pretreated WAS anaerobic fermentation |
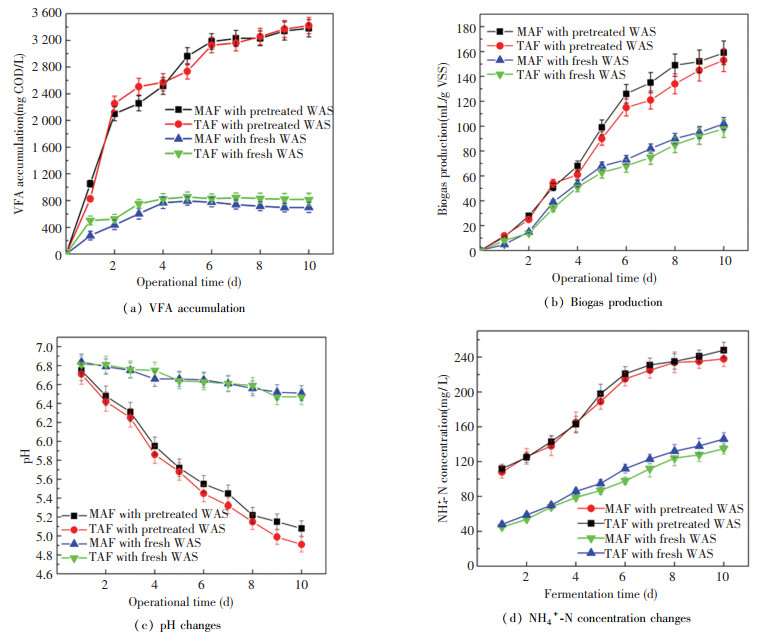
|
Fig.2 Performance of the WAS anaerobic fermentation pretreated by enzymolysis |
Moreover, the fermentative temperature affected these organic substances' distribution in sludge flocs layers obviously. Li et al.[16] reported that soluble protein and carbohydrate contents in slime layer at 35 ℃ was lower than that at 55 ℃, because recalcitrant organic substances could be accumulated at 55 ℃ easily and could not be utilized by bacteria successfully in the 55 ℃ environment. This might be a key reason for the similar VFA accumulation in MAF and TAF in this study. Additionally, the gradual reduction of VSS/TSS (from 0.56 to 0.50 in MAF and from 0.56 to 0.54 in TAF approximately) (Fig. 1(d)) implied that considerable sludge organic matters were solubilized and converted into liquid phase (Fig. 1(d)), which was responsible for VFA production.
3.2 VFA Accumulation and Biogas ProductionCompared with the control test, the total VFA concentration in MAF (fed with pretreated WAS) increased rapidly with more than 3200 mg COD/L from day 6 in the 10-day batch assay (Fig. 2 (a)), while the VFA content reached 3400 mg COD/L on day 8 in TAF. The moderate temperature facilitated VFA effective accumulation, which might be caused by the fact that TAF (55 ℃) might impair some hydrolytic enzymes activities in acid-forming bacteria and constrained further WAS hydrolysis and acidification, because soluble protein solubilization was weaker at 55 ℃ than at 35 ℃[16]. Zhuo et al.[15] proposed that 37 ℃ was the key point with the maximal VFA production after 72 h in ultrasonic-pretreated WAS fermentation. It was speculated that the enzymes catalysis for WAS pre-hydrolysis presented equal VFA accumulation efficiency roughly between MAF and TAF.
The composition of VFA did not vary significantly from the MAF to TAF (data did not show). The acetic acid was the predominant VFA both in MAF and TAF, followed with the propionate. In MAF, the sequence of individual VFA content was acetate > propionate > N-butyrate > iso-valerate> N-valerate > iso-butyrate. It changed as acetate > propionate > N-butyrate > N-valerate > iso-valerate > iso-butyrate in TAF. The single VFA content changes might be caused by the hydrolysis efficiency difference of carbohydrate and protein. Propionic, acetic, iso-butyric, and n-butyric acids could be formed directly from soluble carbohydrate and small molecule-protein fermentation[18]. The VFAs with more molecular weight are largely relevant to the soluble macromolecular-protein bioconversion by single amino acids' reductive deamination or oxidation-reduction between pairs of amino acids via Stickland reaction[19].
The biogas production performance was shown in Fig. 2 (a). The cumulative biogas yield reached over 150 mL/g VSS in the fermentation fed with pretreated WAS, which mainly consisted of CO2 and H2 (over 90%). It indicated that the mathanogenesis was almost inhibited. Total VFA accumulation led to a clear decrease of pH (below 6.0 from day 4) (Fig. 2(c)) and restricted the methanogenesis remarkably. Moreover, the NH4+-N content was accumulated obviously in the liquid phase during the fermentation with over 240 mg/L levels (just 120-140 mg/L in control test, higher NH4+-N levels in TAF than that in MAF, Fig. 2(d)). The ammonia substance could be divided into two forms: unionised ammonia or free ammonia (NH3) and ionised ammonia or ammonium (NH4+) in sludge anaerobic fermentation. The ammonium ion played a negative role on methane production directly with 100% methanogenesis inhibition in TAF by the critical value of 8-13 g NH4+-N/L[50-51].
3.3 Bacterial Community ProfilesDetailed microbiome evolutionary trajectories in MAF and TAF are depicted in Fig. 3. In MAF (Fig. 3(a)), dominant T-RFs contained 88 bp (30 %- 45 % relative abundance), 57 bp (20 %-25 %), 63 bp (10 %-20 %), 84 bp (40 % on day 4), 160 bp (10 %-20 %), 165 bp (30 % on day 8), and 66 bp (10 %-20 %). However, the bacterial community shifted clearly in TAF (Fig. 3(b)) to 75 bp (20 %-40 %), 88 bp (10 %-20 %), 60 bp (10 %-40 %), 148 bp (5 %-10 %), 175 bp (5 %-10 %), and 290 bp (20 %-30 %). The detailed taxonomic composition of T-RFs in bacterial community was depicted in Table 4. The fermentation temperature played a critical role in modulating bacterial community evolutions from seeding sludge to stable fermentation state[52]. It was reported that microbial community structure and dynamics were related to performance stability due to variations of microbial community composition, which were often related to the changes of functional capabilities of those communities[53]. It was speculated that VFA accumulation and proportion were linked with microbiome evolutions caused by fermentation temperature changes (Table 4).
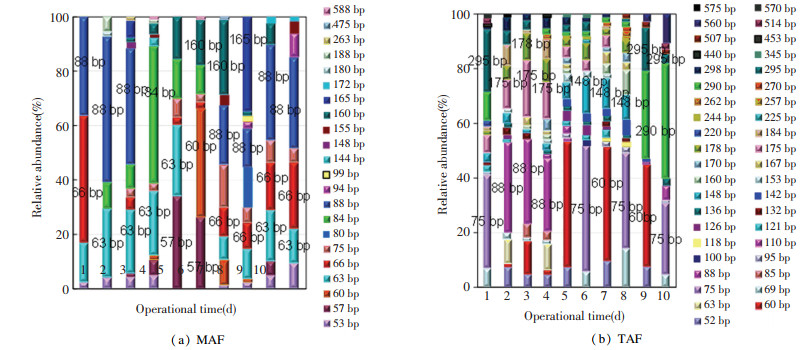
|
Fig.3 T-RFLP depiction of the bacterial community evolutions in pretreated WAS anaerobic fermentation |
| Table 4 Taxonomic compositions of bacterial communities corresponding to T-RFs retrieved from samples |
In MAF, bacterial T-RF of 57bp (Firmicutes) was the major common phylum in anaerobic fermentation systems and responsible for carbohydrates degradation[54]. Meanwhile, T-RFs of 62 bp and 198 bp were affiliated to Anaerotruncus and Synergistaceae, respectively, which might be associated with the bioconversion from soluble carbohydrates and proteins to VFAs[52]. The phylum of Bacteroidetes (related to 160 bp) mainly emerged in MAF, responsible for glucose degradation[55].
In TAF, the 178 bp of Lactobacillus was responsible for the hydrolysis of proteins and lipids[22]. Moreover, T-RFs of 184 bp and 290 bp were related to Clostridium, which was able to degrade proteins to amino acids, and then transferred to fatty acids and NH4+-N[56]. The succession of main actors for organics degradation and bioconversion was from Proteiniborus, Actinobacteria, and Bacteroidetes in MAF to Gallicola, Lactobacillus and Clostridium in TAF. The microbes of Olsenella, Anaerotruncus, Pelotomaculum, and Firmicutes were the common players for organics degradation and VFA production both in MAF and TAF (fed with pretreated WAS).
3.4 Community Evenness Changes and Correlation of Community Composition with Impact FactorsBacterial community evenness changes (represented by PL curves) affected by fermentation temperature are shown in Fig. 4 (a) and (b). Red vectors represented impact factors. Blue arrows stood stand for main T-RFs detected by T-RFLP. Bright colored solid points represented sample communities from various operational days in MAF and TAF, which were marked by "M-" and "T-", respectively. In PL curves, the x-axis stood for the cumulative normalized number of T-RFs, and the y-axis represented the cumulative normalized intensities of T-RFs. According to the Pareto's law, the value of vertical axis was in accordance with 20 % abscissa axis, which was used to explain the meaning of PL curves[40]. The PL curve deviating from 45° diagonal (theoretical perfect evenness line) represented lower community evenness, while the theoretical perfect line hinted the 100% evenness (absolutely even distribution)[24]. It was obvious that the PL curves on day 5 (Fig. 4 (a)) and day 10 (Fig. 4 (b)) in TAF tests tended to be more far off the theoretical perfect evenness line than those in MAF process, which indicated that a greater evenness emerged in communities on day 5 and day 10 in MAF compared with that in TAF. It showed that moderate temperature contributes considerably to construction of intermediate community evenness.

|
Fig.4 Bacterial community evenness changes and correlation of bacterial community composition with impact factors |
In terms of ecology, the rejuvenation of ecosystem function is associated with the restoration of species evenness[57]. The shortage of selective pressure usually resulted in high evenness within a given community, in which a well-defined internal structure was not constructed sufficiently in terms of species dominance since no obvious species displayed at high levels (such as concentration), while a relatively long lag phase was needed to counteract a sudden shock (such as temperature increase)[24]. Correspondingly, the PL curves in TAF represented a small amount of the species dominating within a specialized community, and the remaining species were presented in low numbers, which was fragile to disturbances because longer recovery time was needed[24]. A previous study also corroborated that the community evenness (depicted by PL curves in this study) played a crucial role in retaining the functional stability of an ecosystem with stating that the corresponding functioning was less resistant to environmental stress when communities were extremely uneven (one or a few species are extreme dominance)[23]. Moreover, the intermediate evenness favored a robust function of microbial community which ensured an adequate distribution of dominant microbes and resilient ones and improved the versatility of their metabolic pathways[58]. The intermediate bacterial community evenness might contribute to nearly equal VFA accumulation between MAF and TAF in this study.
Microbial community structure played important roles in VFA production in the fermentation process. It was necessary to reveal the correlation between microbial community composition with impact factors (such as individual VFA) during the sludge fermentation.Detrended correspondence analysis (DCA) via Canoco 4.5 software showed that the correlation of bacterial community composition with impact factors could be properly revealed by redundancy analysis (RDA) (Fig. 4(c)). Monte Carlo permutation tests confirmed the distributions were nonrandom (P < 0.05). The species-environment correlations on axis 1 (horizontal axis) and axis 2 (vertical axis) were 0.827 and 0.797. The communities in MAF (grouped together from M-Day 1 to M-Day 10) and TAF (from T-Day 11 to T-Day 20) were distinctly separated by the horizontal axis, which indicated that temperature had a vital influence on bacterial community evolutions. Moreover, an acute angle emerges between the red vectors of acetate and propionate, which implied that both of two factors exerted a synergetic impact in affecting communities of T-Day 2 to T-Day 9. Besides, the vector of N-butyrate had a negative effect on communities of T-Day 6, T-Day 7, and T-Day 10 with containing main T-RFs of 514 bp, 75 bp, and 560 bp. However, temperature vector (located in the first quadrant) played a positive role on MAF communities (M-Day 2 to M-Day 8) with containing main T-RFs of 57 bp, 63 bp, 475 bp, 160 bp, 80 bp, 289 bp, and 588 bp. Results above implied that fermentation temperature had a closer correlation with bacterial communities in MAF process.
3.5 Electric Energy Harvest and ApplicationEnzymolysis pretreatment prompted potential electricity conversion efficiency of WAS fermentation products by prompting VFA production. The evaluated potential electricity conversion efficiency of the accumulated VFAs from pretreated WAS via MAF and TAF reached 0.75 and 0.82 kW·h/kg VSS (3.9-times higher than that in control test approximately) respectively, which accounted for nearly 60% of theoretical electric energy conversion efficiency through CH4 combustion (Fig. 5, 1 kg COD is assumed to generate 350 L methane by anaerobic digestion).
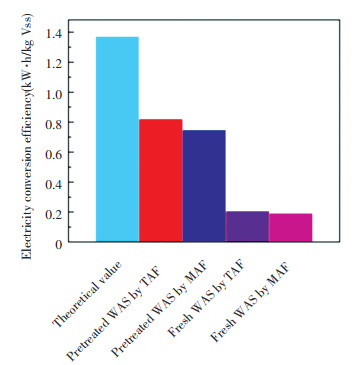
|
Fig.5 Evaluated potential electric energy conversion efficiency of WAS fermentation products via MAF and TAF with/without enzymes catalysis pretreatment |
About 2966000 dry metric tonnes of sewage sludge was produced from wastewater treatment plants (WWTPs) in China in the year of 2008[59], which comprised 1631300 dry metric tonnes of VSS (55% of VSS/TSS ratio was adopted roughly). Based on the electricity conversion efficiency of 0.75 kW·h/kg VSS via MAF by enzymes catalysis pretreatment, the sewage sludge production in China in 2008 was able to generate 1223.5 million kW·h electric energy theoretically, which equaled to a commercial value of about 734.1 million RMB with the unit residential electricity price of 0.6 RMB by MAF.
The current wastewater treatment is energy intensive. For example, the Changi Water Reclamation Plant in Singapore has a designed treatment capability of 0.8 million m3 wastewater per day, i.e., 292 million m3/year with the unit electric energy consumption of 0.52 kW·h/m3 according to public information[60], equaled to about 151.8 million electric energy per year. The electric energy produced from the total sewage sludge of China in 2008 by enzymolysis-pretreatment via MAF can meet the power demand of over eight WWTPs like Changi Water Reclamation Plant, which can compensate for the cost of enzymolysis-pretreatment in practice to a great extent. It is expected to be a promising solution for enhancing acidogenesis via MAF with boosting recoverable values of WAS organics.
Based on the above discussion, a conceptual illustration was proposed by co-locating WAS anaerobic fermentation and MFC plant for electricity generation, which could provide power for the normal operation of full-scale WWTPs. As can be seen in Fig. 6, the collected WAS was firstly pretreated by proper pretreatment method (such as enzymollysis), then the disintegrated WAS entered the anaerobic fermentation plant for acidogenesis with VFA accumulation in 5-8 days[35]. After that, the solid and liquid was separated. The solid residue could be reused for making building materials (such as blended cements)[61], while the liquid (mainly VFA liquor) was fed into MFC plant for electric energy production to sustain the normal running of full-scale WWTPs, supported by a previous study[60]. Additionally, the discharged WAS from WWTPs could be fed into anaerobic fermentation plant after enzymatic pretreatment. It formed a virtuous cycle of WAS pretreatment→anaerobic acidogenesis→electricity generation→sustain WWTPs running→WAS discharge and pretreatment, then the cycle circulated again. It offered new thinking on future WAS treatment/disposal towards energy recovery and energy-sufficient wastewater treatment by co-locating WAS anaerobic fermentation plant and MFC plant with wastewater treatment plant(s).
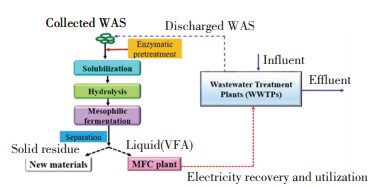
|
Fig.6 Conceptual illustration of co-located WAS anaerobic fermentation and MFC plant with wastewater treatment plants |
4 Conclusions
Enzymes catalysis pretreatment facilitated WAS solubilization remarkably (about 8700 mg/L SCOD increase just within 3 h) and narrowed the effectiveness difference between MAF and TAF significantly with more than 3200 mg COD/L VFA accumulation in six days. Bacterial community shifted with main T-RFs evolutions in MAF and TAF. Formation of intermediate community evenness in MAF contributed to VFA production comparatively. Enzymatic pretreatment prompted WAS biosolids recoverable value with 0.75 kW·h/kg VSS via MAF, which offset the enzymolysis cost largely. A conceptual illustration was proposed by co-locating WAS anaerobic fermentation and MFC plant for achieving energy-sufficient wastewater treatment, which provides new thinking on future WAS treatment/disposal towards energy recovery.
| [1] |
Yang G, Zhang G M, Wang H C, et al. Current state of sludge production, management, treatment and disposal in China. Water Research, 2015, 78: 60-73. DOI:10.1016/j.watres.2015.04.002 (  0) 0) |
| [2] |
Jiang Y, Chen Y G, Zheng X, et al. Efficient polyhydroxyalkanoates production from a waste-activated sludge alkaline fermentation liquid by activated sludge submitted to the aerobic feeding and discharge process. Environmental Science & Technology, 2009, 43: 7734-7741. DOI:10.1021/es9014456 (  0) 0) |
| [3] |
Jin B D, Wang S Y, Xing L Q, et al. Long term effect of alkali types on waste activated sludge hydrolytic acidification and microbial community at low temperature. Bioresource Technology, 2016, 200: 587-597. DOI:10.1016/j.biortech.2015.10.036 (  0) 0) |
| [4] |
Kleerebezem R, Joosse B, Rozendal R, et al. Anaerobic digestion without biogas?. Reviews in Environmental Science and Bio-technology, 2015, 14: 787-801. DOI:10.1007/s11157-015-9374-6 (  0) 0) |
| [5] |
Freguia S, Teh E H, Boon N, et al. Microbial fuel cells operating on mixed fatty acids. Bioresource Technology, 2010, 101(4): 1233-1238. DOI:10.1016/j.biortech.2009.09.054 (  0) 0) |
| [6] |
Ruffino B, Campo G, Cerutti A, et al. Preliminary technical and economic analysis of alkali and low temperature thermoalkali pretreatments for the anaerobic digestion of waste activated sludge. Waste & Biomass Valorization, 2016, 7: 667-675. DOI:10.1007/s12649-016-9537-x (  0) 0) |
| [7] |
Chen Y G, Luo J Y, Yan Y Y, et al. Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Applied Energy, 2013, 102: 1197-1204. DOI:10.1016/j.apenergy.2012.06.056 (  0) 0) |
| [8] |
Salsabil M R, Prorot A, Casellas M, et al. Pre-treatment of activated sludge: Effect of sonication on aerobic and anaerobic digestibility. Chemical Engineering Journal, 2009, 148: 327-335. DOI:10.1016/j.cej.2008.09.003 (  0) 0) |
| [9] |
Guo L, Zhao J, She Z L, et al. Effect of S-TE (solubilization by thermophilic enzyme) digestion conditions on hydrogen production from waste sludge. Bioresource Technology, 2012, 117: 368-372. DOI:10.1016/j.biortech.2012.04.010 (  0) 0) |
| [10] |
Yu S Y, Zhang G M, Li J Z, et al. Effect of endogenous hydrolytic enzymes pretreatment on the anaerobic digestion of sludge. Bioresource Technology, 2013, 146: 758-761. DOI:10.1016/j.biortech.2013.07.087 (  0) 0) |
| [11] |
Yang Q, Luo K, Li X M, et al. Enhanced efficiency of biological excess sludge hydrolysis under anaerobic digestion by additional enzymes. Bioresource Technology, 2010, 101: 2924-2930. DOI:10.1016/j.biortech.2009.11.012 (  0) 0) |
| [12] |
Xin X D, He J G, Feng J H, et al. Solubilization augmentation and bacterial community responses triggered by co-digestion of a hydrolytic enzymes blend for facilitating waste activated sludge hydrolysis process. Chemical Engineering Journal, 2016, 284: 979-988. DOI:10.1016/j.cej.2015.09.060 (  0) 0) |
| [13] |
Yuan Q, Sparling R, Oleszkiewicz J A, et al. Waste activated sludge fermentation: Effect of solids retention time and biomass concentration. Water Research, 2009, 43: 5180-5186. DOI:10.1016/j.watres.2009.08.019 (  0) 0) |
| [14] |
Jung K W, Kim D H, Kim S H, et al. Bioreactor design for continuous dark fermentative hydrogen production. Bioresource Technology, 2011, 102: 8612-8620. DOI:10.1016/j.biortech.2011.03.056 (  0) 0) |
| [15] |
Zhuo G H, Yan Y Y, Tan X J, et al. Ultrasonic-pretreated waste activated sludge hydrolysis and volatile fatty acid accumulation under alkaline conditions: Effect of temperature. Journal of Biotechnology, 2012, 159: 27-31. DOI:10.1016/j.jbiotec.2012.01.005 (  0) 0) |
| [16] |
Li X L, Peng Y Z, Ren N Q, et al. Effect of temperature on short chain fatty acids (SCFAs) accumulation and microbiological transformation in sludge alkaline fermentation with Ca(OH)2 adjustment. Water Research, 2014, 61: 34-45. DOI:10.1016/j.watres.2014.03.030 (  0) 0) |
| [17] |
Luo J Y, Feng L Y, Zhang W, et al. Improved production of short-chain fatty acids from waste activated sludge driven by carbohydrate addition in continuous-flow reactors: Influence of SRT and temperature. Applied Energy, 2014, 113: 51-58. DOI:10.1016/j.apenergy.2013.07.006 (  0) 0) |
| [18] |
Xiong H, Chen J, Wang H, et al. Influences of volatile solid concentration, temperature and solid retention time for the hydrolysis of waste activated sludge to recover volatile fatty acids. Bioresource Technology, 2012, 119: 285-292. DOI:10.1016/j.biortech.2012.05.126 (  0) 0) |
| [19] |
Hao J X, Wang H. Volatile fatty acids productions by mesophilic and thermophilic sludge fermentation: Biological responses to fermentation temperature. Bioresource Technology, 2015, 175: 367-373. DOI:10.1016/j.biortech.2014.10.106 (  0) 0) |
| [20] |
Chen Y, Cheng J J, Creamer K S, et al. Inhibition of anaerobic digestion process: a review. Bioresource Technology, 2008, 99(10): 4044-4064. DOI:10.1016/j.biortech.2007.01.057 (  0) 0) |
| [21] |
Pervin H M, Dennis P G, Lim H J, et al. Drivers of microbial community composition in mesophilic and thermophilic temperature-phased anaerobic digestion pre-treatment reactors. Water Research, 2013, 47: 7098-7108. DOI:10.1016/j.watres.2013.07.053 (  0) 0) |
| [22] |
Jang H M, Ha J H, Kim M S, et al. Effect of increased load of high-strength food wastewater in thermophilic and mesophilic anaerobic co-digestion of waste activated sludge on bacterial community structure. Water Reseatch, 2016, 99: 140-148. DOI:10.1016/j.watres.2016.04.051 (  0) 0) |
| [23] |
Wittebolle L, Marzorati M, Clement L, et al. Initial community evenness favours functionality under selective stress. Nature, 2009, 458(7238): 623-626. DOI:10.1038/nature07840 (  0) 0) |
| [24] |
Marzorati M, Wittebolle L, Boon N, et al. How to get more out of molecular fingerprints: practical tools for microbial ecology. Environmental Microbiology, 2008, 10(6): 1571-1581. DOI:10.1111/j.1462-2920.2008.01572.x (  0) 0) |
| [25] |
Tao Y, Gao D W, Wang H Y, et al. Ecological characteristics of seeding sludge triggering a prompt start-up of anammox. Bioresource Technology, 2013, 133: 475-481. DOI:10.1016/j.biortech.2013.01.147 (  0) 0) |
| [26] |
Balvanera P, Kremen C, Martinez-Ramos M, et al. Applying community structure analysis to ecosystem function: examples from pollination and carbon storage. Ecological Applications, 2005, 15: 360-375. DOI:10.1890/03-5192 (  0) 0) |
| [27] |
Jia S T, Dai X H, Zhang D, et al. Improved bioproduction of short-chain fatty acids from waste activated sludge by perennial ryegrass addition. Water Research, 2013, 47: 4576-4584. DOI:10.1016/j.watres.2013.05.012 (  0) 0) |
| [28] |
Xin X D, He J G, Qiu W. Volatile fatty acid augmentation and microbial community responses in anaerobic co-digestion process of waste-activated sludge mixed with corn stalk and livestock manure. Environmental Science and Pollution Research, 2018, 25: 4846-4857. DOI:10.1007/s11356-017-0834-0 (  0) 0) |
| [29] |
Xin X D, Hong J M, He J G, et al. An integrated approach for waste activated sludge management towards electric energy production/resource reuse. Bioresource Technology, 2019, 274: 225-231. DOI:10.1016/j.biortech.2018.11.092 (  0) 0) |
| [30] |
American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater. 20th ed. 2005, Washington D.C., USA. DOI: 10.1007/BF02996984.
(  0) 0) |
| [31] |
Michel D, Gills K A, Hamilton J K, et al. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 1956, 28: 350-356. DOI:10.1021/ac60111a017 (  0) 0) |
| [32] |
Yan Y Y, Feng L Y, Zhang C J, et al. Ultrasonic enhancement of waste activated sludge hydrolysis and volatile fatty acids accumulation at pH 10.0. Water Research, 2010, 44: 3329-3336. DOI:10.1016/j.watres.2010.03.015 (  0) 0) |
| [33] |
Chen X, Yuan H R, Zou D X, et al. Improving biomethane yield by controlling fermentation type of acidogenic phase in two-phase anaerobic co-digestion of food waste and rice straw. Chemical Engineering Journal, 2015, 273: 254-260. DOI:10.1016/j.cej.2015.03.067 (  0) 0) |
| [34] |
Turner S, Pryer K M, Miao V P W, et al. Investigating Deep Phylogenetic Relationships among Cyanobacteria and Plastids by Small Subunit rRNA Sequence Analysis. Journal of Eukaryotic Microbiology, 1999, 46(4): 327-338. DOI:10.1111/j.1550-7408.1999.tb04612.x (  0) 0) |
| [35] |
Xin X D, He J G, li L, et al. Enzymes catalyzing pre-hydrolysis facilitated the anaerobic fermentation of waste activated sludge with acidogenic and microbiological perspectives. Bioresource Technology, 2018, 250: 69-78. DOI:10.1016/j.biortech.2017.09.211 (  0) 0) |
| [36] |
Ma Y Q, Yin Y, Liu Y, et al. A holistic approach for food waste management towards zero-solid disposal and energy/resource recovery. Bioresource Technology, 2017, 228: 56-61. DOI:10.1016/j.biortech.2016.12.090 (  0) 0) |
| [37] |
Gupta R, Basile A, Veziroglu T N, et al. Compendium of Hydrogen Energy: Hydrogen Storage, Distribution and Infrastructure. 2015, Woodhead Publishing.
(  0) 0) |
| [38] |
McCarty P L, Bae J, Kim J, et al. Domestic wastewater treatment as a net energy producer-can this be achieved?. Environmental Science & Technology, 2011, 45: 7100-7106. DOI:10.1021/es2014264 (  0) 0) |
| [39] |
Samson R, LeDuyt A. Detailed study of anaerobic digestion of spirulina maxima algal biomass. Biotechnology and Bioengineering, 1986, 28(7): 1014-1023. DOI:10.1002/bit.260280712 (  0) 0) |
| [40] |
Wittebolle L, Vervaeren H, Verstraete W, et al. Quantifying community dynamics of nitrifiers in functionally stable reactors. Applied and Environmental Microbiology, 2008, 74(1): 286-293. DOI:10.1128/AEM.01006-07 (  0) 0) |
| [41] |
Morowitz M J, Denef V J, Costello E K, et al. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proceedings of the National Academy of Science, 2011, 108(3): 1128-1133. DOI:10.1073/pnas.1010992108 (  0) 0) |
| [42] |
Teo C W, Wong P C Y. Enzyme augmentation of an anaerobic membrane bioreactor treating sewage containing organic particulates. Water Research, 2014, 48: 335-344. DOI:10.1016/j.watres.2013.09.041 (  0) 0) |
| [43] |
Rashed IGA-A, Akunna J, El-Halwany M M, et al. Improvement in the efficiency of hydrolysis of anaerobic digestion in sewage sludge by the use of enzymes. Desalination and Water Treatment, 2010, 21: 280-285. DOI:10.5004/dwt.2010.1575 (  0) 0) |
| [44] |
Arun C, Sivashanmugam P. Solubilization of waste activated sludge using a garbage enzyme produced from different preconsumer organic waste. RSC Advances, 2015, 5: 51421-51427. DOI:10.1039/c5ra07959d (  0) 0) |
| [45] |
Zhao J W, Gui L, Wang Q L, et al. Aged refuse enhances anaerobic digestion of waste activated sludge. Water Research, 2017, 123: 724-733. DOI:10.1016/j.watres.2017.07.026 (  0) 0) |
| [46] |
Dwyer J, Starrenburg D, Tait S, et al. Decreasing activated sludge thermal hydrolysis temperature reduces product colour, without decreasing degradability. Water Research, 2008, 42: 4699-4709. DOI:10.1016/j.watres.2008.08.019 (  0) 0) |
| [47] |
Wett B, Phothilangka P, Eladawy A, et al. Systematic comparison of mechanical and thermal sludge disintegration technologies. Waste Management, 2010, 30: 1057-1062. DOI:10.1016/j.wasman.2009.12.011 (  0) 0) |
| [48] |
Gianico A, Braguglia C M, Cesarini R, et al. Reduced temperature hydrolysis at 134 ℃ before thermophilic anaerobic digestion of waste activated sludge at increasing organic load. Bioresource Technology, 2013, 143: 96-103. DOI:10.1016/j.biortech.2013.05.069 (  0) 0) |
| [49] |
Ruffino B, Campo G, Genon G, et al. Improvement of anaerobic digestion of sewage sludge in a wastewater treatment plant by means of mechanical and thermal pretreatments: performance, energy and economical assessment. Bioresource Technology, 2015, 175: 298-308. DOI:10.1016/j.biortech.2014.10.0 (  0) 0) |
| [50] |
El Hadj T B, Astals S, Galiì A, et al. Ammonia influence in anaerobic digestion of OFMSW. Water Science & Technology, 2009, 59: 1153-1158. DOI:10.2166/wst.2009.100 (  0) 0) |
| [51] |
Sung S, Liu T. Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere, 2003, 53: 43-52. DOI:10.1016/S0045-6535(03)00434-X (  0) 0) |
| [52] |
Guo X H, Wang C, S un, F Q, et al. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresource Technology, 2014, 152: 420-428. DOI:10.1016/j.biortech.2013.11.012 (  0) 0) |
| [53] |
Hai R, Wang Y L, Wang X H, et al. Bacterial community dynamics and taxa-time relationships within two activated sludge bioreactors. Plos One, 2014, 9: 1-8. DOI:10.1371/journal.pone.0090175 (  0) 0) |
| [54] |
Sträuber H, Lucas R, Kleinsteuber S, et al. Metabolic and microbial community dynamics during the anaerobic digestion of maize silage in a two-phase process. Applied Microbiology Biotechnology, 2016, 100: 479-491. DOI:10.1007/s00253-015-6996-0 (  0) 0) |
| [55] |
Ito T, Yoshiguchi K, Ariesyady H D, et al. Identification and quantification of key microbial trophic groups of methanogenic glucose degradation in an anaerobic digester sludge. Bioresource Technology, 2012, 123: 599-607. DOI:10.1016/j.biortech.2012.07.108 (  0) 0) |
| [56] |
Rui J P, Li J B, Zhang S H, et al. The core populations and co-occurrence patterns of prokaryotic communities in household biogas digesters. Biotechnology for Biofuels, 2015, 8: 158. DOI:10.1186/s13068-015-0339-3 (  0) 0) |
| [57] |
Crowder D W, Northfield T D, Strand M R, et al. Organic agriculture promotes evenness and natural pest control. Nature, 2010, 466: 109-112. DOI:10.1038/nature09183 (  0) 0) |
| [58] |
Carballa M, Regueiro L, Lema J M, et al. Microbial management of anaerobic digestion: exploiting the microbiome-functionality nexus. Current Opinion in Biotechnology, 2015, 33: 103-111. DOI:10.1016/j.copbio.2015.01.008 (  0) 0) |
| [59] |
LeBlanc R J, Richard R P, Beecher N, et al. A review of global atlas of "excreta, wastewater sludge, and biosolids management: moving forward the sustainable and welcome uses of a global resource". Proceedings of the Water Environment Federation, 2009, 3: 1202-1208. DOI:10.2175/193864709793846402 (  0) 0) |
| [60] |
Xin X D, Ma Y Q, Liu Y, et al. Electric energy production from food waste: Microbial fuel cells versus anaerobic digestion. Bioresource Technology, 2018, 255: 281-287. DOI:10.1016/j.biortech.2018.01.099 (  0) 0) |
| [61] |
Chen Z, Poon C S. Comparative studies on the effects of sewage sludge ash and fly ash on cement hydration and properties of cement mortars. Construction and Building Materials, 2017, 154: 791-803. DOI:10.1016/j.conbuildmat.2017.08.003 (  0) 0) |
 2020, Vol. 27
2020, Vol. 27


