Color is one of those old but everlasting topics in life and in science. In life, it comes as no surprise to anyone, as color is primary to human perception and experiences. In science though, it may intrigue many that it was the study of colors in connection to a new technological advance[1] that brought us into the quantum era.
The technological advance at the time, around 1900, was the advent of electric lighting. It was readily observed that the light bulb's color changed from red to orange, then to yellow, with increasing voltage. That puzzled some of the greatest minds and prompted a search for an explanation. It culminated in the conceptualization of quanta, by Planck and Einstein, and opened the door to the development of quantum mechanics. In today's terms, the light bulb's color changes are merely an example of a broad range of effects in physical coloration. Different colors can be generated in the same materials by physical means, rather than by different chemicals, dyes, and pigments.
A hundred years later, another major technological advance is upon us-an advance into the nanoscale. It has brought us unprecedented capabilities and means to make structures and devices on the nanoscale. It is particularly relevant to coloration, as the scale is comfortably smaller than the wavelengths of the colors, or the visible light. We are now capable of engineering materials at the subwavelength scale and producing different colors from the same materials and changing them in real time. With such capabilities comes a renewed interest in physical coloration, driven in part by the growing demands from the display and illumination industries, and by the needs in consumer goods for alternatives to chemical coloration. Examples of such needs are plentiful, ranging from phones to cooking wares.
Dyes, phosphors, and pigments can be colorful, but are often not durable enough, and are susceptible to heat, sunlight (UV), and scratches. Additionally, rare earth materials, essential for chemical colorations, are in the lands of a few countries(Fig. 1) and thus in the hands of politics and politicians, and susceptible to the forces of geopolitics. But, the principles of physical coloration and their applications are in the hands of scientists and engineers, and are accessible to everyone everywhere.

|
Fig.1 Global rare earth production distribution[2] |
More generally, colors are part of an innate human need in acquiring and communicating information as well as in perception and emotion. Advances in capabilities of coloration on subwavelength or nanometer scales make us more capable of meeting the everlasting and growing need.
The build-up of pertinent research has made available many promising physical coloration approaches. Some are inspired by Nature[3], some not even found in Nature and referred to metamaterials[4], and some are newly developed[5]. However, challenges remain and are numerous, especially when the approaches are subjected to the more stringent criterion of being scalable, manufacturable, and commercially viable. After all, coloration is to serve the largest market imaginable-the entire world's population.
One challenge is rooted in the fact that physical coloration implies material structuring at a length scale smaller than the wavelengths of visible light, hence by definition nano-engineering. This challenge is being met and such subwavelength material structuring is now readily doable on small surface areas with today's technologies-nano-lithography. However, doing it over large macroscopic areas, e.g. meters, remains difficult for many of the lithography based approaches.
Another challenge that is perhaps more intriguing rises from a subtle conflict between physical coloration and color perception. One is physical and the other is physiological. Physical coloration is the engineering applications of physics principles and is quantitative in nature. Color perception, on the other hand, is a physiological sensation which is rather mysterious to us all and is hardly quantitative.
The challenges go beyond color creation and color perception, reaching into the tough and still open question of what and how color spectra can affect human performance and quality of life. Human circadian rhythms stand out as an example connecting color spectrum and life. Humans evolved over millions of years under natural lighting conditions. Along the way, human body's internal machinery and circadian rhythm have evolved with and adapted to the evolution of the solar spectrum. All that changed abruptly, with the advent of unnatural lighting from light bulbs and LEDs, impacting humans more than perhaps any other species since humans now spend more time indoors under artificial light.
While electric light may serve the purpose of lighting and illumination, it is no substitute for solar light in terms of its spectral-temporal content and therefore its role in human body functions. Electric light simply lacks the daily and seasonal spectral-temporal evolution of the solar light which the human body and its internal machinery have evolved with and adapted to over millions of years. This spectral or color content mismatch between solar light and electric lighting may be subtle, but does vary with the hours of the day, the days of the season, and the seasons of the year. Over a human's life span, artificial lighting's pervasive presence can become impactful. The 'winter-blue' syndrome, or seasonal affective disorder, is one well-known example. Vitamin D deficiency and depression in the elderly is another example in which attribution has been made to the lack of exposure to the blue-UV end of the solar spectrum. The higher cancer rate and health issues among night-shift workers are now also seen as connected to lighting. Given that everyone now lives a life under electric lights, but in a body with its machinery evolved over millions of years under solar light before electricity, it is no wonder that every generation seems to be reporting more and bigger health problems. Electric lighting is not going away any time soon. The question is-would it be possible to restore the bio function in lighting? If possible, it will likely favor physical coloration, given the heat at and near the light source and the need for real-time tuning of spectrum over the full range of visible lights and beyond (to near-UV and near-IR).
There are many more challenges in this broad field of physical coloration, some of which are still difficult to meet but also provide opportunities for new technologies to emerge, develop and evolve. There is a large body of literature in which a number of comprehensive reviews have covered basic properties and methods of physical coloration, especially the plasmonic type such as gratings, nanoaperture arrays, thin films, and Mie resonances and cavity modes[4, 6-9]. Some are more readily available through open access publications[7-8]. In this light, it seems another comprehensive review may not offer much service to the field. Instead, a presentation focusing on some remaining challenges or open opportunities may add more value. In this regard, the scope of this article is intentionally kept narrow to highlight three fronts that have received less attention in the literature and yet present us with great challenges and opportunities: scalable physical coloration, physical colorimetric sensing, and on-command delivery of subscriptions of 'biophilic lighting'. They also happen to be ones that the author has more familiarity with as he and his team has worked on them fairly recently. For relative convenience in data selection and to avoid the otherwise substantial delays in seeking permissions from a large range of sources, the essences of specific findings will also be mostly distilled from our own explorations[4, 9-11]. The emphasis will be placed on underlying mechanisms and on opportunities and issues that are still open.
2 Scalable Physical ColorationToday's methods of coloration are still predominantly chemical in nature, based on dyes and pigments coloration. Chemical colorations are rich in colors, simple to use, and widely available, but they do come with limitations. They are scratch-prone, static, and degrade under exposure to UV light, heat, and the elements, to name a few. The use of toxic chemicals and environmentally unfriendly processes are also of increasing concern. Physical coloration offers an alternative that in principle can help alleviate some of the limitations and problems while meeting the needs for heat- and scratch-resistant colorations. However, not all the proven methods of physical coloration are scalable and commercially viable.
We have worked on developing physical coloration approaches that are scalable and commercially viable on a representative platform of anodized aluminum (AAO). Aluminum is one of the most used metals worldwide, and is extremely versatile with broad applications because of its abundance, low cost, light weight, and high strength. Mobile phones, laptop cases, and more importantly aircrafts and automobiles are prominent examples that could benefit from physical coloration. The aluminum industry in the US accounts for ~1% of the GDP as recently estimated by the Aluminum Association. Current aluminum coating technology serves two main purposes: aesthetics and wear resistance. Needless to say, aesthetic appeal is of vital importance in the fashion, accessory, automobile, and architecture industries. Currently, methods for aluminum surface coloration are made in its oxidized surface layer mostly by chemical means and come with significant limitations. Organic dyes deteriorate under UV irradiation from the Sun, or due to continued exposure to high temperature and other environmental factors (e.g. oxygen, humidity)[12]. On top of that, dye and pigment sourcing, manufacturing, and applications can be energy consuming, hazardous, and environmentally harmful. In order to achieve brighter, more vivid, and purer colors, the aluminum oxide (alumina) layer must be made thicker and more porous so as to contain more dye. This introduces other challenges with respect to the wear-resistance and ductile properties of the aluminum coating. Less obviously, thick chemical coating also reduces the surface thermal conductance because the dye-filled alumina layer not only adds convective thermal impedance to the part's surface, but also has poor temperature stability and surface emissivity for proper radiative heat dissipation, which can be critically important for aluminum-cased electronics such as LED lighting modules in mobile display, TVs, and highway lighting etc.
As a matter of fact, our interest and exploration in aluminum coloration began with a phone call from the consumer products industry. A technology intelligence arm of a "worldwide leader in consumer goods" reached out to me seeking a method to obtain specifically "vivid red color (e.g., RAL color chart 3001-3003) with good thermal resistance (up to 350 ℃ for 30-minute duration), without the use of paint or any hazardous materials such as cadmium."
It was unexpected since I have neither worked on nor had an active interest in coloration at the time. They found me after sieving through the literature and finding our publications from the 90's when we began developing non-lithographic methods for nanoelectronic and optoelectronic devices[13-16]. In response to this request, we began conducting research in that direction. As non-chemist by training, we extended what we did in the nanofabrication of electronic and photonic devices to the development of methods of physical coloration, and in particular their implementation on aluminum (AAO) surfaces. This physical approach built upon electronic and optical mechanisms achievable in nanostructured metals and dielectrics, in ultrathin (~5-10 nm) film coatings, and in metal nanoparticle-dielectric composites (Fig. 2).

|
Fig.2 Physical colors at the nanoscale. (Taken from an NSF proposal by a team of Jimmy Xu, Gustavo Fernandes, Domenico Pacifici. The research lies at the intersection between (1) nanostructured metal surfaces with long-range optical interferences, (2) short-range localized electronic effects residing in metallic nanoparticles showing quantum-plasmonic resonances, and (3) ultra-thin wavelength-selective absorbers. |
AAO has a well-established industry presence as it is robust, heat and scratch resistant, and scalable to very large surface areas. By itself, AAO is colorless, or nearly so, but colors can be created physically by a number of methods and their combined uses. The results[5] of our work and other works demonstrated facile and scalable dye/pigment-free color coatings, proved their scalability and manufacturability, and laid the foundation for its extensions to other substrate materials. Its potential applications include coating for automotive, aerospace, and consumer products, with bright and vivid colors that can withstand heating up to 300-a temperature hardly tolerable by chemical coloration.
One of the mechanisms available for physical coloration is that of long-range interference and diffraction. It is manifested beautifully in the colorful peacock feather, in which a scanning electron microscope image would reveal periodic arrays of subwavelength pores inside. Nanoscale pores can form within the AAO during anodization. Within certain ranges of anodization parameters such as electrolytes and voltages, they can even self-organize into periodic arrays[17]. Other enabling mechanisms could be local such as electron oscillations localized to the surface of metallic nanoparticles (plasmonic resonances) which can be implemented by filling the nanoparticles into the nanopores[18-19]. In between the long-range and local effects, one can also resort to couplings between propagating waves and localized modes as manifested in wavelength-selective extraordinary (e.g. near-perfect) absorption or reflection in ultra-thin layers[20]. All achievable through subwavelength structural engineering. Or, their judicious combinations.
More generally, and not confined to AAO, we have at our disposal a number of mechanisms to enable physical coloration. They can be used separately, or used together since they are complementary. They may be classified into: (1) long-and short-range diffractions and interferences, (2) localized electronic processes in metal nanoparticles, including but not limited to plasmonic resonances, and (3) ultra-thin wavelength-selective absorbers or reflectors with structurally engineered spectral shaping and enhancement-aka, metasurfaces. They can be used in combination, much like mixing ingredients in chemical coloration, to manipulate the intensity, wavelength, or viewing angle dependence. The combination can be not only physically complementary but also spectrally combinatory, where the spectral features of one material/method compensate for the shortcomings of another, and their combination yields the superposed color and appearance. For a maximal effect however, the combination must account for their interplay with other structural dependences in the physical color recipe.
Bands of attractive colors have been demonstrated in physical coloration, but many opportunities or challenges remain open. For example, certain properties such as brightness, contrast, saturation, and iridescence cannot yet be precisely controlled in physical coloration. Making avail, or merely gaining control over these properties shall be achieved via better understanding of the physical mechanisms responsible for the color as well as their scalability for manufacturing. On top of manufacturability, when it comes to specific industrial applications, the task can become more demanding. The example cited earlier highlights that aspect of the challenge in physical coloration-achieving a specific color, e.g., a vivid red color (coded in the RAL color chart as color 3001-3003). Chemical coloration clearly can do it, albeit without the heat- or UV- or scratch-resistances. Can physical coloration do it?
Upon closer inspection of the variety of colors in Fig. 2 or those reported in the literature, it is clear none are red enough to be regarded as the wanted vivid red. So far, this turns out to be also true for most, if not all, colors in the long wavelength end of the visible spectrum to reach color saturation by physical means. This is because an optical structure, be it Fabry-Perot or plasmonic, with a resonance frequency in the red usually would also allow a resonant mode at twice that frequency, which happens to be in the blue. As a result, it would exhibit a mixture of red and blue, certainly not pure red.
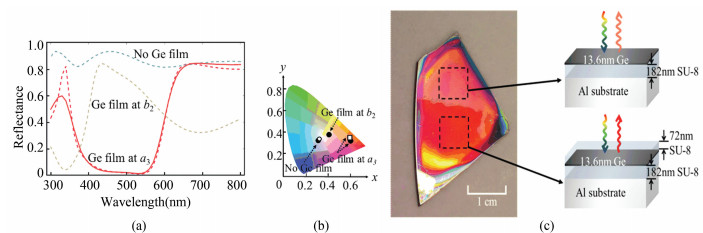
Since this is a physical problem, one can also seek for a physical solution. As we reported earlier[18], indeed that particular red can be obtained by pure physical means. In general, physical coloration permits the use of a strategy referred to as spectral shaping. Spectral shaping is more general than coloration, and is applicable to spectral bands beyond visible colors such as infrared and microwave. It can be implemented as simply as inserting a thin absorbing layer at the antinode of an unwanted mode, as illustrated below (Fig. 3). In this case, a ~14 nm thin germanium layer is inserted into a sub-micron layer structure. Germanium is not essential here, as any material that absorbs in the blue range could be just as good. Placing it at different positions (e.g. marked as b2 and a3 in the figure) would produce different colors in spectrum (Fig. 3(a), 3(b)) and in visual appearance (Fig. 3(c)). And, when inserted in the layered structure at a depth corresponding to the blue mode antinode, which in this layered structure corresponds 72 nm below the surface as determined in simulation and labeled as a3, the desired vivid 'tomato-red' is acquired.
|
Fig.3 The demonstration of the elusive 'tomato red' ascertained in physical coloration by mode-selective spectral shaping. A 14 nm thin germanium layer is inserted into a sub-micron layer structure. Placing it 72 nm below the surface, corresponding to the blue mode antinode as determined in simulation and labeled as a3(a), produces the 'tomato red'(b). Placing it right at the very top gives us pink(c). |
These are simply consequences of a spectral-shaping effect produced by 'spatial modulation of absorption'. It opens the door to an attractive possibility, i.e., 'active coloration'. Displays are a form of 'active coloration'. But, in this case, the active coloration is to happen within an ultrathin surface layer structure of submicron thickness, which is orders of magnitude thinner than current display form factors. The key lies in tuning the 'optical length', or in this case the 'optical depth'. The tuning can be achieved by changing the refractive index of one or more layer(s) dynamically with an externally applied voltage, instead of the physical dimension.
Now that it is shown that the scalability of physical coloration can be ascertained[21] and that we also know specific colors, e.g. this 'vivid red' challenge to physical coloration, can be dealt with by 'spectral shaping', we may turn our attention to another challenge that is more complex and perhaps more intriguing. This challenge rises from an inherent conflict between physical coloration and color perception, or between physics and physiology. The former is about engineering and optimization of physical effects, a quantitative process in nature. The latter is a physiological sensation, rather mysterious and hardly quantitative. This challenge is reflected, solidified, and perhaps amplified, in the next case: colorimetric sensing. Though colorimetric sensing is chemical in origin, physical coloration could be a useful tool to amplify the sensitivity and increase the speed of response of colorimetric sensing.
3 Colorimetric Sensing and Physical AmplificationColorimetric sensing is an effective approach to detecting trace amounts of chemicals in air or in liquid. It reports chemical reactions by color changes, as the name suggests. Because of that, it offers an advantage over other sensing modalities because it is perceptible directly to human eyes, as exemplified by the PH test strips. The color changing and identification mechanisms have been well understood and documented in the literature [22-23].
Colorimetric sensing has evolved from the simple PH test strips to more sophisticated techniques[20], including spectroscopy. Nevertheless, all being equal, it would only be natural to prefer the use of human eyes over spectroscopy instrumentation, as the human eye has some great attributes that are hard to match in engineering: self-powered, portable, real-time detection and reporting, operable in remote or stand-off. However, colorimetric sensing based on human perception does have notable limitations in sensitivity and response time. Part of the limitation is rooted in the fact that color change of the sensing dye is a result of reaction by contact. The reaction happens molecule by molecule and needs to accumulate enough to appreciably affect the light transmitting through or reflecting back and change its color enough to be visually discernable. There lies a fundamental limitation to detection sensitivity, and it is physical. The response time is likewise limited as targeted molecules impinging on the dye surface need time to make their way through the dye layer into its depth. It is a diffusive accumulation process that takes quantity and time which is usually, if not always, much longer than human visual response time (~1/10 s). Why so? The molecules impinging on the surface of a polymer layer that is thicker enough to produce visible color change would have to go through a random-walk process to reach its bottom. The time it takes is not a linear function of the thickness, but scales super-linearly, or quadratically, if the layer is thicker than the molecular mean-free path, or more if it is thicker. Predictive modeling is possible in principle. In practice, it depends on many parameters such as material, porosity, reaction rate, thickness, and the ambient and local temperatures. It is no wonder that with so many variables in play, even machine learning has been recruited to make its entry into the field[24].
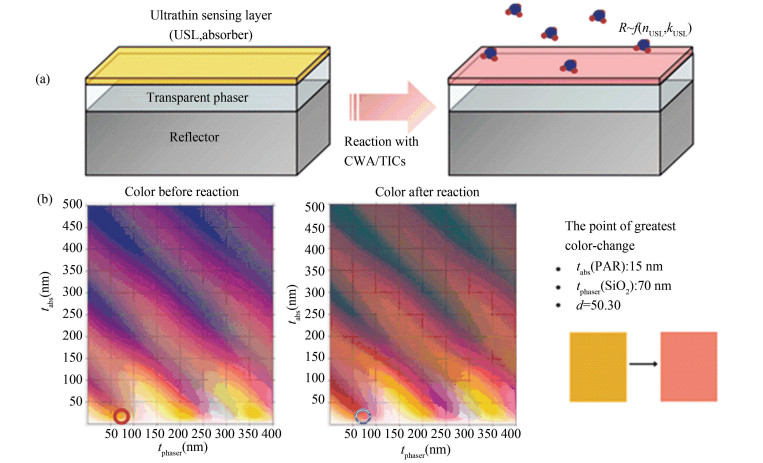
While chemists have been continuously advancing the art of colorimetric sensing, it is clear that sensitivity and speed limitations are physical in nature. That leaves room for physics to join the play and contribute. While chemical reactions are accumulative and can be slow, light propagation and absorption are physical and fast. That gives us a chance to increase the light absorption without increasing either the response time or the dye thickness for the colorimetric sensing. In so doing, one would effectively amplify the sensitivity and speed of response, or introduce a Physical Amplification mechanism. For instance, the unabsorbed portion of light after one pass can be sent back to the same material, however thin, many times to be re-absorbed each time, making the otherwise thin layer of material effectively many times thicker. The embodiment of this physical amplification mechanism can be rather simple, made by inserting a non-absorptive transparent material (a dielectric) in between a thin dye layer on the top and a reflective (metal) on the bottom, as illustrated in Fig. 4.
|
(From B.Sc. Advisee, Keegan Quigley's Brown Physics Honor Thesis, 2018) Fig.4 (a) Concept of physical amplification of colorimetric sensing for a greater sensitivity and faster response. Change of optical constants of ultrathin sensing layer allows chemical colorimetric sensing aided by physical amplification mechanism. (b) Simulated colors of a cavity structures (PAR/SiO2/Al reflector) with different thicknesses of absorber and phaser before (left-hand panel) and after (center panel) reaction with copper ions. The right-hand panel shows the cavity structure having the greatest color change, from "yellow" (red circle in the left panel) to "pink" (blue circle in the center panel), corresponding to a quantitative CIELab color distance of 50.3. |
This structure looks deceivingly simple, and can easily be regarded as a normal Fabry-Perot cavity. A closer examination of the figure, however, would reveal details pointing to the otherwise. The optimized structure with the greatest color change, from yellow to pink corresponding to before and after copper ion reactions with the sensing dye PAR, was achieved in a structure of a 15 nm (tabs) ultrathin dye and a 70 nm (tphasor) dielectric phaser layer (SiO2). Both layers are much thinner than 1/2 or 1/4 wavelength of the corresponding colors. Still so, even when the two thicknesses add together. Clearly, it is not the usual Fabry-Perot mechanism in the play. As a matter of fact, it is not any of the conventional spectroscopy mechanisms in the play. And it shall not be. Fundamentally, spectroscopy is spectrally de-compositional, whereas color perception is spectrally integral. The former takes the light and slices it into small intervals by wavelength and measures the intensity in each. The latter takes the light as a whole and integrate its product with the color receptor's spectra functions.This difference makes the structural design and optimization more complex and more challenging for physical amplification of colorimetric sensing. Unlike for a Fabry-Perot, it is not individual wavelengths but the perceptive responses of the three bands, red, green, and blue, to be optimized. While design and optimization in engineering is necessarily quantitative, human color perception is physiological and sensuous. How shall or could one- an engineer or a physicist- go about reconciling or bridging the difference? Quantifying color is one way and has proved effective.
One of the students of my lab, Keegan Quigley, adopted a color quantification method from the display industry and extended it to a new concept: color distance that quantifies the color distance for colorimetric sensing[25].
The display industry's standards for color quantification are still evolving with greater sophistications for better perceptual satisfaction. But, the essence remains the same. For a given color (over the visible band of 390-780 nm), one can convolve (integrate) it with the illumination spectrum and the spectral sensitivities of the red, green, and blue (RGB) cone receptors of the retina to obtain quantitative coordinates (x, y, z) in the color space. Specifically, the process for computing color coordinates from the measured light spectrum R(λ) of the object and the illuminating spectrum I(λ) proceeds by computing tristimulus values:
| $ X_{i}=\frac{1}{k} \int_{360 \mathrm{nm}}^{780 \mathrm{nm}} R(\lambda) I(\lambda) V_{i}(\lambda) \mathrm{d} \lambda $ |
where i=1, 2, 3, the Vi(λ) are the standard observer's RGB cone receptor spectral functions, and k=
The illumination spectrum however varies with conditions (e.g. indoor vs outdoor). For engineering or a physical structure design, a viable option would be to start with the most general and/or the simplest, such as the daylight spectrum at noon for illumination, because chemical sensing is most likely to take place outdoors. Alternatively, one could use the spectrum of a LED light instead. LEDs make sense for a colorimetric sensor primarily used indoors (e.g. chemical plans, aircraft interior spaces, or chemically treated woods and composites in furniture and floor). The spectrum of a LED is quite different from that of the Sunlight, but also differs from one another.
While its impact on the colorimetric sensor design optimized for human perception is finite, it is likely not as substantial, and as subtle, as on human health-a subject to be discussed in the next section. In principle, regardless of the light source, by convolving the incident light spectrum with the spectral sensitivities of the RGB receptors, the human eye's response to an arbitrary reflection spectrum can be quantified.
Can we then also quantify color changes? Given the fascinating physiological intrigues of human visual perception and our still limited and evolving understanding of them, the actual quantitative computations of color changes can vary in detail with methods, as discussed by Keegan Quigley in his award-winning thesis[25]. This thesis explains how the light spectrum of an object can be translated, via the aforementioned conventional integrals, into a visual color in a color space defined by the International Commission on Illumination / Commission Internationale de l'Eclairage (CIE), the CIE1931 CIEXYZ space. This approach would result in three "color coordinates", x(λ), y(λ), and z(λ), which mimic the response of the RGB receptors in the human retina. Unsurprisingly, it is a non-linear transformation of a light spectrum into the CIE defined color space because of the physiological nature of the RGB coordinates. Nevertheless, it provides us a way to quantify the color change perceived by a person with normal vision, that is, by the Euclidean distance between two points in this X-Y color space. The larger this color distance, the greater the color change. As such, a perceptual color change is translated into a quantity that can be computed and thereby optimized through structural parameter variations. Fig. 4 serves as examples of the colors and color distance computed this way, before and after reaction, for different thicknesses of the top dye layer and the transparent dielectric layer (or phaser) atop a reflective aluminum substrate. The computed maximum color distance, 50.3, gives us the corresponding optimal dye layer thickness (15 nm) and the dielectric layer's (70 nm).
The color quantization lets us quantify the benefit of physical coloration in amplifying the colorimetric sensitivity. One way is to compare the maximum color distance of the traditional pure dye micrometer thick layer before and after reaction with that of the physical coloration structure of the optimized dye layer and phaser thicknesses. It is clear even without calculation that an ultrathin dye layer in the latter would have a much higher speed of response and require a much lower dosage of targeted molecules to reach saturation. With the quantitative calculations, the latter is also shown to produce a much larger color change perceptive to unaided eyes, without altering the chemistry. This was validated in experiments[21] where a 5 nm thick dye produced clearly visible color change within seconds of exposure to ammonia vapor. As a further extension, the physical coloration strategy is now extended to scalable fiber-optic sensing[26] and to industrial aluminum also for proof of salability and a reduced viewing angle dependence[27].
While it is rather satisfying to have a way to address the questions or challenges arising at the intersect between engineering that is quantitative in nature and human vision that is perceptual, it is neither unique nor most accurate. There is room for the development of an alternative or better method. One reason for the cautionary note is that the CIEXYZ space was not developed with perceptual uniformity in mind, that is, equal Euclidean distances in the space correspond to equal differences in perceived color changes. Apparently, CIE has now offered an improved color space-L*a*b* - for use. It expresses color as three values: L* for the lightness from black (0) to white (100), a* from green (-) to red (+), and b* from blue (-) to yellow (+). The CIELab aspires to better approximate human vision and provide perceptual uniformity in its space with its L component closely matching human perception of lightness[28]. One can transform color from the CIEXYZ color space to the CIELab color space using the transformations described by the CIE. We have not done that yet, as our focus at this stage is on the principles of physical coloration and on introducing the concept and mechanism of physical amplification in colorimetric sensing.
4 Spectral Shaping for Biophilic LightingLight and color are also essential to human health beyond just vision. Physical coloration therefore has one more dimension of extension: light-color-life. It is a rather large play of challenges and opportunities. This section introduces a beginning effort of ours in this dimension[29].
Having evolved under solar light over the course of millions of years, the human body has adapted to the solar spectrum. Although the Sun is a constant presence, its light content is a rhymical variable. The spectral intensity content of the sunlight that reaches the Earth's surface is shaped by Earth's atmosphere, rotation, and orbit around the Sun. Its daily and seasonal cycles constitute a natural pacemaker for activity in the terrestrial biosphere. An evolutionary consequence of this rhythmicity is the existence of a circadian rhythm, regulating our sleep-wake cycles. A closer look at the sunlight, as we did and recorded from sunrise to sunset over a period of several months in Providence, Rhode Island, USA (41.8240°N, 71.4128° W), would reveal that its spectral-intensity changes over the hours too. It is likely some of our body's internal machinery has also adapted to the seasonal, daily, and even hourly rhythmicity that are vital to our well-being, such as the production of hormones, mating and feeding times, according to the solar angle and the characteristics of the daylight. The sudden change of human life from being under the solar light to under the electric light, whose constant spectrum sharply peaks in the blue and yellow to fake an artificial 'white', must have impacts on humans. Some may be more subtle than others, as mentioned earlier in the introduction.But it certainly affects people's wellbeing physiologically, psychologically[30-31], and environmentally[26]. Internal to these external observables are molecular and neurological effects of light beyond vision that are currently getting identified and understood in research[32-33].
Not surprisingly but less known is that humans are not alone in this as light impacts insects just as much and 'light pollution' in and around cities is a driver of insect population declines, affecting insect movement, foraging, reproduction, and predation. Pollinating flowers and breaking down waste, insects are crucial to the world we live in. But nowadays some insect species are facing potential extinction, attributable to 'light pollution'[34]. A most recent report supplements the current understanding and offers a glimpse to the impact on animals[35]. Now that we know the problem and even know how big it is and can become, the question to ask is what can be done to address it or bring remedy to it?
The answer may lie in making electric lighting 'biophilic'. That is, make electric lighting capable of delivering time-varied spectral-intensity content that closely resembles, if not reproduces, that of sunlight's along with its rhythmicity. It could start small and simple, for example, spectral programmable lighting and its on-command delivery[36].It can then evolve into on-command delivery of biophilic lighting by prescription-an aim for our joint NSF project with the Sleep Lab of Bradley Hospital, and part of the "emerging challenges and opportunities" also envisaged by others for Using Light to Influence Well-Being[37].
Self-evidently, the technological implementation is unlikely by chemical coloration, but rather by physical coloration, as it is hard to imagine real-time color change and spectral shaping by chemical means. Interestingly, this discussion brings us back to where we started this article with, physical coloration arising from light bulbs some 100 years ago. That was at the advent of the then new technology of electricity. Today's technology and its more recent advance to nanoscale engineering has offered us a lot more tools and means. It so happens that most, if not all, necessary technological pieces have been in place for real-time physical coloration and spectral-shaping, as exemplified by the testbeds under development by Circadian Positioning Systems (a startup by two former members of the team), as shown in Fig. 5. A broader adoption by consumer markets can be expected in time with the lowering cost and rising volume.

|
Fig.5 Programed delivery of biophilic lighting for circadian positioning of mission-critical personnel and personal performance enhancement (permission from Circadian Positioning Systems Ltd., https://sleepcps.com/) |
The real challenge on this front turns out to be less in technology and more in psychiatry and medicine. For example, even if the engineers are able to deliver prescription lighting on-command, it is still not clear what lighting prescription should be for what people. What prescription should it be for night-shift workers? Or, oversea travelers, school children, elderly in nursing homes? Or, people experiencing the onset of dementia and Alzheimer? Conversely, health care providers and doctors could say: we do not have the prescriptions because we have not been given the technology or capability to develop and test them.
Advances on this front will require an integrated approach of adapting the newly developed real-time physical coloration capability to health care needs and developing new health care and treatments with the newly availed capability. It is hopeful that a holistic biophilic lighting technology will be developed to improve our quality life and enhance our performance; and, it will likely be enabled by real-time and full-spectrum physical coloration.
As a closing remark, physical coloration stimulated the development of a new era of science and technology some hundred years earlier, which in return has presented new opportunities and challenges to physical coloration itself. Examples are presented and reviewed on three fronts: scalable nanomanufacturing of physical coloration, physical amplification of sensitivity and speed of response in colorimetric sensing, and provision of biophilic lighting by real-time full-spectrum physical coloration. It is a large multi-dimensional field open and awaiting creative minds and hands. Results can produce broad and direct impacts to humankind.
AcknowledgementThis review is also a summary of the explorations of an extended team in the expanding space that I have had the good fortunate to lead and/or co-lead and that were enabled by support from NSF CMMI-1530547, ARO W911NF1420075, AFOSR A9550-19-1-0355, and ARL CCDC-SC. Much of the findings reviewed here was made in collaboration with Gustavo Fernandes, Mary Carskadon, Eliza van Reen, Joshua Bohar, De He, Zhijun Liu, Do-Joong Lee, Declan Oller, Keegan Quigley, Domenico Pacifici, Jin Ho Kim, R. Odessey, R. Osgood, Brian Demers, and other members of their teams.
| [1] |
Planck constant.https://en.wikipedia.org/wiki/Planck_constant.2020-12-03.
(  0) 0) |
| [2] |
Joseph Gambogi.Rare earths statistics and information. https://www.usgs.gov/centers/nmic/rare-earths-statistics-and-information.
(  0) 0) |
| [3] |
e.g. Peacock feather color is a structural coloration-different colors emerged from the same polymer structured with different periodicities.
(  0) 0) |
| [4] |
Barnes W L, Dereux A, Ebbesen T W. Surface plasmon subwavelength optics. Nature, 2003, 424: 824-830. DOI:10.1038/nature01937 (  0) 0) |
| [5] |
Oller D, He D, Kim J H, et al. Colour gamuts arising from absorber-dielectric-metal optical resonators. Coloration Technology, 2017, 133(6): 441-448. DOI:10.1111/cote.12301 (  0) 0) |
| [6] |
Kristensen A, Yang J K W, Bozhevolnyi S I, et al. Plasmonic color generation. Nature Reviews Materials, 2017, 2: 16088. DOI:10.1038/natrevmats.2016.88 (  0) 0) |
| [7] |
Hedayati M K, Faupel F, Elbahri M, et al. Review of plasmonic nanocomposite metamaterial absorber. Materials, 2014, 7(2): 1221-1248. DOI:10.3390/ma7021221 (  0) 0) |
| [8] |
Yao K, Liu Y M. Plasmonic metamaterials. Nanotechnology Reviews, 2014, 3(2): 177-210. DOI:10.1515/ntrev-2012-0071 (  0) 0) |
| [9] |
Ollera D, Fernandes G E, Siontas S, et al. Scalable physical coloration. Materials Research Bulletin, 2016, 83: 556-562. DOI:10.1016/j.materresbull.2016.07.001 (  0) 0) |
| [10] |
Bohar J, Fernandes G E, Xu J. Spectral-temporal LED lighting modules for reproducing daily and seasonal solar circadian rhythmicities. Proceedings of the 2017 IEEE International Conference on Smart Computing (SMARTCOMP). Piscataway: IEEE, 2017. 16964473. DOI: 10.1109/SMARTCOMP.2017.7947047.
(  0) 0) |
| [11] |
Odessey R, Lee D J, Quigley K, et al. Physical amplification of chemical colorimetric sensing. Proceedings Volume 11007, Advanced Environmental, Chemical, and Biological Sensing Technologies XV. Bellingham: SPIE, 2019.110070G. DOI: 10.1117/12.2518158.
(  0) 0) |
| [12] |
Poinern G E J, Ali N, Fawcett D. Progress in nano-engineered anodic aluminum oxide membrane development. Materials, 2011, 4(3): 487-526. DOI:10.3390/ma4030487 (  0) 0) |
| [13] |
Routkevitch D, Bigioni T, Moskovits M, et al. Electrochemical fabrication of CdS nanowire arrays in porous anodic aluminum oxide templates. Journal of Physical Chemistry, 1996, 100(33): 14037-14047. DOI:10.1021/jp952910m (  0) 0) |
| [14] |
Routkevitch D, Tager A A, Haruyama J, et al. Nonlithographic nanowire arrays: fabrication, physics, and device applications. IEEE Transactions on Electron Devices, 1996, 43(10): 1646-1658. DOI:10.1109/16.536810 (  0) 0) |
| [15] |
Yin A J, Li J, Jian W, et al. Fabrication of highly ordered metallic nanowire arrays by electrodeposition. Applied Physics Letters, 2001, 79: 1039-1041. DOI:10.1063/1.1389765 (  0) 0) |
| [16] |
Li J, Papadopoulos C, Xu J. Growing Y-junction carbon nanotubes. Nature, 1999, 402: 253-254. DOI:10.1038/46214 (  0) 0) |
| [17] |
Chik H, Xu J. Nanometric superlattices: non-lithographic fabrication, materials, and prospects. Materials Science and Engineering: R: Reports, 2004, 43(4): 103-138. DOI:10.1016/j.mser.2003.12.001 (  0) 0) |
| [18] |
Yin A, Tzolov M, Cardimona D, et al. Fabrication of highly ordered anodic aluminium oxide templates on silicon substrates. IET Circuits, Devices & Systems, 2007, 1(3): 205-209. DOI:10.1049/iet-cds:20060101 (  0) 0) |
| [19] |
Kossyrev P A, Yin A, Cloutier S G, et al. Electric field tuning of plasmonic response of nanodot array in liquid crystal matrix. Nano letters, 2005, 5(10): 1978-1981. DOI:10.1021/nl0513535 (  0) 0) |
| [20] |
He D, Liu Z J, Fernandes G E, et al. High-purity red coloration via mode-selective absorption in a layered thin-film cavity. AIP Advances, 2018, 8(6): 065226. DOI:10.1063/1.5016990 (  0) 0) |
| [21] |
Ollera D, Fernandes G E, Siontas S, et al. Scalable physical coloration. Materials Research Bulletin, 2016, 83: 556-562. DOI:10.1016/j.materresbull.2016.07.001 (  0) 0) |
| [22] |
Feng L, Musto C J, Kemling J W, et al. Colorimetric sensor array for determination and identification of toxic industrial chemicals. Analytical Chemistry, 2010, 82(22): 9433-9440. DOI:10.1021/ac1020886 (  0) 0) |
| [23] |
J. Plumitallo et al., An Analytic and Experimental Treatment of Fiber Optic Chemical Sensing: Results on Evanescent Wave Spectroscopy, arXiv: 2010.00691v1.
(  0) 0) |
| [24] |
Brocke S A, Degen A, MacKerell Jr J D, et al. Prediction of membrane permeation of drug molecules by combining an implicit membrane model with machine learning. Journal of Chemical Information and Modeling, 2019, 59(3): 1147-1162. DOI:10.1021/acs.jcim.8b00648 (  0) 0) |
| [25] |
Quigley Keegan. Physics Honor Thesis "Physical Amplification of Chemical Colorimetric Sensing and a Transfer Matrix Analysis".Providence: Brown University, USA, 2018.
(  0) 0) |
| [26] |
Osgood Ⅲ R M, Giardini S, Dinneen S, et al. Lightweight longitudinal colorimetric fiber sensing platform with optimized dye/polymer thickness for remote sensing and reconnaissance (Conference Presentation). Proceedings of SPIE Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XXI. Bellingham, WA: SPIE, 2020. 114160N. DOI: 10.1117/12.2569948.
(  0) 0) |
| [27] |
Odessey R, Shen T Y, Oller D, et al. Reduced angle sensitivity of structural coloration on an industrial aluminum platform. Coloration Technology, 2020, 136(3): 296-301. DOI:10.1111/cote.12466 (  0) 0) |
| [28] |
CIELAB color space.https://en.wikipedia.org/wiki/CIELAB_color_space.2020-12-27.
(  0) 0) |
| [29] |
Bohar J, Fernandes G E, Xu J, et al. Spectral-temporal LED lighting modules for reproducing daily and seasonal solar circadian rhythmicities. Proceedings of 2017 IEEE International Conference on Smart Computing (SMARTCOMP). Piscataway: IEEE, 2017. 16964473. DOI: 10.1109/SMARTCOMP.2017.7947047.
(  0) 0) |
| [30] |
Aries M B C, Aarts M P J, van Hoof J. Daylight and health: a review of the evidence and consequences for the built environment. Lighting Research and Technology, 2015, 47(1): 6-27. DOI:10.1177/1477153513509258 (  0) 0) |
| [31] |
Wirz Justice A, Fournier C. Light, health and wellbeing: implications from chronobiology for architectural design. World Health Design: Architecture, Culture, Technology, 2010, 3: 44-49. (  0) 0) |
| [32] |
Commission Internationale de l' Eclairage. CIE S 026/E-2018. CIE System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light. Vienna, Austria: CIE Central Bureau, 2018.
(  0) 0) |
| [33] |
Lucas R J, Peirson S N, Berson D M, et al. Measuring and using light in the melanopsin age trends. Trends in Neurosciences, 2014, 37(1): 1-9. DOI:10.1016/j.tins.2013.10.004 (  0) 0) |
| [34] |
Owens A C S, Cochard P, Durrant J, et al. Light pollution is a driver of insect declines. Biological Conservation, 2020, 241: 108259. DOI:10.1016/j.biocon.2019.108259 (  0) 0) |
| [35] |
Amano T, Ripperger J A, Albrecht U. Changing the light schedule in late pregnancy alters birth timing in mice. Theriogenology, 2020, 154(15): 211-222. DOI:10.1016/j.theriogenology.2020.05.032 (  0) 0) |
| [36] |
Van Reen E, Fernandes G E, Xu J M, et al. Lighting System for Circadian Control and Enhanced Performance. U.S.: US 2018/0339127 A1, 2018-11-09.
(  0) 0) |
| [37] |
Veitch J A. Light for life: emerging opportunities and challenges for using light to influence well-being. Information Display, 2015, 31(6): 16-21. DOI:10.1002/j.2637-496X.2015.tb00856.x (  0) 0) |
 2021, Vol. 28
2021, Vol. 28
