Phenolic compounds derived from different industries are a significant class of hazardous substances, which are potentially threatening to human as they are cancerogenic, teratogenetic, and mutagenic[1-2].In general, the content of phenolic compounds in water is in trace amounts, while phenol concentrations range from 1000 to 3000 mg/L in industrial wastewater[3-4]. It is crucial to remove them from the environment. Numerous reported methods (membrane, adsorption, oxidation, bio-oxidation, etc.) have been applied to degrade the pollutants[5-6]. Ultraviolet (UV)-based advanced oxidation processes (AOPs) are identified as the most thorough and efficient methods, which could produce strong-oxidizing hydroxyl radicals (·OH)[7-9]. An annular photo-reactor was applied by Imoberdorf and Mohseni[10] to degrade natural organic matter (NOM) under the radiation of vacuum ultraviolet (VUV). The removal rate of total organic carbon (TOC) reached 93.9% after 180 min irradiation. Zhu et al.[11] revealed that VUV is effective for the elimination and degradation of carbamazepine (CBZ). The second-order rate constant between CBZ and ·OH is confirmed, and the value is 1.4×109 M-1s-1. Additionally, 97.6% decay of CBZ was obtained.
Recently, quantum chemical calculations (QCCs) have been used by various researchers and achieved outstanding results[12-13]. These methods are beneficial to investigate the degradation mechanisms and the characteristics of phenolic pollutants in the environment. Massive literature has proved that phenolic compounds are easily oxidized by radicals. In addition, the radical scavenging ability of phenolic compounds is defined as antiradical potential[14-15], which presents the inherent characteristic. For the elimination of phenolic pollutants by radical reactions, it is important to study their antiradical potential. Plentiful literature suggests that the structural characteristics of phenolic compounds are relevant to the radical scavenging ability, namely antiradical potential. DFT/M05-2X method, together with 6-31G(d, p) and 6-31+G(d, p) basis sets, was applied by Vargas-Sánchez et al.[16] to investigate the structure-antiradical properties relationships of phenolic acid and flavonoids. Besides the structural properties, three free-radical inhibition mechanisms were also taken into account, namely hydrogen-atom transfer (HAT), sequential proton loss electron transfer (SPLET), and stepwise electron and proton transfer (SEPT). Results of these calculations indicated that studied compounds exhibited a trend to donate electrons. Moreover, the analysis of Fukui indices confirmed that reactive sites could suggest the antiradical activity differences of compound. A similar investigation was carried out by the team of Delgado Alfaro[17]. Several researchers considered that a series of quantum-chemical descriptors are useful to reveal the structure-antiradical properties relationships. Such descriptors include bond dissociation enthalpy (BDE), adiabatic ionization potential (AIP), and Fukui function[18-19]. Furthermore, some relevant chemical descriptors are also important to reflect the mechanism of the antiradical activity of phenolic compounds, such as energy of highly occupied molecular orbital (EHOMO), energy of lowest unoccupied molecular orbital (ELUMO), energy gap (ΔE) between EHOMO and ELUMO, electron affinity (A), hardness (η), and electrophilic index (ω)[20-24].
In this work, the photo-degradation of phenolic compounds was investigated experimentally in a vacuum ultraviolet (VUV) system, and analyzed by QCCs to identify the degradation discrepancy through the comparison of molecular structure and relevant quantum chemistry descriptors. In addition, phenol, ODB, MDB, PDB, PNP, and OCP were selected to study the antiradical potential from the aspect of position and substituent type. Medium effects (in the gas phase, water solution, and benzene solution) were investigated to study the antiradical properties of the six phenolic compounds. The molecular structures of the referred phenolic compounds are shown in Fig. 1.
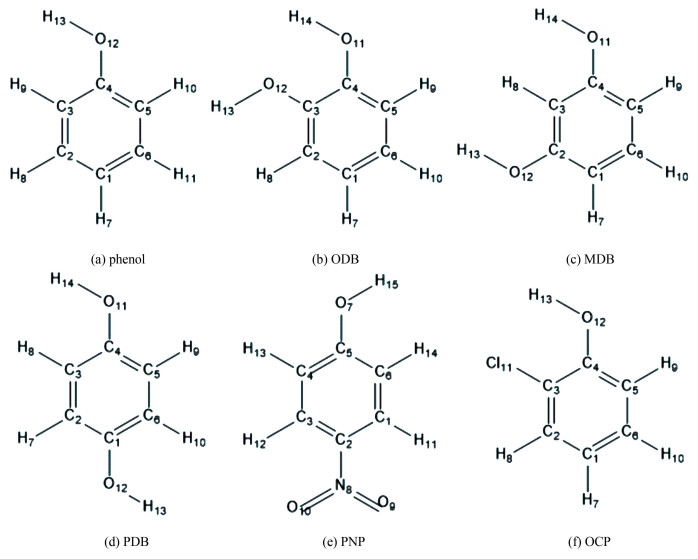
|
Fig.1 Molecular structures of the six chosen phenolic compounds |
1 Experimental Section 1.1 Chemicals
Involved reagent-grade chemicals were purchased and used without further purification. All solutions were prepared with Milli-Q water (18.2 MΩ cm). Phenol, ODB, MDB, PDB, PNP, and OCP were all obtained from Sigma-Aldrich.
1.2 Experimental EquipmentPhoto-oxidation experiments were carried out in a cylindrical glass reactor with a 14 W low-pressure mercury lamp (GCL300T5VH/4) purchased from Light-Tech, USA. The schematic diagram has been depicted in our previous study[25].
1.3 Theory and Computational DetailsNumerous researches prefer the DFT/B3LYP method with a 6-31G* basis set, which is supposed to be available to scientific energies, molecular structures, and so on[26]. In this work, the fundamental structures of the studied phenolic compounds were firstly constructed by using Chemstraw14.0, and then pre-optimized by MM2 method. Finally, these structures were further optimized using the Gaussian 09 program package. All calculations reported in the present study were performed with the DFT/B3LYP method in conjunction with a 6-311G(d, p) basis set in the gas phase, water solution, and benzene solution.
Two main mechanisms were employed to account for the antiradical properties of phenolic compounds. The thermodynamic parameter BDE was calculated based on the HAT mechanism (Eq. (1)). AIP was calculated on account of the single electron transfer mechanism (Eq. (2))[27-28].
| $ \mathrm{R}+\mathrm{ArOH} \rightarrow \mathrm{RH}^{+}+\mathrm{ArO}^{-} $ | (1) |
| $ \mathrm{R}+\mathrm{ArOH} \rightarrow \mathrm{R}^{+}+\mathrm{ArOH}^{-} $ | (2) |
Through charges analysis of atoms in a molecule, the condensed Fukui indices were determined by considering the finite-difference approximations, which are depended on the direction of electron transfer. The relevant equations and attack types are presented in Table 1[29]. The Fukui indices f+ mapped on the density of the six phenolic compounds were calculated by the Multiwfn program[30].
| Table 1 Relevant equations and attack types of Fukui indices |
Furthermore, the chemical indices, such as energy gap (ΔE), electronic affinity (A), ionization potential (I), electronegativity (χ), hardness (η), and electrophilic index (ω), were calculated from the following formulas:
| $ \Delta E=E_{\text {HOMO }}-E_{\text {LUMO }} $ | (6) |
| $ A=-E_{\text {LUMO } } $ | (7) |
| $ I=-E_{\text {HOMO }} $ | (8) |
| $ x=(I+A) / 2 $ | (9) |
| $ \eta=(I-A) / 2 $ | (10) |
| $ \omega=\chi^{2} / 2 \eta $ | (11) |
The fitted curve between ln (Ct/C0) and time of the studied compounds is presented in Fig. 2.
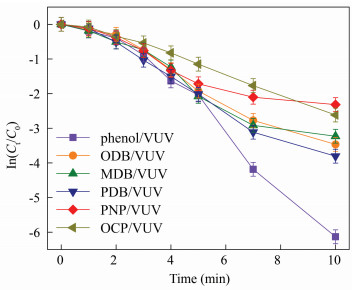
|
Fig.2 Photo-degradation of phenolic compounds under VUV irradiation |
The initial concentration of phenolic compounds is 0.5 mg/L. All the experiments were carried out at room temperature, and all the photo-degradation obeyed pseudo-first-order reaction, which is consistent with previous reports[31-32]. In addition, remarkable differences among the degradation of the six compounds within 10 min are presented. The degradation of phenol, ODB, MDB, and PDB were quicker than those of PNP and OCP. Numerous researches prove that ·OH is mainly oxidative species in the VUV system[7, 33]. Therefore, it is confirmed that the degradation variability between phenolic compounds and ·OH may derive from the structure discrepancy of the studied ones. In other words, the antiradical potential of phenolic compounds may play a crucial role.
2.2 DFT Studies on the Antiradical Potential of the Six Phenolic Compounds 2.2.1 Molecular structural propertiesThe electrostatic potential (ESP) of the six phenolic compounds are depicted in Fig. 3. The six phenolic compounds all had planar structure. The Mulliken charges are presented in Table 2.

|
Fig.3 Illustration of studied phenolic compounds with the numbering of atoms using the B3LYP/6-311G(d, p) method (The gray area stands for the negative charge, and the white area stands for the positive charge) |
| Table 2 Mulliken charges of the six phenolic compounds at 6-311G (d, p) basis set in water-phase |
All oxygen atoms shared negative charges, and the values ranged from -0.4117 to -0.2749. However, the nitrogen atom shared a positive charge with 0.1737, and chlorine atom shared a slightly negative charge with -0.0858. The negative charges were scattered around O and partial C atoms. It suggests these atoms are equipped with electron-donating capacity, which can react with hydroxyl radicals. On the contrary, the positively-charged N and a slightly-negative Cl atom are inherently equipped with electron-withdrawing capacity, resulting in difficulty to react with hydroxyl radicals. Thus, the substitutional functional group on the benzene ring can result in the difference in the antiradical properties of the compounds.
2.2.2 Chemical indicesTable 3 presents the chemical indices of the chosen compounds in the gas phase, water solution, and benzene solution. According to the frontier orbitals theory, EHOMO and ELUMO represent the ability to donate and accept electrons, respectively. High value of EHOMO represents high electron-donating ability, which exhibits high radical scavenging potential, while ELUMO is the opposite. Observed from Table 3, the EHOMO and ELUMO orders of the six phenolic compounds are both PDB > ODB > MDB > phenol > OCP > PNP, no matter what the solvent is. It indicates that PDB, ODB, MDB, and phenol tend to donate electrons, while PNP and OCP tend to accept electrons. Particularly, the location of the substituents also affects the antiradical potential. The calculations show that para-compound and ortho-compound exhibited high antiradical ability.
| Table 3 Chemical indices for phenolic compounds in the gas phase, water solution, and benzene solution using the B3LYP/6-311G(d, p) method |
To further explore the antiradical properties of these phenolic compounds, other relevant quantum-chemical descriptors, such as A, I, x, η, and ω were also obtained based on Koopman's theorem and are presented in Table 3. Low values of χ, I, and ω represent a superior capacity to donate electrons. Their intrinsic properties are associated with the antiradical potential. The calculations prove that phenol, ODB, MDB, and PDB have a low value of χ, I, and ω, which indicate that these compounds with electron-donating groups provide high antiradical ability. In addition, high value of A stands for high capacity of accepting electrons, namely weak antiradical potential, and high value of hardness (η) implies strong resistance to charge transfer. Based on these theories, it is observed that phenol, ODB, MDB, and PDB are more easily to donate electrons, namely their antiradical ability is higher than PNP and OCP. In other words, the molecular with electron-donating groups exhibits excellent antiradical ability.
Moreover, the medium effect was also taken into account by selecting the gas phase, water solution, and benzene solution. The greater the dipole value, the stronger the polarity. Based on the calculations, the dipole order of the three-phases is as follows: water > benzene > gas. Take phenol for example. EHOMO values in the three phases conformed to the following order: benzene (-6.1890 eV) > gas (-6.2194 eV) > water (-6.2662 eV). The same phenomenon can be observed in the value of x, I, and ω of ODB, MDB, and PDB. Low value of them means high antiradical ability. Therefore, for the compound with an electron-donating group, the antiradical ability will decrease with the enhancement of solvent polarity. On the contrary, the opposite consequence is shown by the compound with an electron-withdrawing group like PNP or OCP. In general, phenolic compounds with electron-withdrawing groups in the polar solvent will slightly strengthen the antiradical properties. It is crucial to direct the degradation of phenolic pollutants.
2.2.3 Thermochemical propertiesTo study and compare the antiradical potential of the six phenolic compounds, thermodynamic properties consisting of BDE and AIP values in different solvents were calculated. Results are shown in Table 4 and Table 5, respectively. A good deal of research results indicate that thermos-dynamical parameters are important in understanding the radical scavenging ability of phenolic compounds. These theoretical researches also prove that the antiradical potential of phenolic compounds is widely related to the phenolic O-H bond. Observed from Table 4, the BDE values in the gas-phase result in the following order: phenol < PDB < MDB < ODB < OCP < PNP, in water solution the order is PDB < ODB < OCP < MDB < phenol < PNP, and in benzene solution the order is PDB < ODB < MDB < phenol < OCP < PNP. Take water solution as an example. BDE value of phenol, ODB, MDB, PDB, OCP, and PNP are 383.3230, 363.1067, 381.4852, 353.9174, 411.6784, and 379.1222 kJ/mol, respectively. Phenol, ODB, MDB, and PDB possess higher BDE values compared with those of OCP and PNP. A similar result can be found in the water solution and benzene solution. Generally, phenolic compounds with electron-donating group have lower BDE value and higher antiradical ability, especially for para-compound. Nevertheless, the BDE value of the studied compounds increased with the increase of the solvent polarity, resulting in the reduction of antiradical ability. The discrepancy of BDE values ranged from 4.7259 kJ/mol to 87.1666 kJ/mol.
| Table 4 BDE values of the studied phenolic compounds in the gas phase, water solution, and benzene solution using the B3LYP/6-311G(d, p) method |
| Table 5 AIP values of the studied phenolic compounds in gas phase, water solution, and benzene solution using the B3LYP/6-311G(d, p) method |
Besides, a significant descriptor of phenolic compounds revealing the complexity to donate a single electron was determined by calculating the AIP value, which abides by the SET-PT mechanism. The calculation results are presented in Table 5. AIP values of the studied compounds follow this order in any medium: PDB < ODB < MDB < phenol < OCP < PNP. The discrepancy of AIP values in different solvents ranged from 216.3412 kJ/mol to 241.5460 kJ/mol. The AIP value decreased with the increase of polarity. In other words, phenolic compounds in the polar solvent strengthened antiradical ability. Moreover, it is obvious that compounds with electron-donating group had lower AIP value, exhibiting higher antiradical ability. Take the value in water solution as an example. The AIP values of phenol, ODB, MDB, PDB, OCP, and PNP were 565.0076, 530.8761, 542.9534, 510.1347, 587.3244, and 623.8188 kJ/mol, respectively. A similar result can be seen in gas phase and benzene solution. In conclusion, low BDE and AIP value indicate high antiradical ability.
2.2.4 Fukui indicesThe reactivity of each chosen phenols was confirmed via Fukui indices calculations, so as to explore the reactivity of each atom in these investigated molecules and to evaluate the most active sites for the nucleophilic attack (f+), electrophilic attack (f-), and radical attack (f0). The calculations are shown in Table 6.
| Table 6 Chemical reactivity sites of the studied phenolic compounds obtained by Fukui indices using the B3LYP/6-311G(d, p) method |
The following data analysis is mainly with respect to the values in water solution. The data of phenol show that the main susceptible site for the nucleophilic attack was O12 atom with f+ value of 0.1844; the main susceptible site for the electrophilic attack was C5 atom with f- value of 0.1706; and the main susceptible site for the radical attack was C3 atom with f0 value of 0.1340.
The data of ODB show that the main susceptible site for the nucleophilic attack was O11 atom with f+ value of 0.1505; the main susceptible site for the electrophilic attack was C2 atom with f- value of 0.2190; and the main susceptible site for the radical attack was C2 atom with f0 value of 0.1386.
The data of MDB indicating the main susceptible regions for the nucleophilic attack were C1 and C5 atoms with the same f+ value of 0.1330; the main susceptible region for the electrophilic attack was C3 atom with f- value of 0.2145; and the main susceptible site for the radical attack was C3 atom with f0 value of 0.1433.
The data of PDB show that the main susceptible sites for the nucleophilic attack were O11 and O12 atoms with the same f+ value of 0.1497; the main susceptible sites for the electrophilic attack were C2 and C3 atoms with the same f- value of 0.1700; and the main susceptible sites for the radical attack were C2 and C3 atoms with the same f0 value of 0.1224.
The data of PNP show that the main susceptible site for the nucleophilic attack was O7 atom with f+ value of 0.1777; the main susceptible site for the electrophilic attack was O9 atom with f- value of 0.2427; and the main susceptible site for the radical attack was O9 atom with f0 value of 0.1550.
Moreover, the data of OCP show that the main susceptible site for the nucleophilic attack was O12 atom with f+ value of 0.1741; the main susceptible site for the electrophilic attack was C5 atom with f- value of 0.1790; and the main susceptible site for the radical attack was C2 atom with f0 value of 0.1345.
The susceptible sites for the nucleophilic attack were mainly around the oxygen, while the electrophilic attack and radical attack mainly occurred on the benzene ring. This indicates the antiradical ability of phenolic compounds has a great relationship with oxygen. Furthermore, the solvent effect was also considered to predict the most active sites of phenolic compounds. The results in Table 6 demonstrate that the solvent polarity distinctly altered the distribution of active sites and the Fukui indices values. For phenol, the susceptible region for the nucleophilic attack in the gas phase, water solution, and benzene solution was C4 (0.4970), O12 (0.1844), and O12 (0.1477), respectively. More intriguingly, the vulnerable site for the nucleophilic attack, electrophilic attack, and radical attack of PDB remained unchanged in any solvent except the value, which may be associated with the completely symmetric structure. It could be the primary cause for the high antiradical ability of para-compound.
Fig. 4 shows the Fukui function f+ mapped on the density of the six phenolic compounds. High value of f+ is shown in white (Equivalent value=0.05). The nucleophilic attack reaction region can be observed visually. It is apparent that most positive part of phenol's f+ function is localized in O12, which means the lone pair electrons of oxygen is the preferential reactive section for the nucleophilic attack. For PNP, the positive part of f+ function was only localized in the around oxygen atoms, which indicates that the lone pair electrons of oxygen are the effortless reactive sites for the nucleophilic attack. Nevertheless, the favored reactive regions for the nucleophilic attack of OCP were distributed in O2 and Cl11, which means oxygen and chlorine atoms are vulnerable to the nucleophilic attack. Concerning ODB, MDB, and PDB, the lone pair electrons of oxygen are the vulnerable reactive region for the nucleophilic attack. In general, the region of nucleophilic attack between phenolic compounds and radicals are mainly localized in the around oxygen atoms.
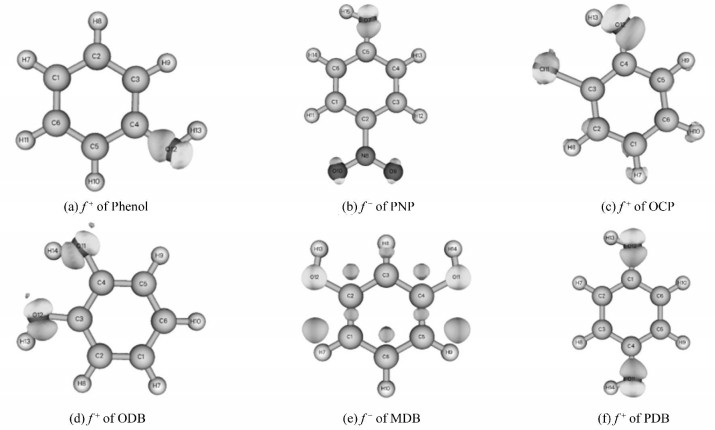
|
Fig.4 Fukui function f+ mapped on the density of the chosen phenolic compounds using the B3LYP/6-311G(d, p) method (High value of f+ is shown in white.) |
3 Conclusions
The degradation rate order of the six studied compounds in the VUV system was phenol > ODB > PDB > MDB > OCP > PNP. Simultaneously, the complete quantum computational investigations using the DFT/B3LYP method with a 6-311G(d, p) basis set have been performed. A series of DFT calculations reveal that phenol, ODB, MDB, and PDB with an electron-donating group exhibited high antiradical potential, while PNP and OCP with an electron-withdrawing group exhibited low antiradical potential. The results are demonstrated by the analysis of chemical indices, thermos-dynamical parameters, and Fukui indices. It is worth nothing that low BDE and AIP value can stand for the high antiradical ability of phenolic compounds. Moreover, the calculations of Fukui indices indicate that the main nucleophilic attack site in the water phase were O12, O11, C1 (C5), O11 (O12), O7, and O12, respectively. The antiradical ability of phenolic compounds has a great relationship with oxygen. Furthermore, the medium effect can also impact the antiradical ability of phenolic compounds through the computational analysis of chemical indices, thermochemical properties, and Fukui indices. Phenolic compounds inclining to donate one electron in polarity solution exhibited lower radical scavenging ability, namely lower antiradical ability. Thus, for the degradation of phenolic compounds, not only the substituent group but also the existing solvent should be considered.
| [1] |
Duan W Y, Meng F P, Cui H W, et al. Ecotoxicity of phenol and cresols to aquatic organisms: a review. Ecotoxicology and Environmental Safety, 2018, 157: 441-456. DOI:10.1016/j.ecoenv.2018.03.089 (  0) 0) |
| [2] |
Miklos D B, Remy C, Jekel M, et al. Evaluation of advanced oxidation processes for water and wastewater treatment-A critical review. Water Research, 2018, 139: 118-131. DOI:10.1016/j.watres.2018.03.042 (  0) 0) |
| [3] |
Gholamreza M, Behnam B, Maryam M. The removal of high concentrations of phenol from saline wastewater using aerobic granular SBR. Chemical Engineering Journal, 2010, 158: 498-504. DOI:10.1016/j.cej.2010.01.038 (  0) 0) |
| [4] |
Bajaj M, Gallert C, Winter J. Treatment of phenolic wastewater in an anaerobic fixed bed reactor (AFBR)-Recovery after shock loading. Journal of Hazardous Materials, 2009, 162: 1330-1339. DOI:10.1016/j.jhazmat.2008.06.027 (  0) 0) |
| [5] |
Alshabob M, Onaizi S A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: current status and potential challenges. Separation and Purification Technology, 2019, 219: 186-207. DOI:10.1016/j.seppur.2019.03.028 (  0) 0) |
| [6] |
Lai T L, Lee C C, Wu K S, et al. Microwave enhanced catalytic degradation of phenol over nickel oxide. Applied Catalysis B: Environmental, 2006, 68: 147-153. DOI:10.1016/j.apcatb.2006.07.023 (  0) 0) |
| [7] |
Zoschke K, Börnick H, Worch E. Vacuum-UV radiation at 185 nm in water treatment-A review. Water Research, 2014, 52: 131-145. DOI:10.1016/j.watres.2013.12.034 (  0) 0) |
| [8] |
Boyjoo Y, Sun H Q, Liu J, et al. A review on photocatalysis for air treatment: from catalyst development to reactor design. Chemical Engineering Journal, 2017, 310: 537-559. DOI:10.1016/j.cej.2016.06.090 (  0) 0) |
| [9] |
Gagol M, Przyjazny A, Boczkaj G. Wastewater treatment by means of advanced oxidation processes based on cavitation-A review. Chemical Engineering Journal, 2018, 338: 599-627. (  0) 0) |
| [10] |
Imoberdorf G, Mohseni M. Degradation of natural organic matter in surface water using vacuum-UV irradiation. Journal of Hazardous Materials, 2011, 186: 240-246. DOI:10.1016/j.jhazmat.2010.10.118 (  0) 0) |
| [11] |
Zhu S M, Dong B Z, Wu Y T, et al. Degradation of carbamazepine by vacuum-UV oxidation process: kinetics modeling and energy efficiency. Journal of Hazardous Materials, 2019, 368: 178-185. DOI:10.1016/j.jhazmat.2019.01.043 (  0) 0) |
| [12] |
Issa T B, Sayari F, Ghalla H, et al. Synthesis, crystal structure, DFT calculations and molecular docking of l-pyroglutamic acid. Journal of Molecular Structure, 2019, 1178: 436-449. DOI:10.1016/j.molstruc.2018.10.033 (  0) 0) |
| [13] |
Arunagiri C, Anitha A G, Subashini A, et al. Synthesis, X-ray crystal structure, vibrational spectroscopy, DFT calculations, electronic properties and Hirshfeld analysis of (E)-4-Bromo-N'-(2, 4-dihydroxy-benzylidene)benzohydrazide. Journal of Molecular Structure, 2018, 1163: 368-378. DOI:10.1016/j.molstruc.2018.03.023 (  0) 0) |
| [14] |
Amarowicz R, Shahidi F. Antioxidant activity of broad bean seed extract and its phenolic composition. Journal of Functional Foods, 2017, 38B: 656-662. DOI:10.1016/j.jff.2017.04.002 (  0) 0) |
| [15] |
Amić A, Marković Z, Klein E, et al. Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chemistry, 2018, 246: 481-489. DOI:10.1016/j.foodchem.2017.11.100 (  0) 0) |
| [16] |
Vargas-Sánchez R D, Mendoza-Wilson A M, Torrescano-Urrutia G R, et al. Antiradical potential of phenolic compounds fingerprints of propolis extracts: DFT approach. Computational and Theoretical Chemistry, 2015, 1066: 7-13. DOI:10.1016/j.comptc.2015.05.003 (  0) 0) |
| [17] |
Delgado-Alfaro R A, Ramos-Organillo A A, Flores-Moreno R, et al. Antiradical capacity of a series of organotin(Ⅳ) compounds: a chemical reactivity study in the Density Functional Theory framework. Inorganica Chimica Acta, 2014, 413: 143-148. DOI:10.1016/j.ica.2014.01.008 (  0) 0) |
| [18] |
Hussan K P S, Thayyil M S, Rajan V K, et al. DFT studies on global parameters, antioxidant mechanism and molecular docking of amlodipine besylate. Computational Biology and Chemistry, 2019, 80: 46-53. DOI:10.1016/j.compbiolchem.2019.03.006 (  0) 0) |
| [19] |
Suhaj M. Spice antioxidants isolation and their antiradical activity: a review. Journal of Food Composition and Analysis, 2006, 19(6-7): 531-537. DOI:10.1016/j.jfca.2004.11.005 (  0) 0) |
| [20] |
Yu S S, Wang Y S, Ma Y J, et al. Structure, thermal stability, antioxidant activity and DFT studies of trisphenols and related phenols. Inorganica Chimica Acta, 2017, 468: 159-170. DOI:10.1016/j.ica.2017.07.022 (  0) 0) |
| [21] |
Parr R G, Pearson R G. Absolute hardness: companion parameter to absolute electronegativity. Journal of the American Chemical Society, 1983, 105(26): 7512-7516. DOI:10.1021/ja00364a005 (  0) 0) |
| [22] |
Yang W T, Parr R G. Hardness, softness, and the Fukui function in the electronic theory of metals and catalysis. Proceedings of the National Academy of Sciences of the United States of America, 1985, 82(20): 6723-6726. DOI:10.1073/pnas.82.20.6723 (  0) 0) |
| [23] |
Roy R K, Krishnamurti S, Geerlings P, et al. Local softness and hardness based reactivity descriptors for predicting Intra-and intermolecular reactivity sequences: carbonyl compounds. The Journal of Physical Chemistry A, 1998, 102(21): 3746-3755. DOI:10.1021/jp973450v (  0) 0) |
| [24] |
Parr R G, Szentpály L V, Liu S. Electrophilicity index. Journal of the American Chemical Society, 1999, 121(9): 1922-1924. DOI:10.1021/ja983494x (  0) 0) |
| [25] |
Cao T T, Xu T F, Zhao M N, et al. Application of vacuum-ultraviolet (VUV) for phenolic homologues removal in humic acid solution: efficiency, pathway and DFT calculation. Journal of Hazardous Materials, 2020, 384: 121464. DOI:10.1016/j.jhazmat.2019.121464 (  0) 0) |
| [26] |
Chermette H. Chemical reactivity indexes in density functional theory. Journal of Computational Chemistry, 1999, 20: 129-154. DOI:10.1002/(SICI)1096-987X(19990115)20:1<129::AID-JCC13>3.0.CO;2-A (  0) 0) |
| [27] |
Sykula A, Kowalska-Baron A, Dzeikala A, et al. An experimental and DFT study on free radical scavenging activity of hesperetin Schiff bases. Chemical Physics, 2019, 517: 91-103. DOI:10.1016/j.chemphys.2018.09.033 (  0) 0) |
| [28] |
KancheVa V D, Saso L, Angelova S E, et al. Antiradical and antioxidant activities of new bio-antioxidants. Biochimie, 2012, 94(2): 403-415. DOI:10.1016/j.biochi.2011.08.008 (  0) 0) |
| [29] |
Elhorri A M, Belaid K D, Zouaoui-Rabah M, et al. Theoretical study of the azo dyes dissociation by advanced oxidation using Fukui indices. DFT calculations. Computational and Theoretical Chemistry, 2018, 1130: 98-106. DOI:10.1016/j.comptc.2018.03.018 (  0) 0) |
| [30] |
Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer. Journal of Computational Chemistry, 2012, 33(5): 580-592. DOI:10.1002/jcc.22885 (  0) 0) |
| [31] |
Kiattisaksiri P, Khan E, Punyapalakul P, et al. Photodegradation of haloacetonitriles in water by vacuum ultraviolet irradiation: mechanisms and intermediate formation. Water Research, 2016, 98: 160-167. DOI:10.1016/j.watres.2016.04.010 (  0) 0) |
| [32] |
Huang L, Jing H Y, Cheng Z H, et al. Different photodegradation behavior of 4-tert-octylphenol under UV and VUV irradiation in aqueous solution. Journal of Photochemistry and Photobiology A: Chemistry, 2013, 251: 69-77. DOI:10.1016/j.jphotochem.2012.10.014 (  0) 0) |
| [33] |
Xie P C, Yue S Y, Ding J Q, et al. Degradation of organic pollutants by Vacuum-Ultraviolet (VUV): kinetic model and efficiency. Water Research, 2018, 133: 69-78. DOI:10.1016/j.watres.2018.01.019 (  0) 0) |
 2021, Vol. 28
2021, Vol. 28


