2. College of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150000, China;
3. Cosmonaut Training Center, Beijing 100094, China
Known as the lightest and thinnest matter in the world[1], graphene is the essential structural unit of fullerene, carbon nano-tube, and graphite, and is the first genuine two-dimension fullerene[2-3], which is manufactured artificially at present. Characterized by small density, high strength, resistance to heat and chemical corrosion, high electrical and thermal conductivity, and small thermal expansivity, graphene is honored as the fourth industrial material[4-8]. However, due to the extremely strong Van de Waals' force and no motion of electric charges between the slices of graphene, the intercalation of monomers cannot be materialized through ion exchange reaction between layers, which restricts the nano composition of graphene with some matters[9-10]. A common way is to make graphene into graphene oxide (GO) through oxidized modification and then perform nano composition[11]. GO is a functionalized graphene, whose surface contains massive oxygen-containing groups[12]. Nano composite of oxidized graphene can be used as electrode materials and energy-saving materials[13-14]. Stankovich et al.[15] first conducted grafted modification on GO via isocyanate by grafting a series of diverse side chains onto the surface of GO. The modified GO is difficult to be peeled off in water. This procedure is easy to operate under moderate conditions and with satisfactory grafting effectiveness. The functionalized GO is steadily dispersed through the organic solvent, which provides security for its further processing and application. Such reduced graphene oxidized sheet has a vast prospect of application. For example, it can save hydrogen and act as an electrical conducive filling material in composites.
Over the years, the bioactivity of GO nano composite has attracted much attention. With great specific area and good absorptive capacity, GO nano composite can be mainly applied in carrying drugs, treating tumors, biosensing, materials of medical apparatus and instruments, antibacterial, and antiviral[16-20]. Zhang et al.[21] successfully modified glucan on the carboxyl group of oxidized graphene by forming amido links. Compared with the unmodified oxidized graphene, the biocompatibility of the glucan/graphene oxide nano composite was significantly enhanced, and the toxicity of cells as well as the inhibitory action on the growth of cells were dramatically reduced. GO nano materials were used as tissue engineering scaffolds to support cellular attachment, proliferation, and differentiation[22-24]. Other studies indicated that GO nano composite possesses high toxicity which can cause pulmonary diseases to human[25].
Lauryl betaines are a class of natural compounds with the structure of quaternary ammonium salt, which are mostly applied in the surface active agents of betaine type. They have excellent hydrophilicity and lipophilicity as well as good thermal stability and resistance to oxidation, and their solubility is less subject to pH and electrolytes[26-27]. Lauryl betaine can be applied in food, medicine, textile, daily chemical engineering, and other domains, which has good sterilizing and anti-inflammation performances, exhibits temperate nature, and is less irritative to human eyes, skin, and mucous membrane tissues. However, the washing of lauryl betaines is a painstaking job that requires repeated efforts[28-29]. Thus, though with excellent performances, they fail to meet the demand of various fields due to the single structure and low quality. Therefore, GO is adopted in this paper to modify lauryl betaines in order to improve their functions and enlarge their application range. It was found that compared with lauryl betaines, the lauryl betaines compounds are much easier to rinse.
In recent years, the sailing of hulls in the ocean is seriously polluted by mussels, seaweed, and other pollution organisms, which causes severe damages to the hull structure and the material structure, seriously affecting the combat effectiveness of the ship. In the 19th century, people used cuprous oxide and arsenious oxide with resin for antifouling, but it had little effect and polluted the environment seriously. In the 20th century, self-polishing copolymer antifouling paint was extensively used as detergents. However, the main component of the detergent, organic tin, is pernicious to the mollusk and thus was prohibited from using. Nowadays, detergent needs to be environmentally friendly and with low toxic, making the natural antifouling compounds gradually become the hotspot of research. Therefore, it has always been essential to find effective and environmentally friendly detergents to solve the problems. This study utilized the easily available lauryl betaine, which has good hydrophilicity and lipophilicity, to modify GO, change its hydrophobic surface, and provide with surface activity. Meanwhile, the study attempted to address the issue of difficulty in washing lauryl betaines and the inconvenience for people. The pollution of marine hull was simulated and the cleaning capacity of the material was tested. A skin irritation and allergy test was conducted over the prepared new-type graphene oxide nano composite to observe its irritation and produce the new-type graphene oxide/lauryl betaine composite, which possesses the nature of surface active agents, has fine decontamination ability, and is with low irritation. The results of the study proved that the new-type nano composite synthesized from natural compounds could be used availably and safely, which had a few side effect or pollution to the marine life and the environment. Further, other than being used as the detergent for ship, this new surfactant could be applied in other fields with its excellent surfactivity, such as oil, environment, and chemical industry, and its application value can be developed in other domains in the future. Moreover, there was hardly any environmental pollution, making it safe to be used in large quantities.
1 Materials and Methods 1.1 MaterialsGraphite powder, 3-aminopropyltriethoxysilane, lauryl betaine, potassium permanganate (KMnO4), ammonium nitrate (NaNO3), oil of vitriol, hydrogen peroxide (H2O2), and absolute ethanol were obtained from Harbin Sengda Laboratory Equipment. Kunming Mice were purchased from the Second Hospital of Harbin Medical University. Mussels and Uiva pertusa seaweed were purchased from Harbin Aquatic Company.
1.2 Synthesis1) Synthesis of GO by hummers. 20 g graphite powder and 10 g sodium nitrate were poured into a three-necked flask, and then added with 98% sulfuric acid. The mixture was transferred to a 4 ℃ ice water bath and stirred until complete mixing for 30 min. 60 g potassium permanganate was added in sequence, and the mixture was slowly kneaded with small forceps. The reaction was stirred for 2 h in an ice water bath, and then transferred to a hot water bath at 35 ℃ for 2 h, which was later added with 100 mL deionized water and heated to 98 ℃. The obtained solution was stirred for 15 min and then diluted by adding a large amount of deionized water. At the same time, 25 mL of a 30% hydrogen peroxide solution was added to neutralize the excess potassium permanganate in the reaction. At this point, the solution was khaki. The solution was stirred for 30 min and filtered while hot. Then, it was washed repeatedly in 5% hydrochloric acid and thoroughly with deionized water until the filtrate became neutral. The precipitate was completely dried in a lyophilizer and the product is GO, which was mixed with deionized water and dispersed in deionized water by ultrasonication. The GO suspension was treated and lyophilized[30].
2) Synthesis of graphene oxide/lauryl betaine (GO-LB). 0.1 g GO was dispersed in 100 mL deionized water and sonicated for 40 min to produce a uniformly stable GO solution. After heated to 80 ℃, 2 g dodecyl betaine was slowly dripped into the GO solution under reflux in water bath for 8 h. With the completion of the reaction, the mixture was cooled to room temperature and dried at 80 ℃ for 4 h to obtain a GO-LB nano composite.
3) Synthesis of modified GO by 3-aminopropyl triethoxysilane (GO-KH550). 0.1 g GO was dispersed in 100 mL deionized water and sonicated for 40 min to produce a uniform GO solution. 1.7 g KH550 was added to 105 mL absolute ethanol, which was stirred for 30 min and slowly dropped into the GO solution. The mixture was stirred under reflux at 80 ℃ for 8 h, and after the completion of the reaction, it was cooled to room temperature and allowed to stand for 24 h. It was washed for 3 times with absolute ethanol, then 3 times with deionized water, and finally dried at 80 ℃ for 24 h to obtain a solid, which is a GO-KH550 compound.
4) Synthesis of modified GO-KH550 by lauryl betaine (GO-KH550-LB). 0.1 g GO-KH550 solid was dispersed in 100 mL deionized water and sonicated for 60 min to obtain a homogeneous stable GO-KH550 suspension. 0.5 g dodecyl betaine was added to the GO-KH550 dispersion. The mixture was stirred under reflux at 80 ℃ for 6 h, and after completion of the reaction, it was cooled to room temperature and allowed to stand for 24 h. After washed for 3 times with absolute ethanol, GO-KH550-LB was dried at 80 ℃ for 24 h to produce a solid.
1.3 MethodsStep 1 Characterization. Modifications of the GO particles with lauryl betaine were analysed using the FTIR spectroscopy (Spectrum One B, PerkinElmer, USA). The surface element analysis by X-ray photoelectric spectroscopy (XPS, ESCALAB 250XiThermo Fisher Scientific, USA) 1H NMR was performed on a 400 MHz Bulter NMR spectrometer (Varian, USA) at 25 ℃ in deuterium oxide as a solvent. Element surface analysis was performed by scanning electron microscopy (SEM; SU8010, Hitachi, Japan). The amount of the attached organic substances was determined by thermogravimetric analysis TGA/DTG (STA449-C, Netzcsh, Germany) with heating rate of 10 ℃/min in nitrogen atmosphere.
Step 2 Contact angle measurement. The contact angles of distilled water on GO, GO-LB, and GO-KH550-LB surfaces were measured with static contact angle analyzer (JCY-4, Fangrui, China) on 3 different locations for each material, and the obtained values were averaged as the water static contact angle value for each material.
Step 3 Measurement of KRAFFT Point. GO-LB, GO-KH550-LB, and LB were prepared as 1 wt% solutions in large test tubes. The solutions were heated in water bath to raise the temperature slowly, and stirred to be checked with thermometer. The temperature at the time when the solution turns into transparent and clear is the KRAFFT Point for the solution to be checked, which was recorded in the study. The process was repeated for 3 times, and the values were averaged.
Step 4 Foaming test. GO-LB, GO-KH550-LB, and LB were prepared as 1 wt% solutions. 10 mL solution was poured into a 20 mL measuring cylinder to be checked and stirred at a speed of 12000 r/min for only 60 s. Then the height of the foam was measured, and the the maximum foam volume and the half-life time foam were recorded. The foam was silenced for 1 min, and the height was then re-measured. The foam stability of these materials was calculated. The measurement was carried out for 3 times and the averaging value was calculated.
Step 5 Emulsion ability test. Emulsion is an effect that a liquid in the form of extremely micro droplets evenly disperses in another liquid which is unmixable with it. Emulsion is a liquid-liquid interfacial phenomenon between two unmixable liquids, such as oil and water, which are separate as two layers in the container. Oil in lower density is the top layer and water in larger density is the bottom layer. 40 mL 0.5% GO-LB, GO-KH550-LB, LB aqueous solutions, and 40 mL soybean oil were moved respectively with pipettes to mix cylinders with stopper. The erlenmeyer flask was shaken fiercely up and down for 5 times and then silenced. The time for the bottom aqueous phase to separate a 1 mL layer was recorded. The values were measured for 3 times and the averaging value of the measured figures was recorded.
Step 6 Decontamination capacity test. GO-LB, GO-KH550-LB, and LB were prepared as 1 wt% solutions. Mussel mucus, seaweed, and egg white were evenly applied to steel plate to simulate the marine hull pollution. 20 mL GO-LB, GO-KH550-LB, and LB were placed in the beaker, which was attached with the steel plate containing mussels mucus, Uiva pertusa seaweed, and egg white. Then the absorbance of the solution at 260 nm and 280 nm was measured before and after experiment. Also, the change of protein content in the solution was calculated to determine whether the synthesized GO-LB and GO-KH550-LB had decontaminating ability. The process was repeated for 3 times and the results were averaged.
Step 7 Skin irritation test. All the animal experiments complied with the ARRIVE guidelines and the National Institutes of Health guide for the care and use of laboratory animals. Kunming Mice, equal in number of males and females, are 18-22 g in body weight. They were observed and raised in the laboratory for a week before the test. During the test, they were raised in cages by group, and free to take food and water. Temperature of the animal raising house is 20±4 ℃ and the relative humidity is 50%±10%. A modest amount of graphene oxide/lauryl betaine solid was taken and made into a 1 mg/mL solution for further use. Then, 10 mice were taken and randomly grouped by gender and body weight into an intact skin group and a damaged skin group after raising them for 3 d without anomalies. Next, all the animals were sheared by their hairs on both sides of the spinal column 24 h before the test. Finally, using a scalpel to carve out a "#" character on the position until blood seeped mildly where the damaged skin group was sheared.
Step 8 Acute skin irritation test. The experimented animals shall compare their left side with their right side. 0.5 g tested object was taken and applied directly onto the skin. Next, cover the skin with a piece of two-sheet sterile gauze, and then fix it using a piece of non-irritative rubberized fabric and bandage. Apply the same volume of distilled water onto the other side of the skin as contrast and for 6 h. The residuals of the tested object were scavenged with warm water after the test. 1, 24, 48, and 72 h later, observe with naked eyes and record whether there are blotches, edemas, or the like at the applied position. Observe the skin reaction at the applied position.
Step 9 Repeated skin irritation test. 0.5 g tested object was taken and applied onto one side of skin on a once-per-day basis and for 7 consecutive days. Stop medication for one week after that. Observe and record the blotches, edemas, or other anomalies at the applied position in 1, 24, and 48 h, respectively. From the next day up, shear and scavenge the residual tested object with water or non-irritative solution every time before applying. Observe the result. Process both the contrast and the trial zones in the same way.
Step 10 Scoring by skin irritation level. Calculate the mean cumulative score per animal per day by the following formula: Mean score of reaction = (Total score of blotch formation + Total score of edema formation)/Total number of animals.
Step 11 Skin allergy test. Twenty Kunming Mice were taken. After raising them for 3 d without anomalies, the mice were grouped at random by gender and body weight into a tested object group (GO-LB group, GO-KH550-LB group), a blank control group, and a positive control group. They were then sheared by their hair on the right backside of the spinal column 24 h before the test.
Step 12 Sensitization contact. 0.5 mL distilled water, the tested object, and 0.5% formaldehyde solution (positive contrast) were taken and applied respectively onto the denuded area on the right backside of the animals in each group. Cover this area with a piece of two-sheet gauze, then seal and fix it with a piece of non-irritative rubberized fabric. Scavenge with distilled water until no residual is left after 6 h of medication. Repeat medicating once and irritating three times in total by the same procedure every other day.
Step 13 Excitation contact. At the 14th day after the medication in the last time, adopt the same procedure by applying the corresponding medicine for each group onto the denuded area on the right backside of the mice (The medicine for the positive control group was 0.5% formaldehyde solution). Wash away the tested object with distilled water after 6 h, and observe the skin allergy condition instantly. Continue to observe the skin allergy condition at 1, 24, 48, and 72 h later, respectively. Also, watchfully observe whether the animals have suffered asthma, astasia, shock, or other severe systematic allergic phenomena.
Step 14 Scoring by allergy level. Calculate the mean score of the reaction and sensitization ratio, and determine the level of the experimented animals' allergy to the tested object. Mean score of reaction = (Total score of blotch formation + Total score of edema formation)/Total number of animals. Sensitization ratio = (Number of animals with blotches and edemas on the skin)/Total number of animals.
2 Results and Discussion 2.1 Synthesis and CharacterizationAs shown in Fig. 1(a), the characteristic absorption peaks of GO could be observed and compared with the standard spectra. The absorption peak at the value of 3374 cm-1 was assigned as —OH stretching vibration peak on GO surface, and that at the value of 1726 cm-1 is the C=O vibration absorption peak of carbonyl and carboxyl at the edge of GO. Due to the high moisture absorption ability of the samples, the absorption peak at the value of 1619 cm-1 corresponded with the absorption peak of water molecule deformation vibration. The absorption peak at the value of 1472 cm-1 was from the unoxidized C=C stretching vibration peak. The area near the value of 1390 cm-1 corresponded with the bending vibration peak. The area near the value of 1220 cm-1 is the GO epoxy characteristic peak. The absorption peak at the value of 1067 cm-1 is C—O. There was plenty of oxygen containing functional groups on the GO surface. Fig. 1(b) shows the GO-LB spectrogram, in which the C=O absorption peak and the —COOH absorption peak disappeared at GO, and the replacement was the CO—NH absorption peak at the value of 3326 cm-1. —CO—NH in-plane bending occurred at the value of 1549 cm-1. At the values of 2923 cm-1 and 2853 cm-1, they corresponded with the —CH2 anti-symmetric stretching peak and the —CH2 symmetric stretching peak, respectively. —NH2 deviation angle occurred at the value of 1635 cm-1, CH3 symmetric deformation vibration at 1464 cm-1, COO— absorption peak at 1401 cm-1, alkane C—C in-plane bending vibration at 1341 cm-1, and C—N vibration at 1127 cm-1. Thus it can be concluded that GO and lauryl betaine were successfully grafted. Fig. 2(b) presents the GO-KH550-LB infrared spectrogram, where amid absorption peak occurred at the value of 3273 cm-1, and Si—O—C generated in KH550 hydrolysis at 1116 cm-1 (Fig. 2).
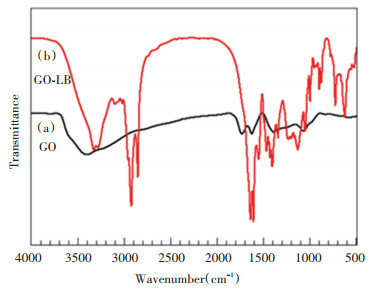
|
Fig.1 FITR spectra for GO and GO-LB |
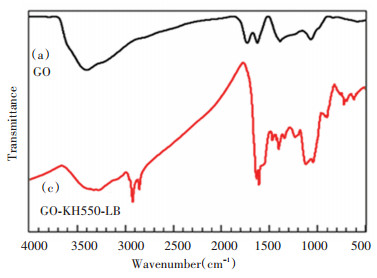
|
Fig.2 FITR spectra for GO and GO-KH550-LB |
Fig. 3 is the C 1s XPS spectrogram for GO before reactions[29]. There are four types of chemical states for GO carbon, i.e., C—C/C=C (283.67eV), C—O (285.9 eV), C=O (286.2 eV), and O—C=O (287.2 eV), while new peaks occurred except the GO quartet in C 1s spectrogram for the composition of GO compounds. As shown in Fig. 3, there was a new C—N peak diverging at the value of 286.62 eV in GO-LB, and a new C—N peak diverging at the value of 286 eV in GO-KH550-LB. It means that GO and lauryl betaine are not an ordinary physical combination, but have successful grafting. Table 1 lists the volume changes of each atom before and after reactions. The C atom volume in GO before the reactions was 44%, which changed into 63.94% in G1O-LB and 59.43% in GO-KH550-LB. It proved that in the reactions, alkane long chains were brought in, which increased the C volume. The oxygen volume was in a significant dropping trend, mainly because GO oxygen containing functional groups were deoxidated, leading to a significant decrease of oxygen volume. By comparing GO-KH550-LB and GO, it was found that there was little element diverging, which was because bridging with KH550 brings in s atoms.
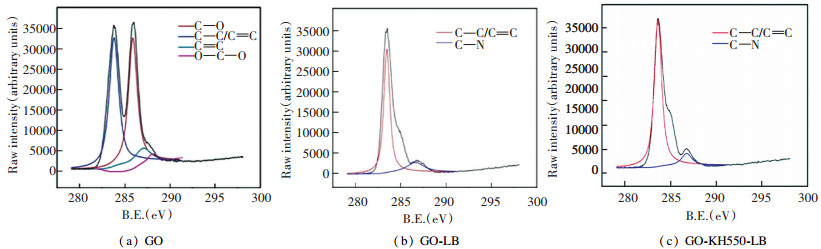
|
Fig.3 XPS spectra for GO, GO-LB, and GO-KH550-LB |
| Table 1 Atomic composition of surface of GO, GO-LB, and GO-KH550-LB determined by XPS analysis |
Fig. 4(a) presents the GO-LB 1HNMR spectrogram. The classification of hydrogen is as follows: δ0.803×10-6-δ0.759×10-6 is the head of CH3 in LB structure; δ1.208×10-6 belongs to (CH2)11 in LB alkane long chain; δ2.165×10-6 belongs to the —CH2 group in LB; δ1.5×10-6 is the GO out-of-plane hydroxyl group; δ1.920×10-6 is H on GO carboxyl six-member ring; δ3.163×10-6 is N+ in LB; and δ3.8×10-6-3.4×10-6 responds with 12 H in GO plane, since GO and N+ in LB connected and formed coupling peak. However, COOH on GO lamella connected to benzene ring should be δ10-13×10-6, which disappeared in the spectrogram. It proved that it was connected with N+ in LB, and LB was attracted by O. Shielding effect of the electron cloud of the nearby H ions resulted in bending folding and thus formed vesicular structure. Fig. 4(b) shows the GO-KH550-LB 1HNMR spectrogram. The classification of hydrogen is as follows: δ3.14×10-6 is N+(CH)2; δ0.789×10-6-0.772×10-6 is head of CH3 in LB; δ1.903×10-6 is H on GO carboxyl six-member ring; δ1.195×10-6 is (CH2)11 in LB; δ2.152×10-6 belongs to —CH2—COOH— group in LB; and δ3.4×10-6-3.7×10-6 is H on the oxidized graphite alkene plane.
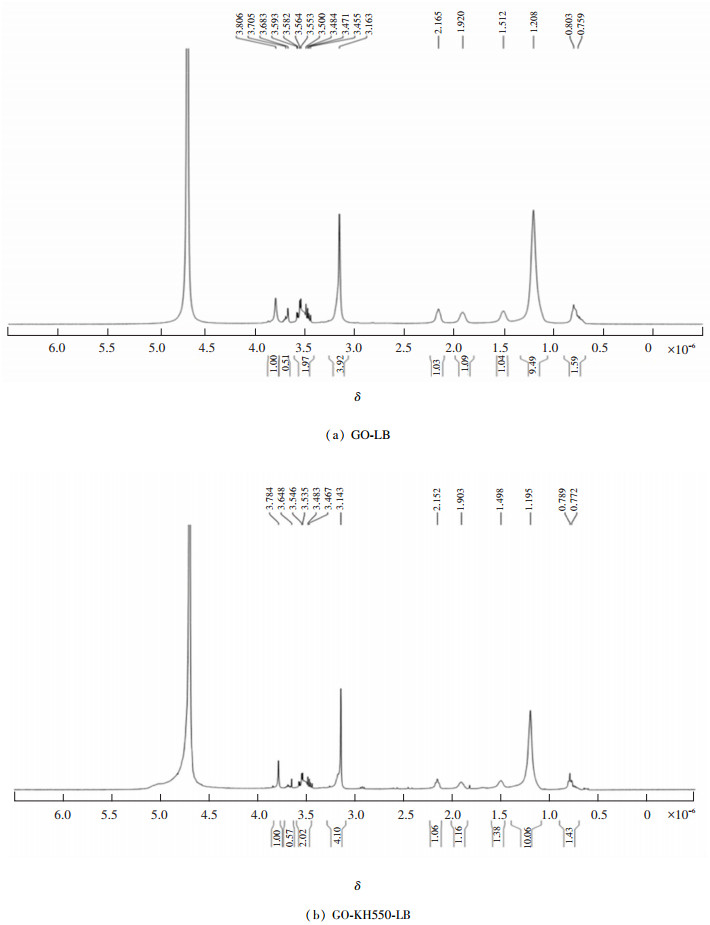
|
Fig.4 1HNMR spectra for GO-LB GO-KH550-LB |
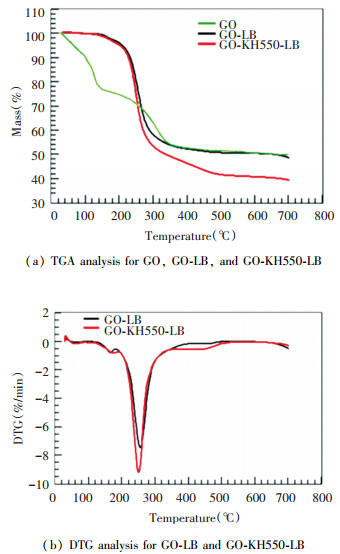
Fig. 5 presents the TG and DTA charts of GO nanometer compound materials formed through two different methods. From the figure, it can be seen that there were all three stages of thermal weight loss process for GO compounds. The first stage was the preliminary heating stage (25-150 ℃). As the temperature increased, the free water and the absorbed water between the lamellas evaporated and caused slight gravimetric loss. The GO-LB weight loss rate was 0.64%, and that of GO-KH550-LB was 1.24%. The weight loss rate mainly related to dryness level, and the initial decomposition temperatures for both were approximately around 30 ℃. The second stage for sharp weight loss occurred between 150-350 ℃. At this stage, unstable oxygen-containing groups on the GO nanometer compound plane decomposed into H2O, which was the major stage of thermal decomposition with a weight loss rate of about 50%. Connecting with the DTG curve, the weight loss rate of GO-LB reached the maximum at 256 ℃, and that of GO-KH550-LB was at 223 ℃.
|
Fig.5 TGA analysis for GO, GO-LB, and GO-KH550-LB, DTG analysis for GO-LB and GO-KH550-LB |
Compared with the DTG curve of GO-KH550-LB, the peak level of that of GO-LB was lower and the peak temperature was higher, which means that the maximum weight loss rate of GO-LB was lower than that of GO-KH550-LB. The third stage was between 300-500 ℃, where the weight loss of GO-KH550-LB continued at a rate of 15%, while GO-LB only experienced slight weight loss at a rate of 5% and approached stability. The last stage was slight weight loss after 500 ℃ at a rate of around 5% through a gradual decomposition process of oxidized graphite olefin carbon structures. When heated to 700 ℃, the weight loss rate of GO-LB was lower than that of GO-KH550-LB, with the values of 56.47% and 72.36%, respectively. From the weight loss rates of the two samples during different stages, the volumes of the GO-KH550-LB interlamination water and unstable oxygen-containing functional groups were all larger than those of GO-LB. It proved from another aspect that the oxidation levels of GO-KH550-LB was larger than that of GO-LB, while the heat stability of GO-KH550-LB was lower than that of GO-LB.
Fig. 6(a) shows the SEM of GO. It can be seen that GO was generally in lamellar structure with relatively course surface and some folds, because plenty of oxygen-containing functional groups were generated on its surface during graphite oxidation. The existence of functional groups damaged the perfect sp2 hybrid orbital of C element in graphite, brought in a lot of sp3 hybrid orbital, which were not in the same plane and generated folding structures. Carboxyl and carbonyl connected at the edge caused slight crimping in GO edges. Fig. 6(c) presents the SEM of lauryl betaine, where the lauryl betaine plane was even and in plate structure. After magnification, folds could be observed.

|
Fig.6 SEM for GO, LB, GO-LB and GO-KH550-LB |
Compound GO and LB are shown in Fig. 6(e). It can be seen that agglomeration appeared and folds increased significantly on the surface, which was possibly because the hydrogen bonds were generated between the plates from GO and LB reactions. After magnification, it can be observed that it was loose with holes and there were relatively large gaps between the GO-LB structures. The reason might be that the compounding increased the absorption capacity of the oxidized graphite alkane and enlarged the holes and gaps. Fig. 6(g) is the SEM for GO-KH550-LB. Because of the electrostatic interaction and interaction between H bond and π-π, the agglomeration level of this material was higher. KH550 equals to crosslinking agent, which stuck GO to LB and generated stacking. After magnification, a fibrous vesicular grid structure could be observed, which helped to increase absorption speed.
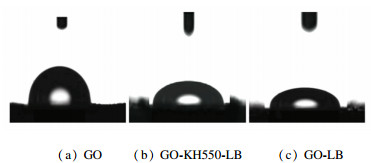
2.2 Decontamination Capability and Safety TestThe contact angle is the intersection angle θ between air-liquid plane tangent line cutting liquid on the intersection point of three phase (air, liquid, solid), and liquid-solid border line, which is a measurement for wetting. If θ < 90°, the solid surface is lyophilic, which means that it is easy for liquids to wet the solid. The smaller the angle is, the higher the wetting level is. If θ>90°, the solid surface is hydrophobic, which means that it is not easy for liquids to wet the solid and liquid tends to move on its surface. The water static contact angle of the oxidized GO was θ=93.3°, which means that it is hydrophobic surface. While the GO surface was not even and with folds, which blocked liquid drops contacting GO surface. GO-LB and GO-KH550-LB showed very strong lyophilic nature with contact angles of 45.8° and 47.2°, respectively. The wetting capacity of GO-LB was better than that of GO-KH550-LB. Besides, liquid spread on the surface of compound materials upon contacting the surface, since they both showed very strong lyophilic natures with small contact angles. Thus, it can be seen that the hydrophobic nature of GO was changed into lyophilic nature after the combination of GO and LB, which increased its wetting capacity (as shown in Fig. 7).
|
Fig.7 Contact angles for GO, GO-KH550-LB, and GO-LB |
The dissolution level of ionic-typed surfactant changed evenly with temperature. When temperature reached one point, the dissolution level of surfactant increased sharply, where the temperature is called KRAFFT Point. From Table 2, it can be seen that the KRAFFT Points of GO-LB, GO-KH550-LB, and LB were around 57.6 ℃, 64.3 ℃, and 43.6 ℃, at which their dissolution levels reached the peak. LB is the control group. The surface activity of GO compound material was not satisfactory under room temperature. The minimum application temperatures as surfactant were above 57 ℃ and 64 ℃, which meant that when the temperatures were higher, they could have effects to a larger extent.
| Table 2 KRAFFT points of GO-LB and GO-KH550-LB |
From Table 3, it can be found that the foam half-life time of GO-LB, GO-KH550-LB, and LB were 29 min, 23 min, and 37 min, respectively. The longer the foam half-life time is, the better the foam stability is. The foam half-life time of GO-LB was longer than that of GO-KH550-LB. According to the foaming heights and 1 min foaming heights of the composites, the foam stability of GO-LB was calculated as around 83.3% and that of GO-KH550-LB was around 55.6%. The foam stability of LB was 85%. Thus, it can be seen that GO-LB was better in foam stability than GO-KH550-LB. Although both GO-LB and GO-KH550-LB were not as good as LB in stability due to the lower foam stability, the new materials are easy to wash.
| Table 3 Foaming property of GO-LB and GO-KH550-LB |
If proper surfactant is added with strong stirring, oil will be dissolved in water and can generate emulsive liquid, which is an emulsion process. The longer the water separation time is, the better the emulsion capacity is. From Table 4, it can be seen that the water separation time of GO-LB was smaller than that of GO-KH550-LB, but could not catch up with LB, indicating that the emulsion capacity of GO-KH550-LB was better than GO-LB.
| Table 4 Emulsification property of GO-LB and GO-KH550-LB |
After the decontamination capacity test, the protein content in the solution before and after the experiment was calculated. LB is the control group. Before the experiment, the protein in GO-LB, GO-KH550-LB, and LB was 0.20, 0.10, and 0.02 mg/mL. GO-LB, GO-KH550-LB, and LB solutions were used to clean steel plate with mussel, and the protein contents in the solution changed to 1.16, 0.81, and 1.66 mg/mL. After cleaned the plate with seaweed, the values changed to 0.42, 0.15, and 0.43 mg/mL. When cleaning the albumen steel plate, the protein in the solutions was 1.28, 0.69, and 1.35 mg/mL. In summary, GO-LB and GO-KH550-LB had cleaning performance, and the decontamination capacity of GO-LB was better than that of GO-KH550-LB.
The result of the acute skin irritation test is shown in Table 5. Through the observation of scoring and comparison, it was found that neither of the two composites caused blotches or edemas among the intact group. In the damaged group, GO-KH550-LB caused some blotches 1 h after being contacted. The blotches on some of the experimented animals disappeared after 24 h and returned to normal after 72 h, which was lightly irritative. Overall, the graphene oxide/lauryl betaine composites were proved mild with low underlying irritation to skin.
| Table 5 Acute skin irritation test |
Table 6 presents the result of the skin irritation test throughout repeated medication. The findings are that neither of the composites caused blotches or edemas after stopping medication in the intact group and both were non-irritative. Likewise, in the damaged group, GO-LB manifested nothing abnormal, while GO-KH550-LB caused blotches at the 1st hour, which disappeared one day later with no anomalies on the skin, and the latter was lightly irritative. Hence, it proved that the GO composites have low irritation to skin over a long term of use.
| Table 6 Repeated skin irritation test |
Table 7 shows the experimental result of the sensitization of graphene oxide/lauryl betaine nano composites to skin. During 6 h of excitation and medication, the animals in the positive medicine group had allergic reactions to different degrees and suffered significant and severe blotches and astasia, with a sensitization ratio of 60%. None of the animals in the other groups suffered abnormal reactions over the tested area of skin. It indicates that the GO composites had no allergic effect. Table 8 shows the experimental result of the excitation test of GO to skin. With the tested object exciting and contacting the experimented animals 14 days after medication in the last time, neither of the composites caused allergic reaction, suggesting that the oxidized graphene/betaine nano-composite could be used safely for low sensitization.
| Table 7 Sensitization contact test |
| Table 8 Excitation contact test |
3 Conclusions
Due to the large number of oxygen-containing functional groups, GO is easy to combine with nano size materials. The active material of the surface of lauryl betaine was used to modify graphene oxide in order to obtain a new type of surface active GO nanomaterials.
The modified GO changed the hydrophobic surface into hydrophilic surface with surface activity. The wetting ability and foaming stability of GO-LB were better than those of GO-KH550-LB, while the emulsifying ability of GO-KH550-LB was better than that of GO-LB. GO-LB and GO-KH550-LB had good decontamination ability for mussel mucus, seaweed, and egg white. Graphene oxide/lauryl betaine was low irritation to skin and low sensitivity, and its preparation method is simple and pollution-free. Therefore, it can replace the traditional surfactant, be used in the field of daily chemical products, and become an important part of antifouling coatings for ships.
| [1] |
Han H S, Jiang Y P, Wang J S. Research and development of US defense structural and functional materials. Aerospace China, 2007(7): 24-27. (in Chinese) (  0) 0) |
| [2] |
Geim A K, Novoselov K S. The rise of graphene. Nature Materials, 2007, 6(3): 183-191. DOI:10.1038/nmat1849 (  0) 0) |
| [3] |
Yang Q H, Lyu W, Yang Y G, et al. Free two-dimensional carbon crystal-single-layer graphene. New Carbon Materials, 2008, 23(2): 97-103. (in Chinese) (  0) 0) |
| [4] |
Huang Y, Chen Y S. Functionalization of graphene and their applications. Science in China Series B-Chemistry, 2009, 39(9): 887-896. (in Chinese) DOI:10.1360/zb2009-39-9-887 (  0) 0) |
| [5] |
Neelgund G M, Oki A, Luo Z P. In situ deposition of hydroxyapatite on graphene nanosheets. Materials Research Bulletin, 2013, 48(2): 175-179. DOI:10.1016/j.materresbull.2012.08.077 (  0) 0) |
| [6] |
Valentini L, Bittolo Bon S, Fortunati E, et al. Preparation of transparent and conductive cellulose nanocrystals/graphene nanoplatelets films. Journal of Materials Science, 2014, 49(3): 1009-1013. DOI:10.1007/s10853-013-7776-9 (  0) 0) |
| [7] |
Yang Y G, Chen C M, Wen Y F, et al. Oxidized graphene and graphene based polymer composites. New Carbon Materials, 2008, 23(3): 193-200. (in Chinese) (  0) 0) |
| [8] |
Kim Y J, Kim B K. Synthesis and properties of silanized waterborne polyurethane/graphene nanocomposites. Colloid and Polymer Science, 2014, 292(1): 51-58. DOI:10.1007/s00396-013-3054-2 (  0) 0) |
| [9] |
Zheng G H, Wu J S, Wang W P, et al. Characterizations of expanded graphite/polymer composites prepared by in situ polymerization. Carbon, 2004, 42(14): 2839-2847. DOI:10.1016/j.carbon.2004.06.029 (  0) 0) |
| [10] |
Allen M J, Tung V C, Kaner R B. Honeycomb carbon: A review of graphene. Chemical Reviews, 2010, 110(1): 132-145. DOI:10.1021/cr900070d (  0) 0) |
| [11] |
Sutter P W, Flege J I, Sutter E A. Epitaxial graphene on ruthenium. Nature Material, 2008, 7: 406-411. DOI:10.1038/nmat2166 (  0) 0) |
| [12] |
He H Y, Klinowski J, Forster M, et al. A new structural model for graphite oxide. Chemical Physics Letters, 1998, 287(1-2): 53-56. DOI:10.1016/S0009-2614(98)00144-4 (  0) 0) |
| [13] |
Mao X J, Xu Z P, Zhao M. The research of the heat dissipation performance for graphite plate and graphite plates with metal film. Energy Conservation Technology, 2013, 31(3): 217-220. (in Chinese) DOI:10.3969/j.issn.1002-6339.2013.03.006 (  0) 0) |
| [14] |
Gao X Q, Guo Q G, Liu L, et al. The study progress on carbon materials with high thermal conductivity. Journal of Functional Material, 2006, 37(2): 173-177. (in Chinese) DOI:10.3321/j.issn:1001-9731.2006.02.002 (  0) 0) |
| [15] |
Stankovich S, Dikin D A, Dommett G H B, et al. Graphene-based composite materials. Nature, 2006, 442: 282-286. DOI:10.1038/nature04969 (  0) 0) |
| [16] |
Shao Y Y, Wang J, Wu H X, et al. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis, 2010, 22(10): 1027-1036. DOI:10.1002/elan.200900571 (  0) 0) |
| [17] |
Zhang L M, Xia J G, Zhao Q H, et al. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small, 2010, 6(4): 537-544. DOI:10.1002/smll.200901680 (  0) 0) |
| [18] |
Akhavan O, Choobtashani M, Ghaderi E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene-tungsten oxide composite under visible light irradiation. Journal of Physical Chemistry C, 2012, 116: 9653-9659. DOI:10.1021/jp301707m (  0) 0) |
| [19] |
Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano, 2010, 4(10): 5731-5736. DOI:10.1021/nn101390x (  0) 0) |
| [20] |
Yang K, Wan J M, Zhang S, et al. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials, 2012, 33(7): 2206-2214. DOI:10.1016/j.biomaterials.2011.11.064 (  0) 0) |
| [21] |
Zhang S, Yang K, Feng L Z, et al. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon, 2011, 49(12): 4040-4049. DOI:10.1016/j.carbon.2011.05.056 (  0) 0) |
| [22] |
Nayak T R, Andersen H, Makam V S, et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano, 2011, 5(6): 4670-4678. DOI:10.1021/nn200500h (  0) 0) |
| [23] |
Park S J, Mohanty N, Suk J W, et al. Biocompatible, robust free-standing paper composed of a TWEEN/graphene composite. Advanced Materials, 2010, 22(15): 1736-1740. DOI:10.1002/adma.200903611 (  0) 0) |
| [24] |
Rodríguez-Lozano F J, García-Bernal D, Aznar-Cervantes S, et al. Effects of composite films of silk fibroin and graphene oxide on the proliferation, cell viability and mesenchymal phenotype of periodontal ligament stem cells. Journal of Materials Science: Materials in Medicine, 2014, 25(12): 2731-2741. DOI:10.1007/s10856-014-5293-2 (  0) 0) |
| [25] |
Luo B, Liu S M, Zhi L J. Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small, 2012, 8(5): 630-646. DOI:10.1002/smll.201101396 (  0) 0) |
| [26] |
del Mar Graciani M, Rodríguez A, Muñoz M, et al. Micellar solutions of sulfobetaine surfactants in water-ethylene glycol mixtures: Surface tension, fluorescence, spectroscopic, conductometric, and kinetic studies. Langmuir, 2005, 21: 7161-7169. DOI:10.1021/la050862j (  0) 0) |
| [27] |
Nong L P, Xiao C L, Zhong Z S. Physicochemical properties of novel phosphobetaine zwitterionic surfactants and mixed systems with an anionic surfactant. Journal of Surfactants and Detergents, 2011, 14(3): 433-438. DOI:10.1007/s11743-011-1259-2 (  0) 0) |
| [28] |
Mazhul L A, Smirnova G V, Lotte V D, et al. Use of the zwitterion detergent Sulfobetain-12 for preparation of virosomes and investigation of their immunogenic and protective properties. Molekuliarnaia Genetika, Mikrobiologiia I Virusologiia, 1988, 9: 144-148. (  0) 0) |
| [29] |
Beckett A H, Kirk G, Virji A S. Surface-active betaines: N-alkyl-NN-dimethylalanine hydrobromides and their critical micelle concentrations. Journal of Pharmacy & Pharmacology, 1967, 19(Suppl): 71S-75S. (  0) 0) |
| [30] |
Hummers W S, Offeman R E. Preparation of graphitic oxide. Journal of the American Chemical Society, 1958, 80(6): 1334-1339. DOI:10.1021/ja01539a017 (  0) 0) |
 2021, Vol. 28
2021, Vol. 28


