Nitrogen removal from wastewater is imperative because it may cause eutrophication of rivers. Conventional biological nitrogen removal processes, such as anoxic/oxic process and sequencing batch reactor, are mainly based on the nitrification-denitrification mechanism[1]. Namely, ammonia in wastewater is converted to nitrate by nitrobacteria in aerobic phase, and then the nitrate is converted to gaseous nitrogen by denitrificans in anoxic phase. In nitrification-denitrification process, it is necessary to keep a long sludge retention time to maintain a sufficient quantity of nitrifying bacteria and denitrifying bacteria in the reactor for the nitrification and denitrification reactions, which will increase the volume of the structure[2]. In addition, the nitrifying bacteria with poor flocculability would flow out with the outflow of the sedimentation tank, causing further reduction of nitrifying bacteria and affecting the final total nitrogen (TN) removal rate.
Anoxic/oxic-membrane bioreactor (A/O-MBR) process is a promising process for nitrogen removal in wastewater[3-4]. It is constructed by the incorporation of membrane filtration and anoxic/oxic process. The HRT and SRT of A/O-MBR can be independently controlled through intercepting microorganisms by membrane. The mixed liquor suspended solids (MLSS) in A/O-MBR are higher than that of the traditional nitrogen removal technology. Compared with traditional nitrogen removal technology, A/O-MBR has high nitrogen removal rate, small sludge yield, and is simple to operate[5]. Dissolved oxygen in aerobic tank (DO), internal mixed liquor recycle rate (IR), and MLSS are the three most significant influencing factors of A/O-MBR for TN removal. DO determines the activities of nitrifying bacteria and denitrifying bacteria, IR determines the amount of nitrate recycled to anoxic tank, and MLSS determines the quantity of nitrifying bacteria and denitrifying bacteria[5-6]. Tan and Ng[4] investigated the influences of DO and IR on the TN removal rate of A/O-MBR, and found that the TN removal rate is jointly affected by DO and IR. Xia et al.[6] found that TN removal efficiency decreases when SRT decreases from 10 d to 3 d, and the decrease of biomass concentration with decreasing SRT may be the main reason that leads to the decrease of TN removal efficiency. Nevertheless, these previous researchers adopted "one variable at a time" to investigate the effects of operating condition on the TN removal rate of A/O-MBR without evaluating the interactive effect of various operating conditions[7]. Therefore, it is requisite to study the interactive influence of operating conditions on the TN treatment efficiency of A/O-MBR.
Response surface methodology (RSM) is a valid statistical method which provides a systematic and effective policy to investigate the interactions of various operating conditions that influence the process[8-9]. It has been extensively employed to optimize the operating condition in wastewater disposal[10-11]. As far as we know, investigation on the interactive influences of operating conditions on the TN removal efficiency of A/O-MBR by RSM has been rarely reported.
In this study, the influence rules of DO, IR, and MLSS on the TN removal rate of A/O-MBR were separately investigated. Then, RSM was used to study the interactive influences of MLSS, IR, and DO on the TN removal efficiency, and to optimize the operating conditions for TN removal. It is anticipated that the optimum condition from this study will provide guidance for the application of A/O-MBR in the future.
2 Materials and Methods 2.1 A/O-MBR System and WastewaterA bench scale A/O-MBR with a working volume of 3.0 L anoxic tank (A) and 9.0 L oxic tank (O) was operated, which was made of plexiglass (48 cm in length, 12 cm in width, and 30 cm in height) (as shown in Fig. 1).
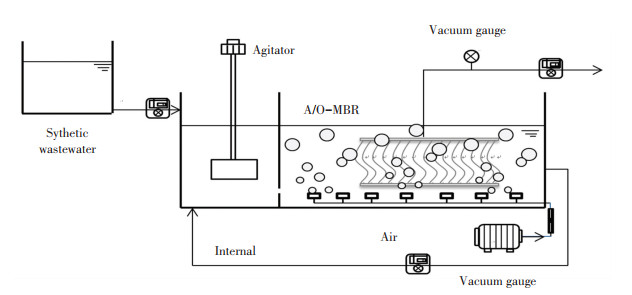
|
Fig.1 Schematic of A/O-MBR |
A membrane module was fixed in the oxic tank, and the membrane flux was set as 5.4 L/(m2·h) by a suction pump with 8 min "on" and 2 min "off". One agitator was placed in each of the anoxic tank to keep sludge mixing. One aeration system was installed underneath the membrane to maintain the DO concentration as experiment needed and the sludge in a suspended state. The operating temperature was maintained at around 23-25 ℃ using a heating element. HRT was 9.26 h. MLSS was controlled as experiment needed by wasting a certain volume of sludge mixture from the oxic tank of A/O-MBR once a day. Except for DO, IR, and MLSS, which were adjusted according to the requirement during the operation of A/O-MBR, other operating conditions remained consistent throughout the study.
Prior to the experiment commencement, the A/O-MBR was firstly run for 60 d for sludge acclimatization.
The inoculated sludge was obtained from Harbin Wenchang sewage treatment plant. The influent is synthetic wastewater, whose composition is shown in Table 1.
| Table 1 Composition of synthetic wastewater |
2.2 Operation of A/O-MBR
After acclimatization, A/O-MBR was operated under different concentrations of DO (1.5 mg/L, 3.0 mg/L, 4.5 mg/L, and 6.0 mg/L in turn) with 300.0% IR and 6000.0 mg/L MLSS. Then, the A/O-MBR was operated at different IRs (150.0%, 450.0%, and 600.0% in turn) with 3.0 mg/L DO and 6000.0 mg/L MLSS. Furthermore, different MLSSs (3000.0 mg/L, 9000.0 mg/L, and 12000.0 mg/L) were conducted with 3.0 mg/L DO and 300.0% IR. After that, a Box-Behnken design[12-13] was conducted for exploring the interactive influences of variables on the response. DO, IR, and MLSS were selected as independent variables, and TN removal efficiency was selected as dependent variable. Influences of DO, IR, and MLSS on TN removal were studied at high (+1), middle (0), and low (-1) levels (Table 2)[14].
| Table 2 Variables and levels employed for optimization |
Seventeen experiments (as shown in Table 3) were conducted randomly according to the Box-Behnken design to avoid system error.
| Table 3 Response surface at various operating conditions |
Each experiment was conducted for over 10 d. Before each test, the A/O-MBR was operated for at least 30 d to ensure the stability of the system. During each experiment, the samples were collected periodically from the corresponding sampling ports for analysis.
The experimental values were analyzed employing the quadratic polynomial model as follows[15]:
| $ \begin{gathered} Y = {\text{ }}{A_0} + {A_1}{x_1} + {A_2}{x_2} + {A_3}{x_3} + {A_{11}}{x_1}^2 + {A_{22}}{x_2}^2 + \hfill \\ {A_{33}}{x_3}^2 + {A_{12}}{x_1}{x_2} + {A_{13}}{x_1}{x_3} + {A_{23}}{x_2}{x_3} \hfill \\ \end{gathered} $ | (1) |
where Y is the predicted TN removal efficiency. x1, x2, and x3 are the coded value of DO, IR, and MLSS, respectively. A0 is a constant, A1, A2, and A3 are the linear coefficient, A11, A22, and A33 are the quadratic coefficient, and A12, A13, and A23 are the interaction coefficient[16]. The quadratic polynomial coefficients were obtained through the Design Expert software.
2.3 Sampling and Analytical MethodsCOD, NH4+-N, NO2--N, TP, and MLSS were analyzed based on the Standard Methods[17]. TN concentration was analyzed with a TN analyzer (TOC-5000A, Shimadzu, Japan).
3 Results and Discussion 3.1 Influence Rules of DO, IR, and MLSS on TN RemovalThe influence rules of the three significant operating variables (i.e., DO, IR, and MLSS) on the TN removal of A/O-MBR were investigated separately.
The TN removal efficiency first increased and then decreased with the increase of DO from 1.5 mg/L to 6.0 mg/L (Fig. 2(a)). In this study, the nitrification was not limited because the NH4+-N and NO2--N contents are very low in the effluent. Thus, the difference of the TN removal efficiency under different DOs was mainly caused by the denitrification process. When DO was increased from 1.5 mg/L to 6.0 mg/L, the dissolved oxygen in the anoxic tank was found increased from 0.1 mg/L to 0.7 mg/L. Under a high DO condition, IR would carry more dissolved oxygen to the anoxic tank and consume the COD in the anoxic tank, resulting in less COD for denitrification[5]. It was verified in the experiment that when the DO was increased from 1.5 mg/L to 6.0 mg/L, the COD concentration in anaerobic tank was decreased from 55.3 mg/L to 49.1 mg/L. Meanwhile, DO in anoxic tank would kinetically affect the denitrifying bacteria[4]. Therefore, when DO was at 3.0 mg/L, the TN removal rate was higher than those of other DO conditions.

|
Fig.2 TN removal efficiency with different conditions |
Changes of the TN removal efficiency with IRs showed similar trend with DOs (Fig. 2(b)). When IR was low, the NO3--N in the aerobic tank could not be timely reduced to nitrogen and led to the low TN removal rate. There are two reasons that result in the low TN removal efficiency under high IR. First, the anoxic environment in the anoxic tank was destroyed, which in turn affected the activity of denitrifying bacteria. This was verified when the IR was 300% and 600%, the DO in the anoxic tank was found to be 0.3 mg/L and 0.5 mg/L, respectively. Second, high NO3--N concentration in IR would exceed the reducing capacity of the denitrifying bacteria in the anoxic tank and could not be reduced adequately, which would affect the final TN removal efficiency. When the IR was 300%, the TN removal efficiency was 79.63%.
Effect of MLSS on the TN removal efficiency with 3.0 mg/L DO and 300.0% IR is presented in Fig. 2(c). Results showed that the TN removal rate first increased and then decreased with increasing MLSS. Under low MLSS, there are two reasons leading to the low TN removal efficiency. For one thing, there were not enough denitrifying bacteria to reduce the nitrate which could affect TN removal. For another, the dissolved oxygen in IR could not be consumed timely, resulting in the high dissolved oxygen concentration in the anoxic tank and in turn affecting the activity of denitrifying bacteria. It was proved that when the MLSS was increased from 3000.0 mg/L to 12000.0 mg/L, the dissolved oxygen in the anaerobic tank was found decreased from 0.4 mg/L to 0.1 mg/L. Under high MLSS, the SMP concentration in the sludge was high, which would affect the activities of nitrifying bacteria and denitrifying bacteria, leading to the decrease of the final TN removal efficiency[18].
The above studies implied that DO, IR, and MLSS influence the TN removal efficiency of A/O-MBR. Furthermore, these factors had substantial relationship, making it imperative to optimize them for improving the TN removal rate.
3.2 Analysis of Variance and Model FittingThe optimum operating condition and the interactive influences of DO, IR, and MLSS on the TN removal rate were investigated using the Box-Behnken design of RSM as shown in Table 3. In order to obtain the optimum operating condition to realize the maximum TN removal rate, a quadratic polynomial model was built and is expressed as
| $ \begin{array}{l} Y = {\rm{ }}80.04 + 2.24A + 5.84B + 4.87C + 1.57AB\\ \;\;\;\;\; + 5.40AC + 2.15BC - 10.73{A^2} - 8.03{B^2} - \\ \;\;\;\;\;5.86{C^2} \end{array} $ | (2) |
The A, B, C in Eq.(2) were coded as (-1, 0, 1). Analysis of variance (ANOVA) of mode for the TN removal rate was completed and the results are shown in Table 4.
| Table 4 ANOVA of response surface quadratic model for TN removal efficiency |
The F-value for TN removal was 182.44 and the probability value was less than 0.0001, attesting that the model was significant and there was only 0.01% chance that the F-value would occur on account of noise[19-20]. The R2 of the model for TN removal was 0.9958, implying that 99.58% of the variability in the response could be explained by Eq.(2)[21-22]. The adj R2 was 0.9903, which was close to R2 (0.9958), indicating that the actual and the predicted TN removal efficiencies fitted well[20]. The P-value less than 0.0500 implied that the corresponding term was significant[21]. The P-value of the linear terms (A1, A2, and A3), the quadratic terms (A11, A22, and A33), and the interaction terms (A12, A13, and A23) were all below 0.0500, indicating that all of these terms were significant and the model was appropriate. In this study, the adequate precision of 32.896 implied an adequate signal, and this model could be employed to navigate the design space[14]. The P-value of the lack of fit was 0.1282, suggesting that this model was adequate[23].
The predicted versus the actual plots for TN removal efficiency are presented in Fig. 3. The predicted values were forecasted by the proposed quadratic polynomial model, while the actual values were determined by the results of the 17 experiments. It can be seen that the predicted values and the actual values were close to the straight line, indicating a satisfactory correlation between the experimental and the predicted values[24].
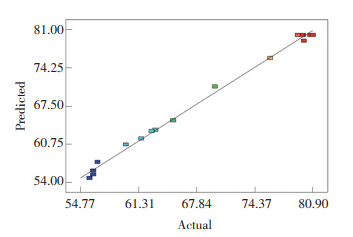
|
Fig.3 TN removal efficiency for predicted and actual plots |
3.3 Interactive Influences of DO, IR, and MLSS on TN Removal Efficiency
The response surface plot for the interactive influences of DO and IR on the TN removal efficiency with MLSS at zero level is presented in Fig. 4(a)[25].
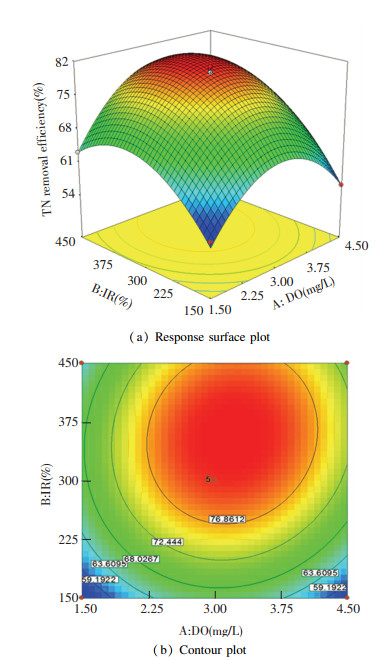
|
Fig.4 Influence of DO and IR, and the corresponding interaction with TN removal efficiency |
As shown in Fig. 4(a), when DO increased from 1.5 mg/L to 3.2 mg/L, TN removal rate increased and then reduced, and the possible cause might be the high dissolved oxygen in IR which disrupted the anoxic environment in the anoxic tank and then reduced the activity of denitrifier[26]. Fig. 4(b) shows the contour plot for the interactive influence of IR and DO when TN removal efficiency was set as response. The elliptic characteristic indicated a significant interactive influence between DO and IR. Previous studies found that elliptical contours will be obtained if there is an interaction between two variables[27]. The long axis of the ellipse along with the IR axis implied that IR was more influential than DO in this study. The cause might be that nitrification could occur when DO was higher than 1 mg/L and the DO in the aerobic tank of A/O-MBR in this study was higher than 1.5 mg/L. Therefore, compared with IR, TN removal efficiency was influenced less significantly by DO. With the increase of IR from 150.0% to 356.4%, TN removal efficiency increased progressively and then decreased. This was attributed to the fact that a high IR would bring abundant dissolved oxygen from the aerobic tank to the anoxic tank and then deteriorated the activity of denitrifying bacteria. Either high DO or high IR would lead to excessive dissolved oxygen in the anoxic tank, which would reduce the TN removal efficiency. When MLSS was 6000.0 mg/L, the optimum DO and IR were 3.2 mg/L and 356.4%, respectively. Under this condition, TN removal efficiency reached the highest value of 81.29%.
Fig. 5(a) shows the response surface plot for the interactive influence of DO and MLSS on TN removal efficiency with IR at zero level. It can be seen that the interactive influence between DO and MLSS on TN removal rate was significant. When MLSS increased from 3000.0 mg/L to 7577.5 mg/L, TN removal rate increased and then reduced. The biomass in the anoxic tank was high with higher MLSS, indicating that the dissolved oxygen brought by IR was quickly consumed in the anoxic tank and the activity of denitrifying bacteria was not affected. Therefore, the TN removal efficiency increased when MLSS increased from 3000.0 mg/L to 7577.5 mg/L. Fig. 5(b) shows the contour plot for the interactive influence of MLSS and DO when TN removal efficiency was set as response. Based on Fig. 5(b), a remarkable elongated maxima along with the MLSS axis indicates that MLSS was more influential than DO in this study. When IR was 300.0%, at 7577.5 mg/L MLSS and 3.4 mg/L DO, TN removal efficiency achieved its highest value of 81.58%.
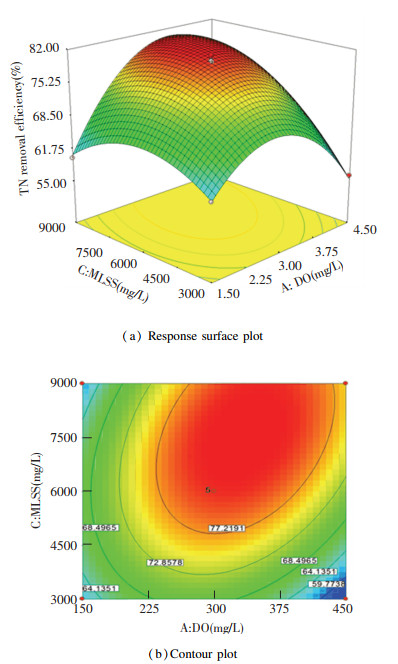
|
Fig.5 Influence of DO and MLSS and the corresponding interaction with TN removal efficiency |
The response surface plot and the homologous counter plot of TN removal efficiency (Fig. 6(a) and Fig. 6(b)) were obtained with DO maintained at 3.0 mg/L, while IR and MLSS were changed within the range of the test.
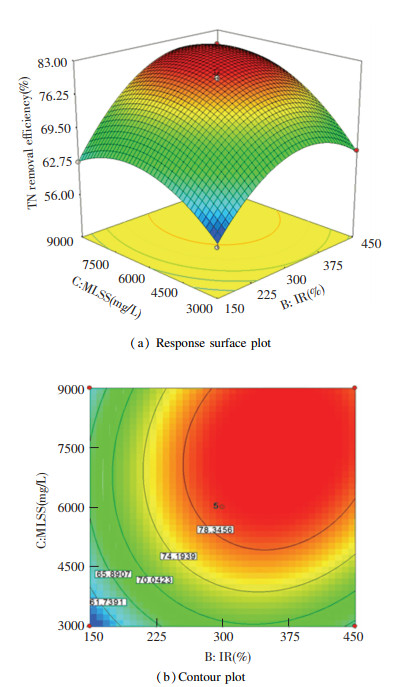
|
Fig.6 Influence of MLSS and IR and the corresponding interaction with TN removal efficiency |
It can be seen from Fig. 6(a) and Fig. 6(b) that TN removal efficiency was influenced more significantly by MLSS, compared with IR. This might be because that TN removal was mainly completed by biological nitrogen removal in A/O-MBR and the biomass relating to biological nitrogen removal in A/O-MBR was proportional to MLSS. Meanwhile, it was influenced greatly by the interaction between IR and MLSS. TN removal efficiency reached a peak value (82.50%) at 364.5% IR and 7487.7 mg/L MLSS.
The elliptic characteristic in Fig. 5(b) was more significant than those in Fig. 4(b) and Fig. 6(b), which implies that the interaction between DO and MLSS had more significant influence than those of the other two. It was confirmed by Eq.(2), which shows that the coefficient of AC was greater than those of AB and BC.
3.4 Optimization and VerificationThe optimum operating condition for TN removal efficiency was predicted from the model obtained from RSM with 7926.6 mg/L MLSS, 371.8% IR, and 3.5 mg/L DO.
A 30-d check test was conducted withthe A/O-MBR to validate the optimum condition (MLSS: 7926.6 mg/L, IR: 371.8%, and DO: 3.5 mg/L) obtained from RSM. The TN removal rate from the experiment under the optimum condition was 83.13%, while the predicted TN removal efficiency under this condition was 83.34%, implying that this optimum strategy was reliable.
Two other 30-d experiments were conducted to validate the accuracy of the model with two operating conditions of A/O-MBR (i.e., MLSS: 9000.0 mg/L, IR: 300.0%, DO: 4.5 mg/L and MLSS: 3000.0 mg/L, IR: 300.0%, DO: 1.5 mg/L). The corresponding TN removal rates of A/O-MBR under the above two conditions were 75.52% and 61.27%, respectively. The predicted TN removal efficiencies under the two conditions were 75.96% and 61.74%, respectively. The results verified the accuracy of the model from a mathematical point of view.
4 ConclusionsThe TN removal efficiency of A/O-MBR increased first and then decreased with increasing DO, IR, and MLSS, respectively, which implies that RSM is a valid and practicable approach to optimize TN removal efficiency. The model obtained from RSM illustrated that the interaction between DO and MLSS had more significant influence on TN removal efficiency than those of the other two. Under the optimum condition (MLSS: 7926.6 mg/L, IR: 371.8%, and DO: 3.5 mg/L), the experimental TN removal efficiency was 83.13%, which was close to the predicted value of 83.34%, suggesting that this optimum strategy is reliable.
| [1] |
Han X, Zhou Z, Mei X, et al. Influence of fermentation liquid from waste activated sludge on anoxic/oxic-membrane bioreactor performance: Nitrogen removal, membrane fouling and microbial community. Bioresource Technology, 2018, 250: 699-707. DOI:10.1016/j.biortech.2017.11.090 (  0) 0) |
| [2] |
Mannina G, Ekama G A, Capodici M, et al. Moving bed membrane bioreactors for carbon and nutrient removal: The effect of C/N variation. Biochemical Engineering Journal, 2017, 125: 31-40. DOI:10.1016/j.bej.2017.05.005 (  0) 0) |
| [3] |
Wen Q, Yang L, Zhao Y, et al. Insight into effects of antibiotics on reactor performance and evolutions of antibiotic resistance genes and microbial community in a membrane reactor. Chemosphere, 2018, 197: 420-429. DOI:10.1016/j.chemosphere.2018.01.067 (  0) 0) |
| [4] |
Tan T W, Ng H Y. Influence of mixed liquor recycle ratio and dissolved oxygen on performance of pre-denitrification submerged membrane bioreactors. Water Research, 2008, 42(4-5): 1122-1132. DOI:10.1016/j.watres.2007.08.028 (  0) 0) |
| [5] |
Tan T W, Ng H Y, Ong S L. Effect of mean cell residence time on the performance and microbial diversity of pre-denitrification submerged membrane bioreactors. Chemosphere, 2008, 70(3): 387-396. DOI:10.1016/j.chemosphere.2007.07.003 (  0) 0) |
| [6] |
Xia S, Jia R, Feng F, et al. Effect of solids retention time on antibiotics removal performance and microbial communities in an A/O-MBR process. Bioresource Technology, 2012, 106: 36-43. DOI: 10.1016/j.biortech.2011.11.112.
(  0) 0) |
| [7] |
Yang S, Guo W, Zhou X, et al. Optimization of operating parameters for sludge process reduction under alternating aerobic/oxygen- limited conditions by response surface methodology. Bioresource Technology, 2011, 102(21): 9843-9851. DOI:10.1016/j.biortech.2011.07.079 (  0) 0) |
| [8] |
Shubham S, Parag R G, Saurabh M J., et al. Ultrasound assisted synthesis of biodiesel from karanja oil by interesterification: Intensification studies and optimization using RSM. Ultrasonics Sonochemistry, 2019, 50: 36-45. DOI:10.1016/j.ultsonch.2018.08.019 (  0) 0) |
| [9] |
Saadat S, Raei E, Talebbeydokhti N. Enhanced removal of phosphate from aqueous solutions using a modified sludge derived biochar: Comparative study of various modifying cations and RSM based optimization of pyrolysis parameters. Journal of Environmental Management, 2018, 225: 75-83. DOI:10.1016/j.jenvman.2018.07.037 (  0) 0) |
| [10] |
Wang G, Mu Y, Yu H Q. Response surface analysis to evaluate the influence of pH, temperature and substrate concentration on the acidogenesis of sucrose-rich wastewater. Biochemical Engineering Journal, 2005, 23(2): 175-184. DOI:10.1016/j.bej.2005.01.002 (  0) 0) |
| [11] |
Chen Z, Cui M, Ren N, et al. Improving the simultaneous removal efficiency of COD and color in a combined HABMR-CFASR system based MPDW. Part 1: Optimization of operational parameters for HABMR by using response surface methodology. Bioresource Technology, 2011, 102(19): 8839-8847. DOI:10.1016/j.biortech.2011.06.089 (  0) 0) |
| [12] |
Box G E P, Behnken D W. Some new three level designs for the study of quantitative variables. Technometrics, 1960, 2(4): 455-475. DOI:10.1080/00401706.1960.10489912 (  0) 0) |
| [13] |
Fu H, Xu P, Huang G, et al. Effects of aeration parameters on effluent quality and membrane fouling in a submerged membrane bioreactor using Box-Behnken response surface methodology. Desalination, 2012, 302: 33-42. DOI:10.1016/j.desal.2012.06.018 (  0) 0) |
| [14] |
Zinatizadeh A A, Mohamed A R, Abdullah A Z, et al. Process modeling and analysis of palm oil mill effluent treatment in an up-flow anaerobic sludge fixed film bioreactor using response surface methodology (RSM). Water Research, 2006, 40(17): 3193-3208. DOI:10.1016/j.watres.2006.07.005 (  0) 0) |
| [15] |
Khuri A I, Cornell J A. Response Surfaces: Design and Analyses (Second Edition). New York: Marcel Dekker, 1996.
(  0) 0) |
| [16] |
Trinh T K, Kang L S. Application of response surface method as an experimental design to optimize coagulation tests. Environmental Engineering Research, 2010, 15(2): 63-70. DOI:10.4491/eer.2010.15.2.063 (  0) 0) |
| [17] |
CEPB (China Environmental Protection Bureau). Standard Methods for Examination of Water and Wastewater. Beijing: Chinese Environmental Science Press, 2002.
(  0) 0) |
| [18] |
Bura R, Cheung M, Liao B, et al. Composition of extracellular polymeric substances in the activated sludge floc matrix. Water Science and Technology, 1998, 37(4-5): 325-333. DOI:10.2166/wst.1998.0657 (  0) 0) |
| [19] |
Guo W, Ren N, Wang X, et al. Optimization of culture conditions for hydrogen production by Ethanoligenens harbinense B49 using response surface methodology. Bioresource Technology, 2009, 100(3): 1192-1196. DOI:10.1016/j.biortech.2008.07.070 (  0) 0) |
| [20] |
Najib T, Solgi M, Farazmand A, et al. Optimization of sulfate removal by sulfate reducing bacteria using response surface methodology and heavy metal removal in a sulfidogenic UASB reactor. Journal of Environmental Chemical Engineering, 2017, 5(4): 3256-3265. DOI:10.1016/j.jece.2017.06.016 (  0) 0) |
| [21] |
Liu J, Weng L, Zhang Q, et al. Optimization of glucose oxidase production by Aspergillus niger in a benchtop bioreactor using response surface methodology. World Journal of Microbiology and Biotechnology, 2003, 19(3): 317-323. DOI:10.1023/A:1023622925933 (  0) 0) |
| [22] |
Tripathi P, Srivastava V C, Kumar A. Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination, 2009, 249(3): 1273-1279. DOI:10.1016/j.desal.2009.03.010 (  0) 0) |
| [23] |
Montgomery D C. Design and Analysis of Experiments (Third Edition). New York: John Wiley & Sons Inc., 1991.
(  0) 0) |
| [24] |
Su S, Nie H, Zhu L, et al. Optimization of adsorption conditions of papain on dye affinity membrane using response surface methodology. Bioresource Technology, 2009, 100(8): 2336-2340. DOI:10.1016/j.biortech.2008.11.048 (  0) 0) |
| [25] |
Yetilmezsoy K, Demirel S, Vanderbei R J. Response surface modeling of Pb(Ⅱ) removal from aqueous solution by Pistacia vera L.: Box-Behnken experimental design. Journal of Hazardous Materials, 2009, 171(1-3): 551-562. DOI:10.1016/j.jhazmat.2009.06.035 (  0) 0) |
| [26] |
Plósz B G, Jobbágy A, Grady Jr. C P L. Factors influencing deterioration of denitrification by oxygen entering an anoxic reactor through the surface. Water Research, 2003, 37(4): 853-863. DOI: 10.1016/S0043-1354(02)00445-1.
(  0) 0) |
| [27] |
Xu H, Sun L, Shi Y, et al. Optimization of cultivation conditions for extracellular polysaccharide and mycelium biomass by Morchella esculenta As51620. Biochemical Engineering Journal, 2008, 39(1): 66-73. DOI:10.1016/j.bej.2007.08.013 (  0) 0) |
 2020, Vol. 27
2020, Vol. 27


