2. College of Chemistry, Chongqing Normal University, Chongqing 401331, China
Iron is a rich metal in our planet. Mild steels that are composed primarily by iron play dominative roles in a wide variety of applications including construction and industry fields due to their super metal mechanical processing nature and the low cost[1-3]. However, mild steels are fragile in air and aggressive media due to high reactivity of iron. In particular, mild steels can be corroded seriously in inorganic acid environments such as in HCl aqueous solution, which is often employed in industry for processing pickling, cleaning, descaling as well as acidizing[4-5]. This requires us to develop efficient strategies to restrain chemical corrosion of mild steels in HCl solution.
It is demonstrated that destruction of mild steels caused by chemical corrosion in hydrochloric acid solution can be effectively suppressed by some organic molecules carrying heteroatoms in molecular skeletons, for instance, oxygen, nitrogen, sulfur, and phosphorus atoms, which is mostly resulted by chemical chelation of iron ions with these molecules[6-10]. Hence, organic adsorption protection layer may be formed on metal surface. For instance, benzimidazole derivatives and Schiff base molecules were demonstrated to be corrosion inhibitors for mild steels in aggressive acid solution[11-12]. On the other hand, most of organic inhibitors are normally gained by multi-step synthetic pathways, which are environmentally threatening and very expensive. In addition, the toxicity of some prepared organic corrosion inhibitors can restrain large scale application potentials.
Therefore, the development of green corrosion inhibitors continues to be one of hot topics in chemical and material engineering field. The environmentally friendly and non-toxic crude extracts from natural plants may be strong potential candidates for anti-corrosion of mild steels in HCl solution.
A number of efforts have been devoted to use crude plant extracts to suppress corrosion of mild steels in harsh acid media[13-19]. For example, the crude plant extracts of Justicia gendarussa and Rollinia occidentalis display corrosion suppression properties to mild steels in HCl solution. In addition, the crude extracts of Musa paradisica peel and bamboo leaf could be employed as corrosion inhibitors for mild steels in aggressive acid medium. On the other hand, the maximal corrosion inhibition efficiency of crude extracts of natural plants to metal in acid media reaches seldomly 90% or above. However, it is hard to find out which crude plant extracts should be employed as neat corrosion inhibitors for mild steels in HCl solution.
It was demonstrated that ionic or non-ionic surfactants show great anti-corrosion capability of mild steels in aggressive acid aqueous solutions[20-23]. Furthermore, molecular aggregates of some organic compounds present good adsorption on metal surface as well as excellent anti-corrosion performance[24-26]. The results inspire us to employ detergent-like crude extracts of natural plants as potential candidates of corrosion inhibitors.
Hence, one of the main concerns in this study aims to propose a new strategy that uses detergent-like crude extracts of natural plants as green corrosion inhibitors for mild steels in HCl solution. For this purpose, the crude Gleditsia sinensis lam extract (GSLE) was employed as green corrosion inhibitor for protecting mild steels in HCl solution.It is well-known that Gleditsia sinensis lam (GSL) is a fruit of Gleditsia sinensis that belongs to macrophanerophytes and is widely grown in earth, which has been used as "soap" for thousands of years. It can even be employed as food and anti-cancer/antibody drug.
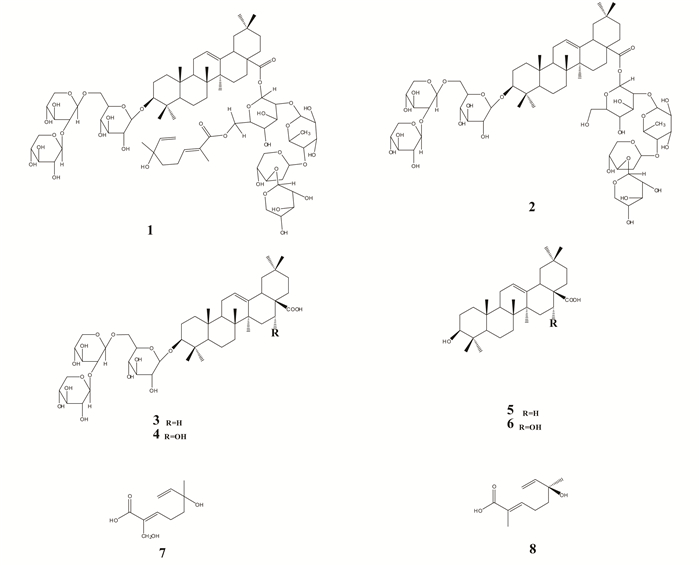
The main chemical constituents in GSLE is shown in Fig. 1 (the molecules 1-8, the chemical names are supplied in Supplementary Materials)[27-29], which carry a number of chemical adsorption centers such as functional groups of -OH, O-C=O, and double π bonds.
|
Fig.1 Chemical structures of the main molecular constituents in GSLE (the chemical names of the molecules 1-8 can be found in Supplementary Materials) |
Thus, the self-assembly nature of GSLE in mixed organic solvents/H2O such as ethanol/H2O (50/50, v/v) is confirmed. The yielded GSLE-assemblies can be tightly adsorbed to the studied mild steel substrate (Q235 steel, national standard in China). The presence of thousands of adsorption centers in GSLE-assemblies may strengthen considerable chemical complexation interaction between GSLE-assemblies and iron ions.
Therefore, this study proposes to employ detergent-like renewable low-cost crude extract of GSL as green corrosion inhibitor for mild steel in HCl solution. A comprehensive survey was performed to study corrosion inhibition nature of formed stable GSLE-assemblies by different means such as potentiodynamic polarization curves (Tafel covers), electrochemical impendence spectroscopy (EIS), and atomic force microscopy (AFM). The Langmuir isotherm plot and molecular modeling were employed to reveal chemical interaction mechanism between the GSLE-assemblies and the surface of mild steel specimen.
2 Experimental Section 2.1 Materials and CharacterizationsThe crude GSLE was prepared from GSL powder by using ethanol as extractant in a Soxhlet extractor[26]. GSL powder was purchased from Guide Chemical Corporation, China. At first, 20 g GSL powder was placed in 120 mL ethanol that was contained in a Soxhlet extractor, which was refluxed for 8 h. The acquired extracted solution was concentrated by a rotary evaporator. The light green solid GSLE was obtained with 18.5% extraction yield after the extracts were fully dried in vacuum oven. In addition, the produced solid crude GSLE was characterized by Fourier transform infrared (FT-IR) spectroscopy with Nicolet iS50 FT-IR spectrophotometer of Thermo Scientific.
The experimental metal samples were processed from a Q235 steel sheet (10 cm × 10 cm × 1 cm) which included 0.16 wt% C, 0.18 wt% Si, 0.29 wt% Mn, < 0.013 wt% S, < 0.014 wt% P, and the remainder was Fe. The working electrodes (WEs) for electrochemical determination were cylinders with a diameter of 1 cm and a thickness of 0.3 cm. The studied mild steel samples were processed into cubic shape specimens with 0.50 cm × 0.50 cm × 0.30 cm and 1.00 cm × 1.00 cm × 0.10 cm dimensions for SEM and AFM measurements, respectively. The metal samples were polished with emery papers of 400-5000 grits before the detection. Then, the metal specimens were ultrasonically cleaned with triply distilled water and absolute alcohol, followed by drying under vacuum.
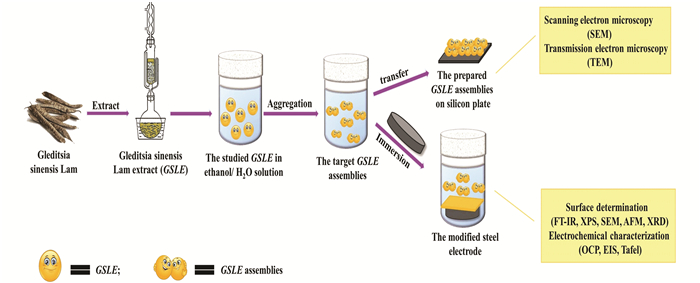
2.2 Preparation of GSLE-Assemblies and Preparation of Mild Steel Electrodes Covered by GSLE-Assemblies Protection LayersThe complete experimental diagrammatic drawing was given in Fig. 2. The GSLE solution with different concentrations from 0.1 g/L to 0.7 g/L in mixed solvents of organic solvents/H2O such as ethanol/H2O and THF/H2O (v/v, 50/50) were prepared, and self-assembly of GSLE was performed at 298 K. The prepared GSLE-assemblies were characterized by scanning electron microscopy (SEM, Jeol-JSM-3.5 CF, Japan) and transmission electron microscopy (TEM, JEM 1200EX microscope, Japan) at an accelerating voltage of 10.0 kV and 120 kV, respectively. The SEM and TEM samples were prepared by slightly titrating GSLE-assemblies solution upon a silicon plate surface. The mixed solvents were evaporated rapidly under vacuum. To enhance the electroconductivity, a tiny amount of gold was sputtered on the produced GSLE assemblies.
|
Fig.2 Schematic diagram of formation of GSLE-assemblies and the preparation of stable GSLE-assemblies protection film on the studied steel specimen surface |
The size distribution of freshly prepared GSLE-assemblies was further investigated by dynamic light scattering (DLS) with an apparatus of Malvern nano-zetasizer (Malvern Instrument, UK), and the corresponding MAS OPTION particle sizing software was employed to collect and analyze DLS data. The stable assembly state was confirmed by zeta potential acquired with Zetasizer (Nano-ZS) of Malvern Instruments, which was analyzed by homologous software of Dispersion Technology Software (DTS).
Then, processed polished mild steel samples were contained promptly into GSLE-assemblies solution for 10 h (electrochemical performance showed that stably covered GSLE-assemblies layer was produced on the surveyed steel surface at 10 h immersion time). Then, the metal samples were taken out, cleaned with ethanol, and dried under vacuum. Eventually, the prepared WEs were kept in dry atmosphere prior to electrochemical measurements.
2.3 Surface AnalysesFT-IR spectra of original GSLE-powder and the studied mild steel surface covered by the stable GSLE-assemblies were collected by Thermo Scientific FT-IR spectrophotometer (Nicolet iS50) with a scanning region of 500-4000 cm-1, respectively. Raman spectroscopy of GSLE-assemblies covered steel sample was performed by Raman spectrometer (Renishaw, UK).
X-ray photoelectron spectroscopy (XPS) determination was carried out by a PHI 5700 spectrometer with Al Kα X-ray source (1486.6 eV), in which the spectra were calibrated by C1s at 285.0 eV. The XPS survey was conducted at vertical direction relative to the studied metal specimen surface. The surface morphologies of the investigated steel specimens without and with the stable GSLE-assemblies protection layer were studied by SEM. The roughness of uncovered and covered stable GSLE-assemblies mild steel surface was investigated by AFM (MFP-3D-BIO, Asylum Research, America), respectively.
2.4 Electrochemical MeasurementsThe typical three electrode cells were employed in electrochemical determination. A processed cylinder mild steel embedded in specimen holder was used as the working electrode (WE), a high purity platinum sheet (area, 2.0 × 2.0 cm2) was employed as the counter electrode (CE), and a saturated calomel electrode included in a Luggin Capillary acted as the reference electrode (RE).
CHI660C working station (Shanghai Chenhua Corporation, China) was employed to perform electrochemical experiments containing Tafel curves and EIS at 298 K. Open-circuit potentials (OCPs) achieved a stable potential value within 1800 s (as shown in Fig. 3). Tafel curves were acquired in a region of ± 250 mV (versus the OCP) with a potential scanning rate of 1 mV·s-1. EIS was obtained in a frequency variation range of 100 kHz to 0.01 Hz, and 5 mV amplitude of alternating voltage was used.

|
Fig.3 OCP versus time curves in 1 M HCl solutions for unmodified steel electrodes and the stable GSLE-assemblies of different concentrations covered steel electrodes |
The corrosion inhibition efficiency achieved by Tafel curves (ηP%) and EIS (ηE%) was shown as Eqs. (1) and (2), respectively[30-31]:
| $ {\eta _{\rm{P}}} = (1 - {\rm{ }}\frac{{{i_{{\rm{corr}}}}}}{{{i_{{\rm{corr, 0}}}}}}) \times 100 $ | (1) |
| $ {\eta _{\rm{E}}} = \frac{{{R_{{\rm{ct}}}}-{R_{{\rm{ct, 0}}}}}}{{{R_{{\rm{ct}}}}}} \times 100 $ | (2) |
where icorr, 0 and icorr are corrosion current densities of unmodified and modified electrodes by the yielded stabled GSLE-assemblies, respectively; Rct, 0 and Rct are charge transfer resistances of electrodes unmodified and modified by the formed stable GSLE-assemblies, respectively.
2.5 Molecular ModelingMolecular modeling was carried out by density functional theory with Gaussian 09 software[32]. The representative constituents in GSLE (the chemicals 1, 3, 5, and 7 shown in Fig. 1) were geometrically optimized at DFT level using B3LYP methods under the basis set of 6-311++G (d, p)[33], and water molecule was employed as solvent in molecular simulation process. In addition, the energy of the highest occupied molecular orbital (EHOMO) and the energy of the lowest unoccupied molecular orbital (ELUMO) along with the energy gap ΔE (ΔE= ELUMO-EHOMO) were computed and analyzed.
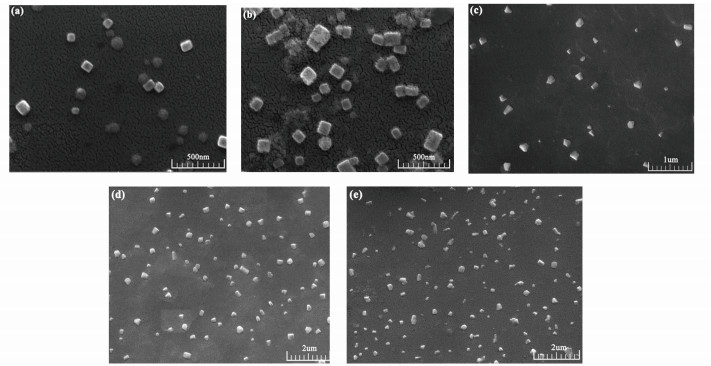
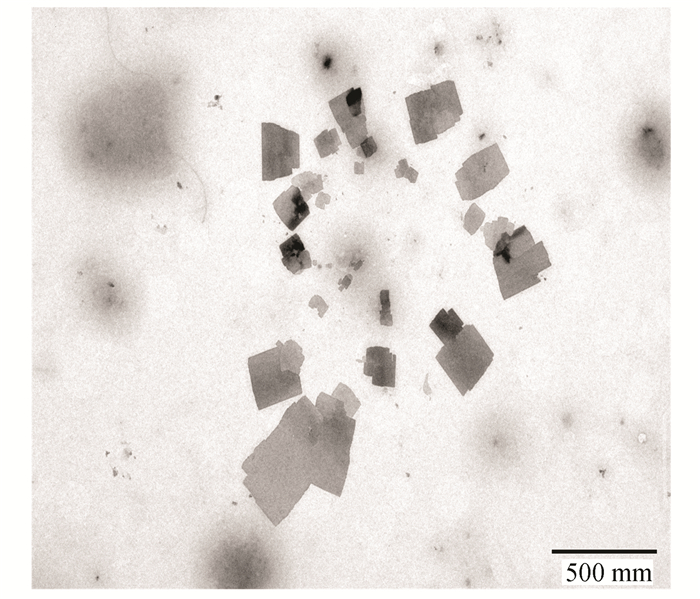
3 Results and Discussion 3.1 Self-Assembly of GSLE in the Mixed Organic/H2O SolutionsThe GSLE-assemblies of 0.6 g/L in mixed organic/H2O solutions such as ethanol/H2O, THF/H2O, and ACN/H2O were determined. SEM images of GSLE-assemblies in mixed ethanol/H2O (v/v, 50/50) solvent formed at different assembly evolving time are shown in Fig. 4. At evolving time of 30 min, the scattered and thin flake assemblies are produced with approximately 75 nm diameter size (Fig. 4(a)). With increasing assembly time at 2 h, the cubic-shape assembled species with about 125 nm average size are formed (Fig. 4(b)). At 6 h aggregation time, the thicker cubic assembled particles with approximately 200 nm average size are yielded (Fig. 4(c)). Furthermore, the much thicker cubic-shaped GSLE-assemblies with 400 nm diameters can be discovered at 10 h evolving time (Fig. 4(d)). In addition, it is shown that the diameter sizes and morphologies of yielded assembled species do not display great changes with increasing assembly time at 24 h (Fig. 4(e)), which shows that GSLE-assemblies arrives in a stable state at 10 h of evolution time. In addition, TEM images also demonstrate that cubic-shape GSLE-assemblies is obtained at 10 h aggregation time (Fig. S1).
|
Fig.4 SEM images of GSLE-assemblies of 0.6 g/L in ethanol/H2O (v/v, 50/50) solution at different evolving time courses (a) 30 min; (b) 2 h; (c) 6 h; (d) 10 h; (e) 24 h |
|
S1 TEM image of the GSLE-assemblies in the mixed ethanol /H2O solution (v/v, 50/50) at 10 h |
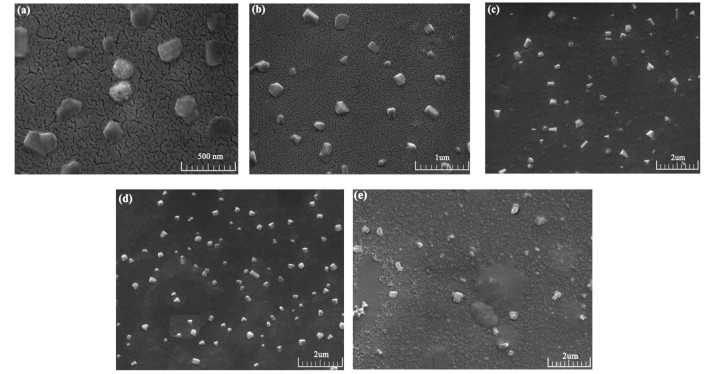
Fig. 5 further shows the GSLE-assemblies of various concentrations (from 0.1 g/L to 0.7 g/L) in mixed ethanol/H2O (v/v, 50/50) solution at 10 h assembly time. Firstly, as the concentrations of GSLE increase from 0.1 g/L to 0.6 g/L, the diameters of assemblies show an increase from 150 nm to 400 nm (Figs. 5(a)-(d)). However, the assembly state of GSLE tends to be amorphous as its concentration increases at 0.7 g/L (Fig. 5(e)), which can be resulted by more disorderly accumulation of GSLE. Thus, GSLE-assemblies reach the greatest stable state at 0.6 g/L with 10 h evolving time. The results indicate that the diameter sizes and shapes of GSLE-assemblies show dependence on concentrations of GSLE.
|
Fig.5 SEM images of GSLE-assemblies in mixed ethanol/H2O (v/v, 50/50) solvent at 10 h evolution time during increasing concentrations from 0.1 to 0.7 g/L (a) 0.1 g/L; (b) 0.3 g/L; (c) 0.5 g/L; (d) 0.6 g/L; (e) 0.7 g/L |
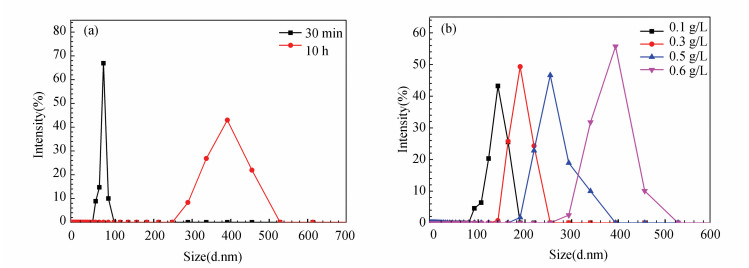
Furthermore, the diameters of GSLE-assemblies at different assembly concentrations and evolving time in mixed ethanol/H2O (v/v, 50/50) solvents were surveyed by DLS. Fig. S2(a) shows that the diameters distribution of GSLE-assemblies of 0.6 g/L is concentrated in a range of 75-400 nm with evolving time increasing from 30 min to 10 h. Furthermore, the diameters of assemblies are located in a range of 150-400 nm with concentrations increasing from 0.1 g/L to 0.6 g/L at 10 h assembly time (Fig. S2(b)). It is shown that the obtained results by DLS survey are in agreement with those acquired by SEM characterization. The corresponding zeta potential is -46.2 mV (Fig. S3), which means that a stable assembly state can be achieved[34].
|
S2 (a) Size distribution of the yielded GSLE-assemblies (at 30 min and 10 h) in the mixed ethanol/ H2O (v/v, 50/50) solution obtained by the DLS; (b) Size distribution of GSLE-assemblies with the different concentrations in the mixed ethanol/H2O solution (v/v, 50/50) obtained by the DLS |

|
S3 Zeta potential of the stable GSLE-assemblies in the mixed ethanol/H2O (v/v, 50/50) solution obtained by the DLS for 10 h |
As discussed above, Fig. 1 shows that GSLE contains a number of hydrophilic and hydrophobic groups in the primary components, and thus amphiphilic GSLE may cause molecular-molecular stacking. In addition, the presence of hydroxyl group and carboxyl group induces formation of hydrogen bonding framework between solute and solvent molecules, and thus molecular assembly of GSLE can occur.
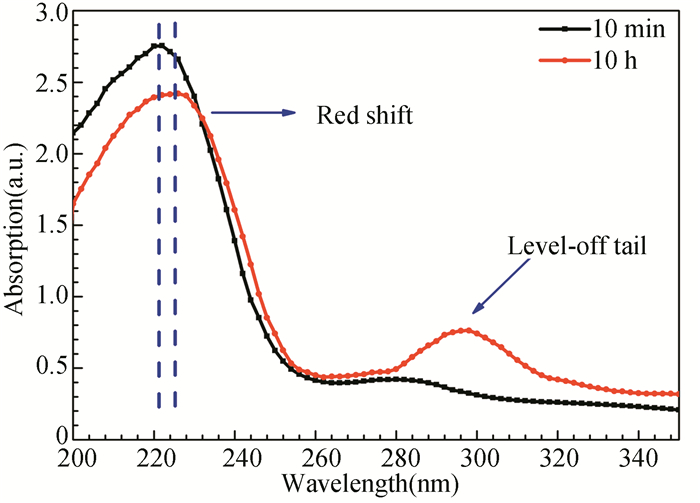
UV/visible absorption spectra of GSLE-assemblies are further investigated in mixed ethanol/H2O solvents (v/v, 50/50) (Fig. S4). The maximal absorption wavelength of GSLE-assemblies presents an approximate 3-4 nm red-shift at 10 h assembly evolution time. In addition, a new absorption band in longer wavelength region appears at 10 h evolving time. The results suggest that J-assembly intermolecular stacking (heat to tail) of GSLE happens[35].
|
S4 UV/visible absorption spectra of the GSLE-assemblies of 0.6 g/L in the mixed ethanol/H2O (v/v, 50/50) solution at assembly time of 10 min and 10 h |
3.2 Experimental Evidence of Chemical Groups in GSLE and Chemical Adsorption of GSLE-Assemblies on the Studied Mild Steel Specimen Surfaces 3.2.1 Chemical groups and chemical adsorption evidence of GSLE-assemblies on mild steel specimen surfaces identified by FT-IR spectroscopy and Raman spectroscopy
FT-IR spectroscopy and Raman spectroscopy of original GSLE-powders and GSLE-assemblies (10 h assembly time, 0.6 g/L) covered metal samples were carried out, respectively, which were used to analyze adsorption mechanism of GSLE-assemblies on mild steel surface.
FT-IR spectra of GSLE-powder and GSLE-assemblies are shown in Fig. 6(a). The strong absorption band located at 3419 cm-1 is attributed to O-H stretching vibration, while the bands situated at 2923 cm-1 may be yielded by C-H stretching vibration. The band at 1638.7 cm-1 can be attributed to C=O stretching vibration, while 1384 cm-1 band may be assigned to C=C stretching vibration. The peaks yielded by C-O stretching vibration are situated at 1077.3 cm-1 and 1045.6 cm-1.

|
Fig.6 FT-IR spectroscopy and Raman spectroscopy of original GSLE-powders and GSLE-assemblies covered metal samples |
Besides, the bands located at a range of 1000-500 cm-1 are corresponding to C-H group in heterocyclic rings. Although GSLE-assemblies adsorbed on the surveyed mild steel sample shows similar FT-IR spectroscopy as the extracted GSLE-powder, the characteristic peak of C=O in the adsorbed GSLE-assemblies is weakened and almost disappeared on the surveyed mild steel surface (1638.7 cm-1, Fig. 6(b)), which indicates that GSLE-assemblies protection film is formed by mainly using C=O group chemical chelation with iron.
Raman spectrum of GSLE-assemblies adsorbed mild steel specimen is depicted in Fig. 6(c). The peak in 3087-2786 cm-1 is stretching of C-H group[36]. The bands located in 1518-1378 cm-1 and 1252-1092 cm-1 are yielded by groups of C=C and C-O[37], respectively. It is discovered that no stretching of C=O group in a range of 1653-1813 cm-1 is presented. Moreover, the stretching of O:Fe group is found at 772-385 cm-1[38]. Raman spectroscopy results further demonstrate that GSLE-assemblies adsorb on the studied mild steel specimen surface based on chemical chelating bonding between oxygen and Fe atoms.
3.2.2 Chemical groups and chemical adsorption evidence of GSLE-assemblies on the studied mild steel specimen surfaces identified by XPS spectroscopyXPS spectroscopy analyses of GSLE-assemblies covered on the studied mild steel specimen surfaces were performed to further elucidate adsorption mechanism. Figs. 7-9 show the detected XPS spectra of Fe, C, and O elements on the studied mild steel specimen surfaces. The adsorbed stable GSLE-assemblies mild steel specimens were included in HCl solution for 1 h. The bare metal substrate (steel-bare) was also contained in 1 M HCl solution for 1 h as the reference.

|
Fig.7 Fe 2p3/2 XPS spectra of steel-bare and steel-GSLE-assemblies |
|
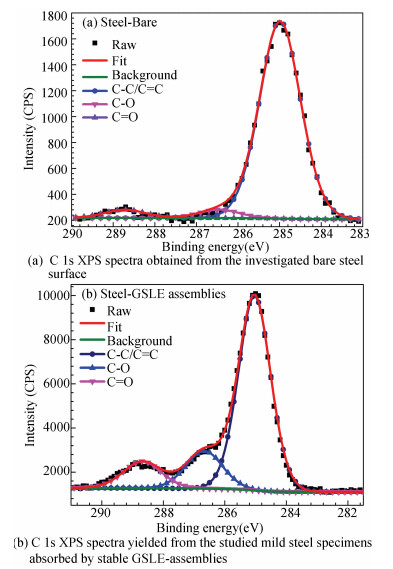
Fig.8 C 1s XPS spectra of steel-bare and steel-GSLE-assemblies |
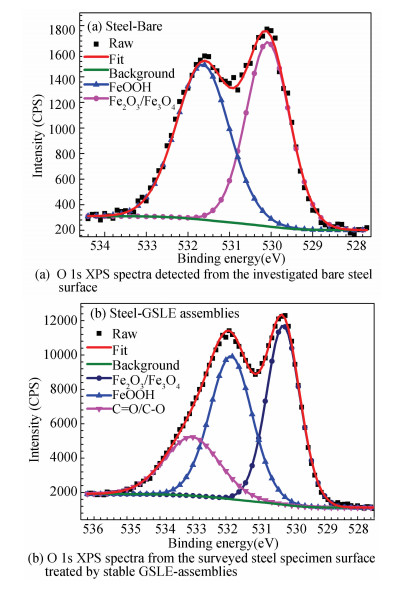
|
Fig.9 O 1s XPS spectra of steel-bare and steel-GSLE-assemblies |
Fig. 7 depicts the peak profiles of Fe 2p3/2 XPS spectra for the studied metal specimens of steel-bare and steel-GSLE-assemblies, respectively. The corresponding resolved parameters including binding energy, chemical states, and full widths at half maximum (FWHMs) of Fe 2p3/2 XPS spectra peaks are presented in Table 1. The resolved Fe 2p3/2 XPS spectrum of steel-bare containing triple main peaks are ascribed to Fe2O3/Fe3O4 (710.28 eV), FeOOH (711.56 eV), and FeCl3 (712.71 eV), respectively. The Fe 2p3/2 XPS spectrum of steel-GSLE-assemblies can be divided into three peaks that are attributed to Fe0(707.58 eV), Fe2O3/Fe3O4 (709.56 eV), and FeOOH(711.32 eV), respectively[39]. It is deduced that steel-bare specimen presents XPS signals of Fe3+ and Fe2+, whereas steel-GSLE-assemblies shows XPS signals of Fe3+, Fe2+, and Fe0 [7, 9, 40-41]. Thus, the results show that GSLE-assemblies covered on the investigated steel surface can effectively inhibit oxidation of iron, which thus leads to great anti-corrosion performance.
| Table 1 De-convolution parameters containing chemical states, binding energies, and FWHMs of Fe 2p3/2 XPS spectra peaks detected from surfaces of the studied bare mild steel and the surveyed mild steel specimens covered by stable GSLE-assemblies |
Fig. 8 presents the de-convoluted C 1s XPS spectra for the investigated mild steel surfaces, and the results are presented in Table 2. It is shown that C 1s spectra of steel-bare may be resolved into C-C/C=C, C-O, and C=O components located at 285.00, 286.46, and 288.72 eV, which can be yielded by adventitious carbon contamination. The C 1s spectrum of steel-GSLE-assemblies sample is de-convoluted into triple peaks, which indicates three chemical forms of C atoms are discovered on the mild steel surface (Fig. 8(b)). The primary peak situating at 285.04 eV is mostly assigned to C-C/C=C bonds contained in GSLE and contaminant hydrocarbons. The other two peaks may be corresponding to C-O and C=O species at 286.67 eV and 288.68 eV, respectively[9, 41]. It is discovered that C 1s XPS spectrum of steel-GSLE-assemblies sample displays more remarkable C-O and C=O peaks than that of steel-bare specimen, which suggests chemical adsorption of GSLE-assemblies on steel surface.
| Table 2 De-convolution parameters containing chemical states, binding energies, and FWHMs of C 1s XPS spectra peaks detected from the studied bare steel specimen surfaces and the investigated mild steel specimens absorbed by stable GSLE-assemblies, respectively |
Fig. 9 exhibits the de-convolution of O 1s spectra for the investigated metal surfaces. The studied steel-bare specimen sample shows adventitious oxygen contamination. Fig. 9(a) suggests that O 1s spectrum determined from the surveyed steel-bare surface can be resolved into Fe2O3/Fe3O4 and FeOOH components, which are found at 530.07 and 531.64 eV (Table 3). In contrast, the O 1s spectrum of steel-GSLE-assemblies sample surface is de-convoluted into triple peaks (Fig. 9(b)), which are attributed to Fe2O3/Fe3O4(530.26 eV), FeOOH (531.84 eV), and C=O/C-O (533.03 eV), respectively. The discovery of O 1s XPS signal ascribed to C=O/C-O further suggests chemical adsorption of GSLE-assemblies on the studied steel surface[7, 40].
| Table 3 De-convolution parameters containing chemical states, binding energies, and FWHMs of O 1s XPS spectra peaks obtained from surfaces of the studied bare mild steel and the surveyed steel specimens covered by stable GSLE-assemblies |
3.2.3 Chemical adsorption evidence of GSLE-assemblies on mild steel specimen surfaces identified by XRD spectroscopy (X-ray diffraction)
The XRD patterns of the studied metal samples are acquired to identify corrosion products (Fig. 10). The XRD pattern of steel-bare specimen shows remarkable peaks located at 27°, 35.3°, and 61.2°, which can be attributed to oxides/oxyhydroxides of iron. The other two peaks can be attributed to iron appearing at 44.7° and 82.3°[42-43]. In contrast, the XRD pattern of GSLE-assemblies covered steel specimen presents triple iron peaks at 44.7°, 82.3°, and 65.0°. However, it is noticed that one more detected peak ascribed to Fe appears, but the peak assigned to oxyhydroxides of iron is lost. Hence, the XRD results suggest that GSLE-assemblies protection film covered on metal surface can inhibit destruction of steel by oxidation.

|
Fig.10 XRD patterns of the investigated bare mild steel specimens and the surveyed mild steel specimens treated by stable GSLE-assemblies |
3.2.4 SEM analyses of the studied mild steel specimen surfaces
Fig. 11 presents the micrograms of the investigated steel sample surfaces. Fig. 11(a) depicts the morphologies of polished mild steel specimen surface. The friction tracks on polished metal surface (SiC abrasive papers caused during polishing) can be discovered. However, Fig. 11(b) shows the mild steel specimen surface is totally destroyed due to metal corrosion in 1 M HCl solution for 6 h, and the tracks on mild steel surface completely disappear.

|
Fig.11 (a) SEM micrograph of freshly polished mild steel specimen; (b) SEM micrograph of polished blank steel included in 1 M HCl solution for 6 h; (c) SEM image of polished mild steel covered by stable GSLE-assemblies of 0.5 g/L contained in 1 M HCl solution for 6 h; (d) SEM micrograph of polished mild steel covered by stable GSLE-assemblies of 0.6 g/L in 1 M HCl solution for 6 h; (e) SEM micrograph of polished mild steel covered by stable GSLE-assemblies of 0.7 g/L in 1 M HCl solution for 6 h |
SEM images of mild steel specimens covered by stable GSLE-assemblies of different concentrations (formed at 10 h assembly) in 1 M HCl solution for 6 h are given in Figs. 11(c)-(e). For the metal covered by stable GSLE-assemblies of 0.5 g/L, the micrograms of the studied mild steel specimen surface show that the polished tracks can be still observed (Fig. 11(c)) after the mild is corroded by HCl solution for 6 h. However, as the metal surface is completely covered by stable GSLE-assemblies of 0.6 g/L, the nicks and lines cannot be found (Fig. 11(d)). Furthermore, the thick GSLE-assemblies adsorption film covered on the studied mild steel surfaces keeps stable after the metal is evenly corroded by 1 M HCl solution for 6 h. Besides, the abrasion marks together with scatter adsorption layer of stable GSLE-assemblies of 0.7 g/L on the studied steel specimen surface are observed after the metal is included in HCl solution for 6 h (Fig. 11(e)). The results suggest that more regular GSLE-assemblies of 0.6 g/L yield more robust protection layer and more notable corrosion inhibition in HCl solution than those of the other concentrations.
3.2.5 AFM analyses of the studied mild steel specimen surfacesThe three dimensional AFM images of polished steel surface, and the steel surface uncovered or covered by stable GSLE-assemblies included in 1 M HCl for 30 min are provided in Figs. 12(a)-(e), and the corresponding height profile graphs are given in Figs. 12(f)-(j). The polished mild steel shows smooth surface morphology (Fig. 12(a)), and the corresponding average roughness (Ra) value is 1.9 nm (Fig. 12(f)). In contrast, the corroded mild steel sample presents considerably rough structure with deep and large pits after it is corroded by aggressive acid medium (Fig. 12(b)), and the higher Ra value of 40.9 nm is also discovered (Fig. 12(g)).
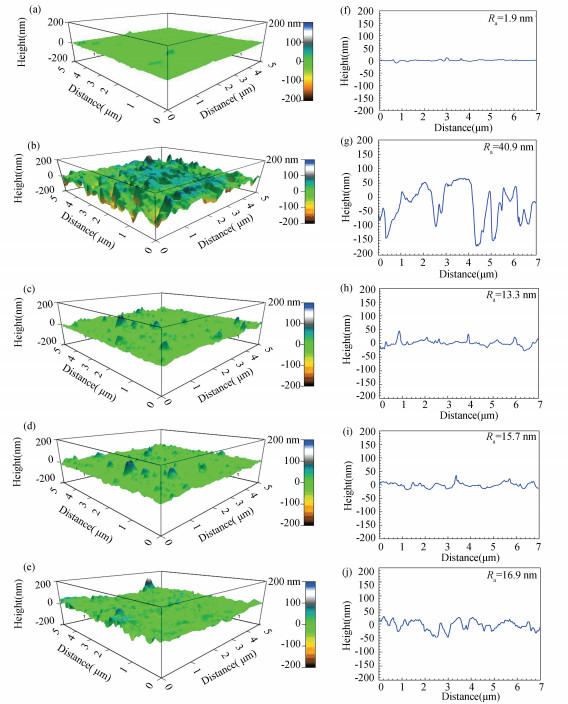
|
Fig.12 (a) AFM micrographs of the studied freshly polished mild steel specimen; (b) AFM micrographs of the studied polished mild steel specimen in 1 M HCl solution for 30 min; (c) AFM micrographs of the studied polished mild steel specimen surface covered by stable GSLE-assemblies of 0.6 g/L in 1 M HCl solution for 30 min; (d) AFM micrographs of the studied polished mild steel specimen surface covered by stable GSLE assemblies of 0.5 g/L in 1 M HCl solution for 30 min; (e) AFM micrographs of the studied polished mild steel specimen surface covered by stable GSLE-assemblies of 0.7 g/L in 1 M HCl solution for 30 min and (f)-(j) the corresponding height profile graphs of the studied mild steel specimen surfaces to (a)-(e), respectively |
It is found that the surveyed mild steel surface covered by stable GSLE-assemblies of 0.6 g/L is flat and smooth (Fig. 12(c)), and the Ra value is only 13.3 nm (Fig. 12(h)). On the other hand, the studied steel sample surfaces modified by stable GSLE-assemblies of 0.5 g/L and 0.7 g/L become rougher. The results reveal that the mild steel can be protected more efficiently by more orderly stable GSLE-assemblies of 0.6 g/L in harsh acid media.
3.3 Potentiodynamic Polarization Measurements of the Studied Mild Steel ElectrodesIn hydrochloric acid solution, the corrosion reaction of mild steel may be presented as the following Eqs. (3)-(9)[44]:
The anodic dissolution of Fe is
| $ \text{Fe + C}{{\text{l}}^{\text{-}}}\rightleftharpoons {{\text{(FeC}{{\text{l}}^{\text{-}}}\text{)}}_{\text{ads }\!\!~\!\!\text{ }}} $ | (3) |
| $ {{\text{(FeC}{{\text{l}}^{\text{-}}}\text{)}}_{\text{ads}}}\rightleftharpoons {{\left( \text{FeCl} \right)}_{\text{ads }\!\!~\!\!\text{ }}}\text{+ }{{\text{e}}^{\text{-}}}\text{ }\!\!~\!\!\text{ } $ | (4) |
| $ {\left( {{\rm{FeCl}}} \right)_{{\rm{ads }}{\rm{ }}}} \to {\rm{FeC}}{{\rm{l}}^{\rm{ + }}}{\rm{ }}{\rm{ + }}{{\rm{e}}^{{\rm{ - }}{\rm{ }}}} $ | (5) |
| $ \text{FeC}{{\text{l}}^{\text{+}}}\text{ }\!\!~\!\!\text{ }\rightleftharpoons \text{F}{{\text{e}}^{\text{2+}}}\text{ }\!\!~\!\!\text{ + C}{{\text{l}}^{\text{-}}} $ | (6) |
While the cathodic evolution of H2 is
| $ \text{Fe + }{{\text{H}}^{\text{+}}}\rightleftharpoons {{\text{(Fe}{{\text{H}}^{\text{+}}}\text{)}}_{\text{ }\!\!~\!\!\text{ ads }\!\!~\!\!\text{ }}} $ | (7) |
| $ {{\text{(Fe}{{\text{H}}^{\text{+}}}\text{)}}_{\text{ }\!\!~\!\!\text{ ads }\!\!~\!\!\text{ }}}\text{+ }{{\text{e}}^{\text{-}}}\to {{\left( \text{FeH} \right)}_{\text{ }\!\!~\!\!\text{ ads}}} $ | (8) |
| $ {{\left( \text{FeH} \right)}_{\text{ }\!\!~\!\!\text{ ads}}}\text{ }\!\!~\!\!\text{ + }{{\text{H}}^{\text{+ }\!\!~\!\!\text{ }}}\text{+ }{{\text{e}}^{\text{-}}}\text{ }\!\!~\!\!\text{ }\to \text{Fe +}{{\text{H}}_{\text{2}}} $ | (9) |
Fig. 13 shows the Tafel curves in 1 M HCl solutions for the bare mild steel electrodes (blank) and the stable GSLE-assemblies of different concentrations adsorbed mild steel electrodes. The results show that as the concentration of GSLE-assemblies increase from 0.1 to 0.6 g/L, the current densities of cathode and anode decrease. In particular, the reduction in anodic branch is more pronounced. The results suggest that stable GSLE-assemblies can inhibit anodic steel dissolution reaction and cathodic hydrogen evolution process at the same while, but the anodic reaction is suppressed more dramatically. In addition, as compared to the bare steel electrode, the variations of Ecorr values for stable GSLE-assemblies adsorbed steel electrodes are smaller than 85 mV, which indicates that stable GSLE-assemblies is a mixed-cathodic/anodic corrosion inhibitor for mild steel in 1 M HCl solution[45].
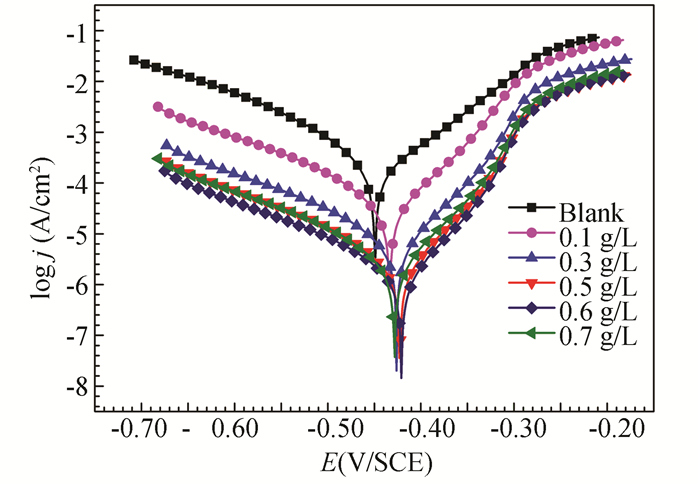
|
Fig.13 Tafel curves in 1 M HCl solutions for the investigated unmodified mild steel electrodes and the stable GSLE-assemblies of the different concentrations modified mild steel electrodes, respectively |
The electrochemical parameters including corrosion current density (jcorr), corrosion potential (Ecorr), anodic and cathodic Tafel slope (βa, βc) as well as inhibition efficiency (ηp) can be deduced from the polarization curves. Table 4 suggests that the jcorr decreases to the minimum and the ηp achieves the maximum at 0.6 g/L of the stable GSLE-assemblies. The results show that GSLE-assemblies protection layer adsorbed on the studied mild steel electrodes tends to be much tougher with increasing concentrations of the assemblies from 0.1 g/L to 0.6 g/L. Thus, the metal mild steel can be protected by 0.6 g/L of GSLE-assemblies more effectively in HCl solution. However, as the concentration of assemblies increases at 0.7 g/L, the corrosion inhibition efficiency (ηp) value shows a decrease. The results reveal that the produced disorderly GSLE-assemblies at 0.7 g/L may not be effectively adsorbed on steel surface, which can be caused by stronger steric hindrance and weaker chemisorption. Thus, the maximal inhibition efficiency of GSLE-assemblies obtained by polarization curves is around 95.0% at 0.6 g/L. It displays much greater anti-corrosion performance than many crude extracts of natural plants (Table S1).
| Table 4 Polarization parameters for the investigated unmodified and modified mild steel specimens by stable GSLE-assemblies of various concentrations in 1 M HCl solution |
| Table S1 The representative inhibition efficiency (η) detected by Tafel of GSLE-assemblies in the present work and the previous published corrosion inhibitor for copper in 1 M HCl |
3.4 Electrochemical Impedance Spectroscopy Measurements
Fig. 14(a) shows the Nyquist diagrams of the surveyed mild steel electrodes uncovered (blank) and covered by stable GSLE-assemblies in 1 M HCl solution. The acquired single depressed semicircle in impedance spectra suggests that corrosion of mild steel in HCl is primarily controlled by charge transfer process[46]. The diameters of semicircle increase with increasing concentrations of GSLE-assemblies from 0.1 g/L to 0.6 g/L, but the diameter decreases at 0.7 g/L. The results suggest that more robust protection layer of stable GSLE-assemblies of 0.6 g/L covered on steel surface yields stronger protection of steel in HCl solution.
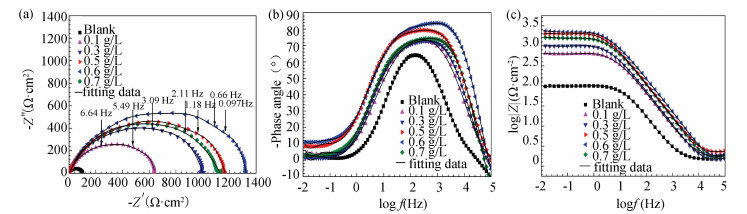
|
Fig.14 (a) Nyquist plots for the investigated naked mild steel electrodes modified by stable GSLE-assemblies of different concentrations; (b, c) Bode plots for the investigated naked mild steel electrodes and stable GSLE-assemblies of different concentrations modified mild steel electrodes |
Figs. 14(b)-(c) give the Bode plots of measured EIS. The results show that the impedance values in entire frequency range show an increase with enhancing concentrations of stable GSLE-assemblies, and the peak value is obtained at 0.6 g/L of GSLE-assemblies. In addition, the maximal phase angle in defined frequency range increases with increasing concentrations of stable GSLE-assemblies (0.1-0.6 g/L). However, the impedance and phase angle values show decrease at 0.7 g/L of stable GSLE-assemblies.
The impedance spectra can be analyzed by equivalent circuit (Fig. 15)[16]. The gained EIS parameters by fitting equivalent circuits are provided in Table 5. Herein, Rs refers to a solution resistance, Rct represents a charge transfer resistance. Besides, CPE is a constant phase angle element that is related to the double-layer capacitance (Cdl).
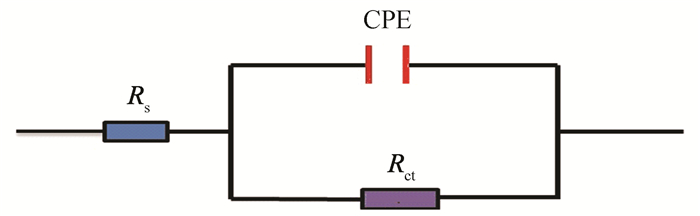
|
Fig.15 Equivalent circuit models fitting EIS experimental data for blank metal sample and stable GSLE-assemblies covered metal sample in this study |
| Table 5 Electrochemical impedance parameters for the studied unmodified and modified mild steel specimens by different concentrations of stable GSLE-assemblies in 1 M HCl solution |
The CPE impedance can be described by the Eq. (10)[47]:
| $ {{Z}_{\text{CPE}}}=\frac{1}{Y{{\left( \text{j}\omega \text{ } \right)}^{n}}} $ | (10) |
where Y is a magnitude of the CPE, ω represents an angular frequency, j shows an imaginary root, n is a deviation parameter relating to the phase shift. Furthermore, the capacitance values of Cdl could be calculated from the CPE parameter values of Y and n according to Eq. (11)[48]:
| $ {C_{{\rm{dl}}}} = \frac{{Y{\omega ^{n - 1}}}}{{{\rm{sin}}\left( {n\pi /2} \right)}} $ | (11) |
Table 5 suggests that Rct values increase with enhancing concentrations of stable GSLE-assemblies, which confirms that organic protection film is adsorbed on the studied mild steel surface. Furthermore, the Rct values of the studied GSLE-assemblies modified electrodes increase with the concentration increasing from 0.1 to 0.6 g/L, but the values decrease at a higher concentration (0.7 g/L). Hence, Rct reaches the peak value at 0.6 g/L of stable GSLE-assemblies along with the maximal ηE value (1337 Ω·cm2, 92.5%).
3.5 Adsorption Isotherm PlotsThe corrosion inhibition of metal in acidic medium by organic inhibitors can be resulted by formed organic adsorption layer on metal surface. The adsorption of organic inhibitors can be divided by physical adsorption or chemisorption[49]. The degree of surface coverage (θ) for the adsorbed stable GSLE-assemblies at various concentrations is determined by electrochemical experiments in 1 M HCl solution.
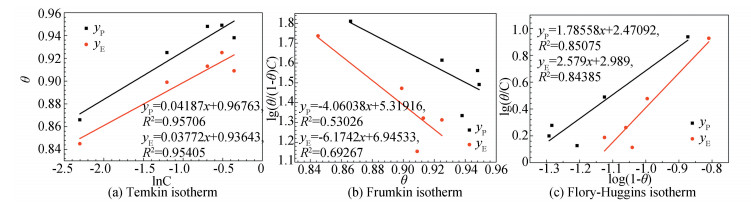
The function between surface coverage and isotherm can be expressed by Temkin (Eq. (12))[50], Frumkin (Eq. (13))[51], Flory-Huggins (Eq. (14))[52], or Langmuir adsorption isotherm models (Eq. (15))[53], respectively. The corresponding fitting results are shown in Fig. 16 and Fig. S6, which indicate that the Langmuir adsorption isotherm is the most suitable model with the linear correlation coefficient (R2) (RP2 =0.99963, RE2 =0.99952) which is approximately 1.0[54].
| $ \theta = \left( {1/f} \right){\rm{ln}}({K_{{\rm{ads}}}}C) $ | (12) |
| $ {\rm{lg}}\left( {\theta /\left( {1 - \theta } \right)C} \right) = {\rm{lg}}{K_{{\rm{ads}}}} + 2a\theta /2.303 $ | (13) |
| $ {\rm{log}}\left( {\theta /C} \right) = {\rm{log}}{K_{{\rm{ads}}}} + x{\rm{log}}(1 - \theta ) $ | (14) |
| $ \frac{c}{\theta } = \frac{1}{{{K_{{\rm{ads}}}}}} + c $ | (15) |
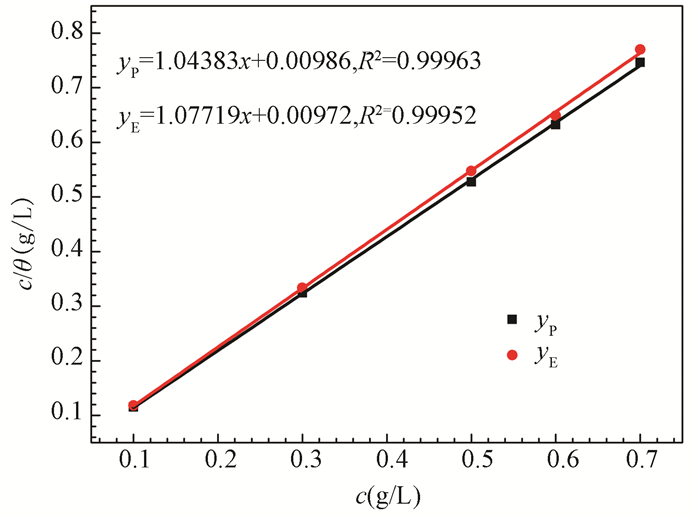
|
Fig.16 Langmuir adsorption isotherm of stable GSLE-assemblies on the studied mild steel specimen surfaces in 1 M HCl solution at 298 K (yP to Tafel curves, yE to electrochemical impedance spectroscopy) |
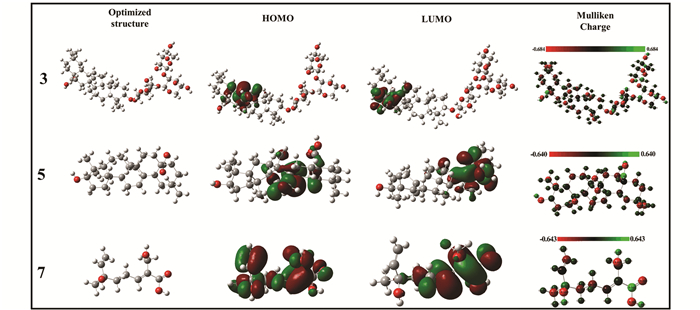
|
S5 Optimized structures, frontier orbital density distribution, and Mulliken charge of the molecules 3, 5 and 7 in the GSLE |
|
S6 (a) Temkin, (b)Frumkin and (c) Flory-Huggins adsorption isotherm of stable GSLE-assemblies on studied mild steel specimens surfaces in 1 M HCl solution at 298 K (yP to Tafel curves, yE to electrochemical impedance spectroscopy) |
where Kads is an equilibrium constant of adsorption, c shows a concentration of the studied GSLE-assemblies, f and a are parameters related to adsorbing interaction, and x is the number of inhibitor molecules occupying an active site (or meaning one water molecule replaced by one inhibitor molecule). Hence, the standard adsorption free energy ΔGads0 can be acquired by the following Eq. (16)[55]:
| $ \Delta G_{{\rm{ads}}}^0 = {\rm{ - }}RT{\rm{ln}}(1 \times {10^3}{K_{{\rm{ads}}}}) $ | (16) |
where R is the universal gas constant, and T shows the absolute temperature.
The adsorption can be considered as physisorption for ΔGads0 values of -20 kJ·mol-1 or less negative, while it may be chemisorption for ΔGads0 values of -40 kJ·mol-1 or more negative[56-57]. Table 6 suggests that the obtained ΔGads0 values are between -40 kJ·mol-1 and -20 kJ·mol-1, which indicates that the adsorption behavior of GSLE-assemblies on mild steel surface involves both the chemisorption and physisorption.
| Table 6 Thermodynamic parameters for adsorption of stable GSLE-assemblies in 1 M HCl solution at 298 K |
3.6 Molecular Modeling and Corrosion Inhibition Mechanism
Some representative chemicals 1, 3, 5, and 7 in GSLE (Fig. 1) were employed for molecular modeling to gain deep insight of chemical adsorption on mild steel surface. Fig. 17 and Fig. S5 present optimized structure, frontier orbital density distribution, and Mulliken charge of 1, 3, 5, and 7.
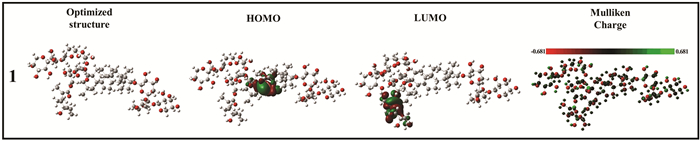
|
Fig.17 Optimized structures, frontier orbital density distribution, and Mulliken charge of 1 in GSLE |
As shown in Fig. 17, electron density distribution in HOMOs of 1 is mainly located at hexatomic rings. Meanwhile, electron density distribution in LUMOs is primarily concentrated in alkyl chain. In addition, Mulliken charge distribution shows that oxygen atoms in molecule 1 present large electronegative (red color)[58]. Hence, the results indicate that oxygen atoms can make major contribution to donation of electrons to steel surface. The similar conclusion may be drawn from molecular modeling of 3, 5, and 7 (Fig. S5).
Furthermore, the calculated values of EHOMO, ELUMO, and ΔE for 1, 3, 5, and 7 are shown in Fig. 18. It is noticed that 1 presents lower HOMO-LUMO energy gap than 3, 5, and 7, which suggests that 1 may display greater chemical chelating capability than the other constituents (3, 5, 7). Overall, the small HOMO-LUMO energy gap values of these molecules mean that a tough chemical coordination of these molecules with metal iron can occur[59], and thus a protection film of GSLE-assemblies may be yielded. Hence, mild steel can be protected well by stable GSLE-assemblies in hydrochloric acid solution.

|
Fig.18 Diagram of calculated parameters EHOMO, ELUMO, and ΔE for the molecules 1, 3, 5, and 7 in GSLE |
Based on the acquired experimental results and theoretical analysis, the possible adsorption mechanism and anti-corrosion mechanism may be proposed. The chemical adsorption of GSLE-assemblies on mild steel surface is confirmed. The XRD analysis further reveals that the corrosion products of GSLE-assemblies covered mild steel surface could be Fe(II) and Fe(III). Thus, the chemical adsorption of GSLE-assemblies on steel slab may be yielded by the complexes formation of ferrous and ferri ions. The molecular modeling suggests that the low values of HOMO-LUMO energy gaps of main chemical components in GSLE-assemblies are favorable for chemical interaction with mild steel surface, and distinct electronegative oxygen atoms in GSLE may make primary contribution to chemical chelation with iron ions. Thus, the stable GSLE-assemblies can produce robust protection layer on mild steel surface. The electrochemical determination reveals that GSLE-assemblies layer can suppress both cathodic and anodic reactions at the same time, thus neat anti-corrosion performance of mild steel in hydrochloric acid aqueous solution is achieved.
4 ConclusionsThis study proposes a new efficient strategy to seek renewable low-cost surfactant-like natural plant extracts as green organic inhibitors for mild steels in aggressive HCl solution. The crude Gleditsia sinensis lam extract (GSLE) was gained by mild condition, which could undergo self-assembly in mixed solvents of organic solvents/H2O such as ethanol/H2O solvent (v/v, 50/50). The yielded GSLE-assemblies could process strong chemical chelation with iron ions through O-contained functional groups, and a tough organic protection layer on the surveyed mild steel surface was formed. A mixed inhibiting cathodic & anodic reaction mechanism by stable GSLE-assemblies film is shown by electrochemical determination, and the anodic reaction inhibition is more pronounced. The stable GSLE-assemblies displayed good corrosion inhibition performance to mild steel in 1 M HCl solution. The chemical adsorption of GSLE-assemblies on steel surface prevailed in yielding organic protection layer, which was further confirmed by Langmuir adsorption model. The molecular modeling results further suggest that the small HOMO-LUMO energy gaps of the main chemical constituents in GSLE are favorable for chemical chelation with mild steel surface, and notable electronegative oxygen atoms can play significant role in chemical chelation with iron ions. The results presented herein would be beneficial for researchers to find out detergent-like natural plant extracts as environmentally friendly renewable corrosion inhibitors with large-scale application potentials for metals in harsh acid media. More renewable detergent-like natural plant extracts can be employed as efficient green corrosion inhibitors, which will be reported elsewhere in the near future.
AcknowledgmentsWe shall express our gratitude to Prof. Xinzheng Li at Peking University for his warm help in the molecular modeling. We also appreciate the great support from the Analytical and Testing Center of Chongqing University.
Supplementary MaterialsThe Supplementary Materials including chemical names of the molecules 1-8, Figs. S1-S6, and Table S1.

|
Molecule 1
3-((3, 4-dihydroxy-5-((5-hydroxy-4-((3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4, 5-dihydroxy-6-((((E)-6-hydroxy-2, 6-dimethylocta-2, 7-dienoyl)oxy)methyl)tetrahydro-2H-pyran-2-yl (6aS, 6bR, 10S, 12aR)-10-(((2R, 3R, 4S, 5S, 6R)-6-((((2S, 3R, 4S, 5R)-4, 5-dihydroxy-3-(((2S, 3R, 4S, 5R)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)methyl)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-2, 2, 6a, 6b, 9, 9, 12a-heptamethyl-1, 3, 4, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-octadecahydropicene-4a(2H)-carboxylate

|
Molecule 2
3-((3, 4-dihydroxy-5-((5-hydroxy-4-((3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4, 5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl (6aS, 6bR, 10S, 12aR)-10-(((2R, 3R, 4S, 5S, 6R)-6-((((2S, 3R, 4S, 5R)-4, 5-dihydroxy-3-(((2S, 3R, 4S, 5R)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)methyl)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-2, 2, 6a, 6b, 9, 9, 12a-heptamethyl-1, 3, 4, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-octadecahydropicene-4a(2H)-carboxylate

|
Molecule 3
(4aS, 6aS, 6bR, 10S, 12aR)-10-(((2R, 3R, 4S, 5S, 6R)-6-((((2S, 3R, 4S, 5R)-4, 5-dihydroxy-3-(((2S, 3R, 4S, 5R)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)methyl)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-2, 2, 6a, 6b, 9, 9, 12a-heptamethyl-1, 3, 4, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-octadecahydropicene-4a(2H)-carboxylic acid
Molecule 4
(4aR, 5R, 6aS, 6bR, 10S, 12aR)-10-(((2R, 3R, 4S, 5S, 6R)-6-((((2S, 3R, 4S, 5R)-4, 5-dihydroxy-3-(((2S, 3R, 4S, 5R)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)methyl)-3, 4, 5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-5-hydroxy-2, 2, 6a, 6b, 9, 9, 12a-heptamethyl-1, 3, 4, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-octadecahydropicene-4a(2H)-carboxylic acid

|
Molecule 5
(4aS, 6aS, 6bR, 10S, 12aR)-10-hydroxy-2, 2, 6a, 6b, 9, 9, 12a-heptamethyl-1, 3, 4, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-octadecahydropicene-4a(2H)-carboxylic acid
Molecule 6
(4aR, 5R, 6aS, 6bR, 10S, 12aR)-5, 10-dihydroxy-2, 2, 6a, 6b, 9, 9, 12a-heptamethyl-1, 3, 4, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-octadecahydropicene-4a(2H)-carboxylic acid
|
Molecule 7
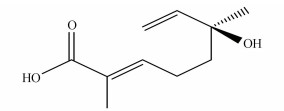
(S, E)-6-hydroxy-2, 6-dimethylocta-2, 7-dienoic acid
|
Molecule 8
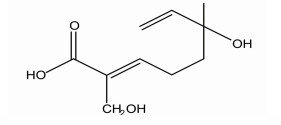
(E)-6-hydroxy-2-(hydroxymethyl)-6-methylocta-2, 7-dienoic acid
| [1] |
Wang H M, Akid R. Encapsulated cerium nitrate inhibitors to provide high-performance anti-corrosion sol-gel coatings on mild steel. Corrosion Science, 2008, 50(4): 1142-1148. DOI:10.1016/j.corsci.2007.11.019 (  0) 0) |
| [2] |
Fayomi O S I, Popoola A P I. An investigation of the properties of Zn coated mild steel. International Journal of Electrochemical Science, 2012, 7(7): 6555-6570. (  0) 0) |
| [3] |
Bayol E, Gürten A A, Dursun M, et al. Adsorption behavior and inhibition corrosion effect of sodium carboxymethyl cellulose on mild steel in acidic medium. Acta Physico-Chimica Sinica, 2008, 24(12): 2236-2243. DOI:10.1016/S1872-1508(08)60085-6 (  0) 0) |
| [4] |
Goyal M, Kumar S, Bahadur I, et al. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: a review. Journal of Molecular Liquids, 2018, 256: 565-573. DOI:10.1016/j.molliq.2018.02.045 (  0) 0) |
| [5] |
Musa A Y, Kadhum A A H, Mohamad A B, et al. Electrochemical and quantum chemical calculations on 4, 4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. Journal of Molecular Structure, 2010, 969(1-3): 233-237. DOI:10.1016/j.molstruc.2010.02.051 (  0) 0) |
| [6] |
Zhang D Q, Tang Y M, Qi S J, et al. The inhibition performance of long-chain alkyl-substituted benzimidazole derivatives for corrosion of mild steel in HCl. Corrosion Science, 2016, 102: 517-522. DOI:10.1016/j.corsci.2015.10.002 (  0) 0) |
| [7] |
Hashim N Z N, Anouar E H, Kassim K, et al. XPS and DFT investigations of corrosion inhibition of substituted benzylidene Schiff bases on mild steel in hydrochloric acid. Applied Surface Science, 2019, 476: 861-877. DOI:10.1016/j.apsusc.2019.01.149 (  0) 0) |
| [8] |
Singh P, Ebenso E E, Olasunkanmi L O, et al. Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. The Journal of Physical Chemistry C, 2016, 120(6): 3408-3419. DOI:10.1021/acs.jpcc.5b11901 (  0) 0) |
| [9] |
Kharbach Y, Qachchachi F Z, Haoudi A, et al. Anticorrosion performance of three newly synthesized isatin derivatives on carbon steel in hydrochloric acid pickling environment: electrochemical, surface and theoretical studies. Journal of Molecular Liquids, 2017, 246: 302-316. DOI:10.1016/j.molliq.2017.09.057 (  0) 0) |
| [10] |
Verma C, Olasunkanmi L O, Ebenso E E, et al. Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. The Journal of Physical Chemistry C, 2016, 120(21): 11598-11611. DOI:10.1021/acs.jpcc.6b04429 (  0) 0) |
| [11] |
Al-Amiery A A, Kassim F A B, Kadhum A A H, et al. Synthesis and characterization of a novel eco-friendly corrosion inhibition for mild steel in 1 M hydrochloric acid. Scientific Reports, 2016, 6: 19890. DOI:10.1038/srep19890 (  0) 0) |
| [12] |
Satapathy A K, Gunasekaran G, Sahoo S C, et al. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corrosion Science, 2009, 51(12): 2848-2856. DOI:10.1016/j.corsci.2009.08.016 (  0) 0) |
| [13] |
Mehdipour M, Ramezanzadeh B, Arman S Y. Electrochemical noise investigation of Aloe plant extract as green inhibitor on the corrosion of stainless steel in 1 M H 2 SO 4. Journal of Industrial and Engineering Chemistry, 2015, 21: 318-327. DOI:10.1016/j.jiec.2014.02.041 (  0) 0) |
| [14] |
Alvarez P E, Fiori-Bimbi M V, Neske A, et al. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. Journal of Industrial and Engineering Chemistry, 2018, 58: 92-99. DOI:10.1016/j.jiec.2017.09.012 (  0) 0) |
| [15] |
Qiang Y J, Zhang S T, Tan B C, et al. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corrosion Science, 2018, 133: 6-16. DOI:10.1016/j.corsci.2018.01.008 (  0) 0) |
| [16] |
Ji G, Anjum S, Sundaram S, et al. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corrosion Science, 2015, 90: 107-117. DOI:10.1016/j.corsci.2014.10.002 (  0) 0) |
| [17] |
Deyab M A. Egyptian licorice extract as a green corrosion inhibitor for copper in hydrochloric acid solution. Journal of Industrial and Engineering Chemistry, 2015, 22: 384-389. DOI:10.1016/j.jiec.2014.07.036 (  0) 0) |
| [18] |
Odewunmi N A, Umoren S A, Gasem Z M. Utilization of watermelon rind extract as a green corrosion inhibitor for mild steel in acidic media. Journal of Industrial and Engineering Chemistry, 2015, 21: 239-247. DOI:10.1016/j.jiec.2014.02.030 (  0) 0) |
| [19] |
Li X H, Deng S D, Fu H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corrosion Science, 2012, 62: 163-175. DOI:10.1016/j.corsci.2012.05.008 (  0) 0) |
| [20] |
Al-Sabagh A M, Nasser N M, Khamis E A, et al. Synthesis of non-ionic surfactants based on alkylene diamine and evaluation of their corrosion inhibition efficiency on carbon steel in formation water. Egyptian Journal of Petroleum, 2017, 26(1): 41-51. DOI:10.1016/j.ejpe.2016.03.001 (  0) 0) |
| [21] |
Mobin M, Aslam R, Zehra S, et al. Bio-/Environment-friendly cationic gemini surfactant as novel corrosion inhibitor for mild steel in 1 M HCl solution. Journal of Surfactants and Detergents, 2016, 20: 57-74. DOI:10.1007/s11743-016-1904-x (  0) 0) |
| [22] |
Fouda A S, El-Askalany A, El-Habab A T, et al. Anticorrosion properties of some nonionic surfactants on carbon steel in 1 M HCl environment. Journal of Bio-and Tribo-Corrosion, 2019, 5: 56. DOI:10.1007/s40735-019-0248-2 (  0) 0) |
| [23] |
El Achouri M, Kertit S, Gouttaya H M, et al. Corrosion inhibition of iron in 1 M HCl by some gemini surfactants in the series of alkanediyl-α, ω-bis-(dimethyl tetradecyl ammonium bromide). Progress in Organic Coatings, 2001, 43(4): 267-273. DOI:10.1016/S0300-9440(01)00208-9 (  0) 0) |
| [24] |
Huang H, Fu Y, Wang X, et al. Nano- to micro-self-aggregates of new bisimidazole-based copoly(ionic liquid)s for protecting copper in aqueous sulfuric acid solution. ACS Applied Materials & Interfaces, 2019, 11(10): 10135-10145. DOI:10.1021/acsami.8b1999 (  0) 0) |
| [25] |
Huang H J, Fu Y, Li F H, et al. Orderly self-assembly of new ionic copolymers for efficiently protecting copper in aggressive sulfuric acid solution. Chemical Engineering Journal, 2020, 384: 123293. DOI:10.1016/j.cej.2019.123293 (  0) 0) |
| [26] |
Wang Z Q, Gong Y L, Zhang L, et al. Self-assembly of new dendrimers basing on strong π-π intermolecular interaction for application to protect copper. Chemical Engineering Journal, 2018, 342: 238-250. DOI:10.1016/j.cej.2018.02.080 (  0) 0) |
| [27] |
Xing Y, Ji L, Liu Z P, et al. Effects of Gleditsia saponin on high-solids enzymatic hydrolysis of furfural residues. Industrial Crops and Products, 2015, 64: 209-214. DOI:10.1016/j.indcrop.2014.09.055 (  0) 0) |
| [28] |
Zhang Z Z, Koike K, Jia Z H, et al. Four new triterpenoidal saponins acylated with one monoterpenic acid from Gleditsia sinensis. Journal of Natural Products, 1999, 62(5): 740-745. DOI:10.1021/np980441k (  0) 0) |
| [29] |
Zhang Z Z, Koike K, Jia Z H, et al. Triterpenoidal saponins acylated with two monoterpenic acids from Gleditsia sinensis. Chemical & Pharmaceutical Bulletin, 1999, 47(3): 388-393. DOI:10.1248/cpb.47.388 (  0) 0) |
| [30] |
Chidiebere M A, Oguzie E E, Liu L, et al. Ascorbic acid as corrosion inhibitor for Q235 mild steel in acidic environments. Journal of Industrial and Engineering Chemistry, 2015, 26: 182-192. DOI:10.1016/j.jiec.2014.11.029 (  0) 0) |
| [31] |
Mallaiya K, Subramaniam R, Srikandan S S, et al. Electrochemical characterization of the protective film formed by the unsymmetrical Schiff's base on the mild steel surface in acid media. Electrochimica Acta, 2011, 56(11): 3857-3863. DOI:10.1016/j.electacta.2011.02.036 (  0) 0) |
| [32] |
Frisch M, Trucks G, Schlegel H, et al. Gaussian 09, Revision A. 02. Wallingford, CT: Gaussian, Inc. 2009.
(  0) 0) |
| [33] |
Gece G. The use of quantum chemical methods in corrosion inhibitor studies. Corrosion Science, 2008, 50(11): 2981-2992. DOI:10.1016/j.corsci.2008.08.043 (  0) 0) |
| [34] |
Bhattacharjee S. DLS and zeta potential-What they are and what they are not?. Journal of Control Release, 2016, 235: 337-351. DOI:10.1016/j.jconrel.2016.06.017 (  0) 0) |
| [35] |
Cai K, Xie J J, Zhao D H. NIR J-aggregates of hydroazaheptacene tetraimides. Journal of the American Chemical Society, 2014, 136(1): 28-31. DOI:10.1021/ja410265n (  0) 0) |
| [36] |
M'hiri N, Veys-Renaux D, Rocca E, et al. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corrosion Science, 2016, 102: 55-62. DOI:10.1016/j.corsci.2015.09.017 (  0) 0) |
| [37] |
Lebrini M, Robert F, Lecante A, et al. Corrosion inhibition of C38 steel in 1M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corrosion Science, 2011, 53(2): 687-695. DOI:10.1016/j.corsci.2010.10.006 (  0) 0) |
| [38] |
O. Cozar, N. Leopold, C. Jelic, et al. IR, Raman and surface-enhanced Raman study of desferrioxamine B and its Fe(Ⅲ) complex, ferrioxamine B. Journal of Molecular Structure, 2006, 788(1-3): 1-6. DOI:10.1016/j.molstruc.2005.04.035 (  0) 0) |
| [39] |
Ituen E, Akaranta O, James A. Evaluation of performance of corrosion inhibitors using adsorption isotherm models: an overview. Chemical Science International Journal, 2017, 18(1): 1-34. DOI:10.9734/CSJI/2017/28976 (  0) 0) |
| [40] |
Boumhara K, Tabyaoui M, Jama C, et al. Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1M HCl solution: electrochemical and XPS investigations. Journal of Industrial and Engineering Chemistry, 2015, 29: 146-155. DOI:10.1016/j.jiec.2015.03.028 (  0) 0) |
| [41] |
Bouanis M, Tourabi M, Nyassi A, et al. Corrosion inhibition performance of 2, 5-bis(4-dimethylaminophenyl)-1, 3, 4-oxadiazole for carbon steel in HCl solution: gravimetric, electrochemical and XPS studies. Applied Surface Science, 2016, 389: 952-966. DOI:10.1016/j.apsusc.2016.07.115 (  0) 0) |
| [42] |
Abboud Y, Hammouti B, Abourriche A, et al. 5-Naphthylazo-8-hydroxyquinoline (5NA8HQ) as a novel corrosion inhibitor for mild steel in hydrochloric acid solution. Research on Chemical Intermediates, 2012, 38: 1591-1607. DOI:10.1007/s11164-012-0486-0 (  0) 0) |
| [43] |
Hussin M H, Rahim A A, Ibrahim M N Mohamad, et al. The capability of ultrafiltrated alkaline and organosolv oil palm (Elaeis guineensis) fronds lignin as green corrosion inhibitor for mild steel in 0.5 M HCl solution. Measurement, 2016, 78: 90-103. DOI:10.1016/j.measurement.2015.10.007 (  0) 0) |
| [44] |
Mazumder M A J, Al-Muallem H A, Faiz M, et al. Design and synthesis of a novel class of inhibitors for mild steel corrosion in acidic and carbon dioxide-saturated saline media. Corrosion Science, 2014, 87: 187-198. DOI:10.1016/j.corsci.2014.06.026 (  0) 0) |
| [45] |
Hussin M H, Jain Kassim M, Razali N N, et al. The effect of Tinospora crispa extracts as a natural mild steel corrosion inhibitor in 1M HCl solution. Arabian Journal of Chemistry, 2016, 9(Supplement 1): S616-S624. DOI:10.1016/j.arabjc.2011.07.002 (  0) 0) |
| [46] |
Ostovari A, Hoseinieh S M, Peikari M, et al. Corrosion inhibition of mild steel in 1M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corrosion Science, 2009, 51(9): 1935-1949. DOI:10.1016/j.corsci.2009.05.024 (  0) 0) |
| [47] |
Siǧircık G, Yildirim D, Tüken T. Synthesis and inhibitory effect of N, N'-bis(1-phenylethanol)ethylenediamine against steel corrosion in HCl Media. Corrosion Science, 2017, 120: 184-193. DOI:10.1016/j.corsci.2017.03.003 (  0) 0) |
| [48] |
Li L J, Zhang X P, Lei J L, et al. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corrosion Science, 2012, 63: 82-90. DOI:10.1016/j.corsci.2012.05.026 (  0) 0) |
| [49] |
Solomon M M, Umoren S A. In-situ preparation, characterization and anticorrosion property of polypropylene glycol/silver nanoparticles composite for mild steel corrosion in acid solution. Journal of Colloid and Interface Science, 2016, 462: 29-41. DOI:10.1016/j.jcis.2015.09.057 (  0) 0) |
| [50] |
Ituen E, Akaranta O, James A, et al. Green and sustainable local biomaterials for oilfield chemicals: Griffonia simplicifolia extract as steel corrosion inhibitor in hydrochloric acid. Sustainable Materials and Technologies, 2017, 11: 12-18. DOI:10.1016/j.susmat.2016.12.001 (  0) 0) |
| [51] |
Ghanbari A, Attar M M, Mahdavian M. Corrosion inhibition performance of three imidazole derivatives on mild steel in 1M phosphoric acid. Materials Chemistry and Physics, 2010, 124(2-3): 1205-1209. DOI:10.1016/j.matchemphys.2010.08.058 (  0) 0) |
| [52] |
Oguzie E E, Unaegbu C, Ogukwe C N, et al. Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives. Materials Chemistry and Physics, 2004, 84(2-3): 363-368. DOI:10.1016/j.matchemphys.2003.11.027 (  0) 0) |
| [53] |
Loto R T, Loto C A, Joseph O, et al. Adsorption and corrosion inhibition properties of thiocarbanilide on the electrochemical behavior of high carbon steel in dilute acid solutions. Results in Physics, 2016, 6: 305-314. DOI:10.1016/j.rinp.2016.05.013 (  0) 0) |
| [54] |
Biswas A, Pal S, Udayabhanu G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Applied Surface Science, 2015, 353: 173-183. DOI:10.1016/j.apsusc.2015.06.128 (  0) 0) |
| [55] |
Chauhan D S, Ansari K R, Sorour A A, et al. Thiosemicarbazide and thiocarbohydrazide functionalized chitosan as ecofriendly corrosion inhibitors for carbon steel in hydrochloric acid solution. International Journal of Biological Macromolecules, 2018, 107(Part B): 1747-1757. DOI:10.1016/j.ijbiomac.2017.10.050 (  0) 0) |
| [56] |
Hu Z Y, Meng Y B, Ma X M, et al. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl. Corrosion Science, 2016, 112: 563-575. DOI:10.1016/j.corsci.2016.08.012 (  0) 0) |
| [57] |
Obot I B, Obi-Egbedi N O. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corrosion Science, 2010, 52(1): 198-204. DOI:10.1016/j.corsci.2009.09.002 (  0) 0) |
| [58] |
Fonseca Guerra C, Handgraaf J W, Baerends E J, et al. Voronoi deformation density (VDD) charges: assessment of the Mulliken, Bader, Hirshfeld, Weinhold, and VDD methods for charge analysis. Journal of Computational Chemistry, 2004, 25(2): 189-210. DOI:10.1002/jcc.10351 (  0) 0) |
| [59] |
Perepichka D F, Bryce M R. Molecules with exceptionally small HOMO-LUMO gaps. Angewandte Chemie (International ed. in English), 2005, 44(34): 5370-5373. DOI:10.1002/anie.200500413 (  0) 0) |
| [60] |
Ostovari A, Hoseinieh S M, Peikari M, et al. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corrosion Science, 2009, 51(9): 1935-1949. DOI:10.1016/j.corsci.2009.05.024 (  0) 0) |
| [61] |
Chidiebere M A, Ogukwe C E, Oguzie K L, et al. Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: experimental and theoretical studies. Industrial & Engineering Chemistry Research, 2012, 51(2): 668-677. (  0) 0) |
| [62] |
Oguzie E E, Enenebeaku C K, Akalezi C O, et al. Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. J Colloid Interface Sci, 2010, 349(1): 283-292. DOI:10.1016/j.jcis.2010.05.027 (  0) 0) |
| [63] |
Umoren S A, Gasem Z M, Obot I B. Natural products for material protection: inhibition of mild steel corrosion by Date palm seed extracts in acidic media. Industrial & Engineering Chemistry Research, 2013, 52(42): 14855-14865. (  0) 0) |
| [64] |
Oguzie E E, Oguzie K L, Akalezi C O, et al. Natural products for materials protection: corrosion and microbial growth inhibition using Capsicum frutescens biomass extracts. ACS Sustainable Chemistry & Engineering, 2012, 1(2): 214-225. (  0) 0) |
| [65] |
Umoren S A, Obot I B, Gasem Z M. Adsorption and corrosion inhibition characteristics of strawberry fruit extract at steel/acids interfaces: experimental and theoretical approaches. Ionics, 2014, 21(4): 1171-1186. (  0) 0) |
| [66] |
Oguzie E E, Iheabunike Z O, Oguzie K L, et al. Corrosion inhibiting effect of Aframomum melegueta extracts and adsorption characteristics of the active constituents on mild steel in acidic media. Journal of Dispersion Science and Technology, 2013, 34(4): 516-527. DOI:10.1080/01932691.2012.682008 (  0) 0) |
| [67] |
Cang Hui, Fei Zhenghao, Xiao Hairong, et al. Inhibition effect of Reed leaves extract on steel in hydrochloric acid and sulphuric acid solutions. International Journal of Electrochemical Science, 2012, 7: 8869-8882. (  0) 0) |
| [68] |
Akalezi C, Enenebaku C, Okolue B, et al. Potentials of Hyppocratea pallens planch leave extract as inhibition towards the corrosion of mild steel in acidic media. Scholars Research Library, 2012, 4(3): 1195-1205. (  0) 0) |
 2020, Vol. 27
2020, Vol. 27


