2. School of Marine Science and Technology, Harbin Institute of Technology (Weihai), Weihai 264209, Shandong, China;
3. Shandong Provincial Engineering Technology Research Center of Marine Health Food, Rongcheng 264300, Shandong, China;
4. College of Food Science and Technology, Bohai University, Jinzhou 121013, Liaoning, China
According to reports, a large number of by-products are produced every year during the processing of fish productions, and part of them can be used for the production of high value-added intermediate foodstuff such as surimi[1], which is consumed world-widely. Surimi cake is a type of high-temperature fried food, which can be eaten directly and has been used widely in catering as foodstuff. However, lipid oxidation is closely associated with the quality of surimi cake, which is composed of a complex chain of reactions. It initially attributes to the primary oxidation product, i.e., the formation of hydroperoxide. Then, after a long-term accumulation in oxidation conditions, the peroxide will be further oxidized to produce secondary oxidation products[2]. However, most of the secondary oxidation products will produce unacceptable odors and participates in other biological reactions, reducing the quality of the food[3]. Therefore, the lipid oxidation of foods is a fundamental issue of food quality control.
Methods for the evaluation of the lipid oxidation of food have been reported[2]. Recently, analysis of volatile compounds has been utilized in food lipid oxidation analyses diffusely[4-6]. Compared with the common chemical methods (such as POV/TBARS), volatile compounds analyses method is fast, simple, and with high sensitivity. Many studies concentrated on the overall aroma of fish products, which not only contribute to the positive sensory attributes but also produce "off-flavors"[7]. The electronic nose (E-nose) and headspace solid-phase microextraction (HS-SPME) with gas chromatography mass spectrometer (GC-MS) technology are usually used in the analyses of volatile compounds of lipid oxidation[2, 8-10]. E-nose has been used to precisely detect and distinguish samples with complex odors, which is at low cost[11]. Researchers utilized HS-SPME-GC-IT/MS and sensory evaluation to analyze the volatile components of the Senegalese sole muscle during different storage periods[7].
In recent years, many studies used antioxidants as synthetic antioxidants and natural antioxidants to extend the shelf-life and the inhabitation of food[12-13]. Nowadays, people are paying more and more attention to food safety, so natural antioxidants are largely demanded[14-15]. Tea polyphenols are widely used in food because they have both antioxidant and antibacterial activities[16-17]. Thus, the objective of this study is to evaluate the effectiveness of tea polyphenols in the prevention and reduction of the lipid oxidation of surimi cake during ice storage.
1 Materials and Methods 1.1 Sample PreparationIn this study, samples of fresh surimi cake were collected immediately from Taixiang (Rongcheng, China) after production. Tea polyphenols(0% and 0.2%), purchased from Rhawn Co., Ltd. (Shanghai, China), were dissolved in water and added to the samples of the same weight. The samples were then fried at 160 ℃ for 6-8 min and stored at 4 ℃ for 0, 2, 4, 6, 8, 10, 12, 14, and 16 days for analysis. All the experiments were performed with replicates.
1.2 Determination of Lipid OxidationLipids were extracted from surimi cakes using the modified method of Floch[18]. First, the samples(5.0 g) were mixed with 40 mL chloroform and 20 mL methanol. The mixture was then shaken for 30 min and stood for 12 h. Next, 20 mL water was added, and the mixture was filtered after being shaken for 30 min. The lower phase which was treated with excessive anhydrous sodium sulfate was filtered again, and the lipid in the supernatant was rotationally evaporated and concentrated using water bath at 40 ℃. POV of the primary lipid oxidation products was measured in the form of lipid hydroperoxide. The products of the secondary lipid oxidation were measured using the TBARS assay, and the results based on the absorbance were at 532 nm and 600 nm.
1.3 Analysis of Volatile Compounds by E-noseThe German PEN3 E-nose for the surimi cake odor identification was equipped with 10 metal sensors based on different surimi cake odors. The E-nose test time is 120 s, the headspace temperature is 25 ℃, and the internal flow and sample flow rate are 300 mL/min. Each sample was repeated for three times.
1.4 HS-SPME-GC-MS AnalysisIn this part, 3 g surimi cake samples were weighed and sealed up with cap equipped with silicon/PTFE septa. Three types of coating fibers for HS-SPME were tested, i.e., 75 μm Carboxen/poly- dimethyl-siloxane coating (CAR/PDMS), 65 μm poly-dimethyl- siloxane/divinyl-benzene coating(PDMS/ DVB), and 50/30 μm Carboxen/poly-dimethyl-siloxane/ divinyl- benzene (CAR/PDMS/DVB) coating fibers. The samples were then exposed for 30 min for the analysis of HS-SPME. Volatile compounds were analyzed using the Agilent GC-MS (7890A/5975C, USA) with the flow rate of 1.0 mL/min.
1.5 Statistical AnalysisAn analysis of variance (ANOVA) was applied for experimental data, and the significant differences were defined as p < 0.05.
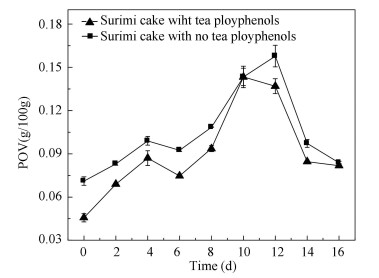
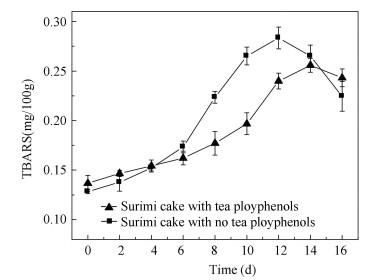
2 Results and Discussion 2.1 Nemipterus virgatus Surimi Cake Storage ExperimentPOV has been utilized in the determination of the primary oxidation products[2]. Fig. 1 reveals the POV changes of the surimi cake during ice storage in 16 days. It showed that POV increased significantly (p < 0.05) in the two groups on the 8th day, and the POV of the experimental group added with tea polyphenol was always lower than that of the control group without tea polyphenol. The highest value of the control samples was 0.158 g/100 g and that of the experimental samples (with tea polyphenols) was 0.143 g/100 g, which then decreased until the end of the processing. In comparison with the experimental group, the control group (untreated surimi cake) exhibited higher POV during the whole storage process, and the initial POV was not stable, which might be due to the instability of the hydrogen peroxide. It was the initial product of lipid oxidation that was formed by the oxidation of fatty acids. Meanwhile, the hydrogen peroxide decomposed to the secondary products or interacted with proteins or other substances[19].Variations of POV content indicated that the treatment with tea polyphenols as antioxidants provided the most effective protection against lipid peroxidation compared with that of the control group. The addition of tea polyphenols to surimi cake would inhibit the accumulation of the hydrogen peroxide[20]. TBARS content is a measure of lipid peroxidation. Participates in TBARS reaction substances, which are generated by peroxide, are oxidized to aldehyde and ketone[12]. As monitored by TBARS, lipid oxidation in surimi cake with and without tea polyphenols blend is shown in Fig. 2. The TBARS value in surimi cake significantly increased on the 8th day of the refrigerated storage (p < 0.05), which indicated that tea polyphenols could inhibit the production of the secondary oxidation products, thus reducing the content of the secondary oxidation products[21]. The highest values of the control group and the experimental group were 0.283 mg/100 g and 0.256 mg/100 g, respectively. In particular, the TBARS value of the surimi cake with tea polyphenols increased more slowly than that without tea polyphenols, and the highest value of the surimi cake with tea polyphenols was lower than that without tea polyphenols. It indicated that tea polyphenols in surimi could inhibit the next reaction of the secondary oxidation products, which was in good agreement with the research in Ref. [22], reporting that natural antioxidant can inhibit lipid oxidation. Then, the TBARS values in both groups decreased. It is noteworthy that the change of the TBARS values for the control group and the experimental group was similar for all the samples. The experimental group with tea polyphenols changed gently compared with that without tea polyphenols. The decrease in the TBARS value of the control and experimental groups was probably due to some of the substances reacting with the secondary oxidation products, such as amines and protein.
|
Fig.1 Change of POVs of surimi cake during refrigerated storage |
|
Fig.2 Change of TBARS values of surimi cake during refrigerated storage |
2.2 Analysis of E-nose
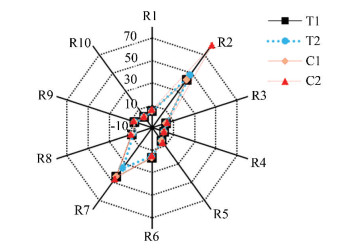
E-noses are growing in popularity as objective automated non-destructive techniques to analyze food flavors[11]. The volatile compounds radar fingerprint chart of different surimi cakes during refrigerated storage is shown in Fig. 3, in which five sensor response values, i.e., R2 (W5S), R6 (W1S), R7 (W1W), R8 (W2S), and R9 (W2W), were higher than others. While the response values of R2 (W5S) and R7 (W1W) for the experimental group with the addition of tea polyphenols were significantly lower than those of the control group. Moreover, R1 (W1C), R3 (W3C), R4 (W6S), R6 (W1S), and R10 (W3S) almost overlapped, which indicated that the volatile ingredients in the surimi cake were similar in composition[6]. Additionally, the outcomes of E-nose displayed that there were some changes of the fingerprints outline of the aroma of the surimi cake during the ice storage.
|
Fig.3 Radar fingerprint chart of volatile compounds in different surimi cakes |
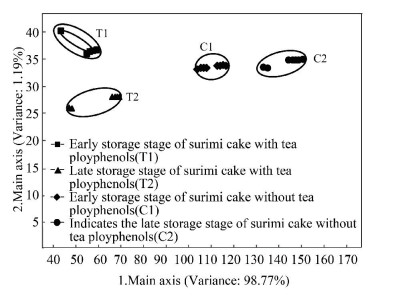
Principal component analysis (PCA) is a simple approach that can transform multidimensionally possibly correlated variables into lowdimensional linearly uncorrelated variables[6, 23]. The use of PCA statistics for different samples of volatile components can obviously lead to different results. Fig. 4 shows the results of PCA for the surimi cake samples stored at 4 ℃ with or without tea polyphenols. The higher the contribution rate is, the better the reflection of the original multi index information on the principal components can be[6]. Generally, the method is feasible when the total contribution rate is over 84%. In this study, the contribution rates of the first and second principal components were identified to be 98.77% and 1.19%, respectively. The accumulative variance contribution rate was 99.96% (more than 85.0%), which indicated that both of the principal components involved most of the information of the surimi cake volatile compounds. The measurements corresponding to samples T1, T2, C1, and C2 were grouped around their barycenter, and in particular for surimi cake samples T2 and C2. Furthermore, most of the data points of the intra and inter groups were well dispersed (Fig. 4), which demonstrated that the differences of the samples along the vertical axis were less significant than those along the horizontal axis[24]. Besides, the scattering on the horizontal and vertical axes might be caused by the occurrence of more new species of the volatile compounds in the headspace of the surimi cake without tea polyphenols samples, which characterized the deterioration of the surimi cake stored at 4 ℃.
|
Fig.4 PCA of E-nose data for surimi cakes in refrigerated storage with and without tea polyphenols |
2.3 Analysis of HS-SPME-GC-MS
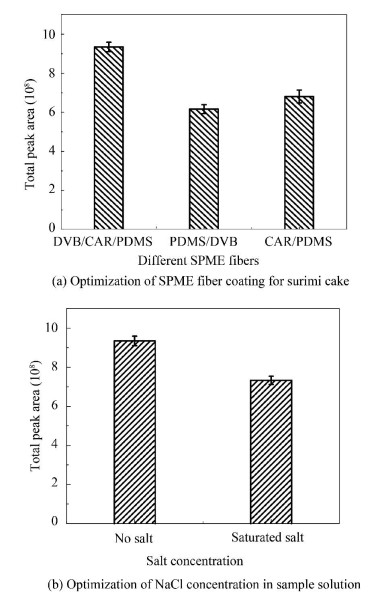
The type of SPME fiber coating and the ionic concentration (NaCl concentration in the experiment) are the main factors in HS-SPME[4, 9]. The optimized extraction efficiency and analytical sensitivity of the three types of coating fibers were analyzed. Fig. 5(a) shows the different fibers corresponding to volatiles, which were extracted from surimi cakes. CAR/PDMS/DVB fiber showed a better result in the light of the highest total peak area in GC-MS chromatogram than PDMS/DVB and CAR/PDMS fibers. Therefore, 50 μm CAR/PDMS/DVB was selected as the fiber for the next research. Usually, adding salt could improve the efficiency of extraction[9], however, it was found in this study that the experiment group which was added with saturated NaCl had relatively low peak area and the efficiency of the extraction was reduced (Fig. 5(b)).
|
Fig.5 Optimization of the headspace solid-phase microextraction (HS-SPME) sampling conditions for Nemipterus virgatus surimi cake volatiles |
HS-SPME-GC-MS was used to analyze the volatile compounds of the surimi cake, and 52 compounds were selected in this study (Table 1), including aldehydes, ketones, alcohols, hydrocarbons, acids, heterocycles, and amines. The major components among the volatile compounds were aldehydes, alcohols, and heterocycles, followed by amines. The content lower than other compounds was ketones, and hydrocarbons and acids were minor in the surimi cake with or without tea polyphenols during ice storage. Most of the compounds detections in this study were also reported for other fish and seafood, such as turbot, horse mackerel, and anchovies[9, 25-26]. High contents of ethanol, (E, E)-2, 4-decadienal, hexanal, nonanal, 2-pentyl-furan, 2-methyl-1, 4-benzenediamine, and 2, 5-dimethyl-pyrazine were in both experimental group and control group in the early stage of storage. However, the contents of aldehydes, ketones, alcohols, and acids were all decreased in the late stage.
| Table 1 Volatile components identified in surimi cake with or without tea polyphenols during cold storage |
Aldehydes are formed by polyunsaturated fatty acid oxidation. In this study, 8 aldehydes were identified in all samples, and the 3 saturated aldehydes were hexanal, nonanal, and heptanal. Hexanal is representative substance of lipid oxidation, which is only saturated aldehydes that are mainly from 13-hydroperoxide of linoleate[27]. It was reported that hexanal significantly contributes to the characteristic aroma of being fish-like, grassy, sour, and rancid[9, 28]. During the late period of the storage, the hexanal content of the experimental group with tea polyphenol surimi cake reduced from 4.56% to 3.88%. In comparison with the untreated surimi cake, the hexanal content of the experimental group decreased significantly (p < 0.05). It showed that tea polyphenols might inhibit 13-hydroperoxide of linoleate to hexanal, thus reducing the off-odors of the surimi cake. According to the previous studies associated with lipid, heptanal was generated from n-6 polyunsaturated fatty acid (PUFA) oxidation, while nonanal with rancid fruity aroma was generated from the oxidation of n-9PUFA[9].
Two ketones were detected in surimi cake, i.e., 2, 3-octanedione and 3-nonen-2-one (Table 1). Among them, 2, 3-octanedione only presented in the surimi cake which has no tea polyphenols, and it was not found in that with tea polyphenols. 3-nonen-2-one was found in the early period of the storage in all samples and the content in experimental groups was lower than that in control groups, which indicated that ketones were the primary products of lipid oxidation and was not stable, and tea polyphenols could delay the generation of ketones.
In total, 6 alcohols were identified in all samples by HS-SPME-GC-MS, and ethanol always existed in surimi cake (with or without tea polyphenols). However, the content of 1-octen-3-ol and ethanol decreased significantly during storage (p < 0.05). Cis-4-methyl-cyclohexanol only presented at the initial period of the storage, while 2, 2-dimethyl-1-octanol at the late of storage. 2-(vinyloxy)-ethanol and 2-methyl-(Z)-3-octen-2-ol were only detected in the surimi cake without tea polyphenols, whose contents increased in the late stage. In general, the unsaturated alcohols were generated by the autoxidation of unsaturated fatty acids and they could contribute to mushroom or metal flavor[29]. 1-Octen-3-ol might have originated from linoleic acid decomposition of surimi cake, which is associated with mushroom, fresh, and plant-like odors[9].
Since hydrocarbons have high odor threshold values, they insignificantly contribute to the flavor. Hydrocarbons consist of alkenes and alkanes, and the content of alkanes was higher than alkenes. In this study, long chain aliphatic hydrocarbons were mainly identified. D-limonenenene was detected in all samples and the content was relatively high. However, it has been reported that D-limonene may be formed from the surrounding environment[30].
Heterocycles involve pyrazines and furans, and five pyrazines were identified (Table 1), among which 2, 5-dimethyl pyrazine exhibited the highest content in surimi cake compared with other pyrazines. In contrast to our results, no pyrazine was found in raw fish or fish-products[9, 26]. The heterocyclic compounds of the surimi cake with tea polyphenol were lower than those in control group during the whole storage (p < 0.05). Pyrazines compounds are Maillard reaction products[31]. Furans are mainly 2-pentyl-furan, and the formation of 2-pentyl furan mainly involves singlet oxygen[32]. Furans and pyrazines mostly exist in cooked (with fried) food. These findings were in agreement with the report of Lu[30], which also found furans in cooked meat.
Nitrogenous compounds are mainly generated from spoilage microorganisms, such as Pseudomonas spp.[26] Two acids were detected in this study, and acetic acid existed in all of the surimi cake samples, which was originated by the action of lactic acid bacteria. In the later stage of the storage, the content of acetic acid decreased obviously in the experimental group (p < 0.05), which indicated that tea polyphenols had a certain antibacterial activity.
3 ConclusionsThe lipikd oxidation of Nemipterus virgatus surimi cake and the change of the volatile compounds during storage were investigated. According to the results, changes of the POV and the TBARS values of the samples, which were treated with tea polyphenols, were significantly lower than those of the untreated samples. According to the consequence, the main flavor compounds of surimi cake were identified as ethanol, hexanal, 2-hydroxy-propanamide, 2, 5-dimethyl-pyrazine, 2-methyl-1, 3-benzenediamine, 2-pentyl-furan. In the control group (without tea polyphenols), the contents of hexanal, acetic acid, 2- hydroxy-propanamide, and 2-methyl-1, 4-Benzenediamine were increased. In contrast, with the addition of tea polyphenols, the contents decreased in these compounds. Compared with the control group, the tea polyphenols promoted the reduction of alcohols. The results provide theoretical basis for the antioxidant mechanism of the surimi cake treated with tea polyphenols during ice storage process, which needs to be further studied.
| [1] |
Fogaça F H S, Trinca L A, Bombo A J, et al. Optimization of the surimi production from mechanically recovered fish meat (MRFM) using response surface methodology. Journal of Food Quality, 2013, 36(3): 209-216. DOI:10.1111/jfq.12019 (  0) 0) |
| [2] |
Barriuso B, Astiasarán I, Ansorena D. A review of analytical methods measuring lipid oxidation status in foods: a challenging task. European Food Research and Technology, 2013, 236(1): 1-15. DOI:10.1007/s00217-012-1866-9 (  0) 0) |
| [3] |
Kanner J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Molecular Nutrition and Food Research, 2007, 51(9): 1094-1101. DOI:10.1002/mnfr.200600303 (  0) 0) |
| [4] |
Iglesias J, Medina I. Solid-phase microextraction method for the determination of volatile compounds associated to oxidation of fish muscle. Journal of Chromatography A, 2008, 1192(1): 9-16. DOI:10.1016/j.chroma.2008.03.028 (  0) 0) |
| [5] |
Damerau A, Kamlang-ek P, Moisio T, et al. Effect of SPME extraction conditions and humidity on the release of volatile lipid oxidation products from spray-dried emulsions. Food Chemistry, 2014, 157: 1-9. DOI:10.1016/j.foodchem.2014.02.032 (  0) 0) |
| [6] |
Yang W J, Yu J, Pei F, et al. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME-GC-MS and electronic nose. Food Chemistry, 2016, 196: 860-866. DOI:10.1016/j.foodchem.2015.09.097 (  0) 0) |
| [7] |
Moreira N, Valente L M P, Castro-Cunha M, et al. Effect of storage time and heat processing on the volatile profile of Senegalese sole (Solea senegalensis Kaup, 1858) muscle. Food Chemistry, 2013, 138(4): 2365-2373. DOI:10.1016/j.foodchem.2012.11.135 (  0) 0) |
| [8] |
Soto V C, Maldonado I B, Jofré V P, et al. Direct analysis of nectar and floral volatile organic compounds in hybrid onions by HS-SPME/GC-MS: relationship with pollination and seed production. Microchemical Journal, 2015, 122: 110-118. DOI:10.1016/j.microc.2015.04.017 (  0) 0) |
| [9] |
Xu Y X, Liu Y, Jiang C C, et al. Determination of volatile compounds in turbot (Psetta maxima) during refrigerated storage by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Journal of the Science of Food and Agriculture, 2014, 94(12): 2464-2471. DOI:10.1002/jsfa.6581 (  0) 0) |
| [10] |
Liu Q, Sun G F, Wang S, et al. Analysis of the variation in scent components of Hosta flowers by HS-SPME and GC-MS. Scientia Horticulturae, 2014, 175: 57-67. DOI:10.1016/j.scienta.2014.06.001 (  0) 0) |
| [11] |
Peris M, Escuder-Gilabert L. A 21st century technique for food control: electronic noses. Analytica Chimica Acta, 2009, 638(1): 1-15. DOI:10.1016/j.aca.2009.02.009 (  0) 0) |
| [12] |
Li T T, Hu W Z, Li J R, et al. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control, 2012, 25(1): 101-106. DOI:10.1016/j.foodcont.2011.10.029 (  0) 0) |
| [13] |
Shi C, Cui J Y, Yin X F, et al. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: effect on lipid and protein oxidation. Food Control, 2014, 40: 134-139. DOI:10.1016/j.foodcont.2013.12.001 (  0) 0) |
| [14] |
Maqsood S, Benjakul S, Abushelaibi A, et al. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: a detailed review. Comprehensive Reviews in Food Science and Food Safety, 2014, 13(6): 1125-1140. DOI:10.1111/1541-4337.12106 (  0) 0) |
| [15] |
Senanayake S P J N. Green tea extract: chemistry, antioxidant properties and food applications-A review. Journal of Functional Foods, 2013, 5(4): 1529-1541. DOI:10.1016/j.jff.2013.08.011 (  0) 0) |
| [16] |
Medina I, Gallardo J M, González M J, et al. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. Journal of Agricultural and Food Chemistry, 2007, 55(10): 3389-3895. DOI:10.1021/jf063498i (  0) 0) |
| [17] |
Pezeshk S, Ojagh S M, Alishahi A. Effect of plant antioxidant and antimicrobial compounds on the shelf-life of seafood-A Review. Czech Journal of Food Sciences, 2015, 33(3): 195-203. DOI:10.17221/593/2014-CJFS (  0) 0) |
| [18] |
Folch J, Lees M, Sloane Stanley G H. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry, 1957, 226(1): 497-509. (  0) 0) |
| [19] |
Jongjareonrak A, Benjakul S, Visessanguan W, et al. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocolloids, 2008, 22(3): 449-458. DOI:10.1016/j.foodhyd.2007.01.002 (  0) 0) |
| [20] |
Maqsood S, Benjakul S. Effect of bleeding on lipid oxidation and quality changes of Asian seabass (Lates calcarifer) muscle during iced storage. Food Chemistry, 2011, 124(2): 459-467. DOI:10.1016/j.foodchem.2010.06.055 (  0) 0) |
| [21] |
Sae-Leaw T, Benjakul S. Fatty acid composition, lipid oxidation, and fishy odour development in seabass (Lates calcarifer) skin during iced storage. European Journal of Lipid Science and Technology, 2014, 116(7): 885-894. DOI:10.1002/ejlt.201300381 (  0) 0) |
| [22] |
Camo J, Beltrán J A, Roncalés P. Extension of the display life of lamb with an antioxidant active packaging. Meat Science, 2008, 80(4): 1086-1091. DOI:10.1016/j.meatsci.2008.04.031 (  0) 0) |
| [23] |
Rattray N J W, Hamrang Z, Trivedi D K, et al. Taking your breath away: metabolomics breathes life in to personalized medicine. Trends in Biotechnology, 2014, 32(10): 538-548. DOI:10.1016/j.tibtech.2014.08.003 (  0) 0) |
| [24] |
Souza H A L, Bragagnolo N. New method for the extraction of volatile lipid oxidation products from shrimp by headspace-solid-phase microextraction-gas chromatography- mass spectrometry and evaluation of the effect of salting and drying. Journal of Agricultural and Food Chemistry, 2014, 62(3): 590-599. DOI:10.1021/jf404270f (  0) 0) |
| [25] |
Dehaut A, Himber C, Mulak V, et al. Evolution of volatile compounds and biogenic amines throughout the shelf life of marinated and salted anchovies (Engraulis encrasicolus). Journal of Agricultural and Food Chemistry, 2014, 62(32): 8014-8022. DOI:10.1021/jf5021736 (  0) 0) |
| [26] |
Miyasaki T, Hamaguchi M, Yokoyama S. Change of volatile compounds in fresh fish meat during ice storage. Journal of Food Science, 2011, 76(9): C1319-C1325. DOI:10.1111/j.1750-3841.2011.02388.x (  0) 0) |
| [27] |
Panseri S, Soncin S, Chiesa L M, et al. A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME-GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chemistry, 2011, 172(2): 886-889. DOI:10.1016/j.foodchem.2010.12.150 (  0) 0) |
| [28] |
da Cunha Veloso M C, da Silva V M, Santos G V, et al. Determination of aldehydes in fish by high-performance liquid chromatography. Journal of Chromatographic Science, 2001, 39(5): 173-176. DOI:10.1093/chromsci/39.5.173 (  0) 0) |
| [29] |
Zhang Z M, Li G K, Luo L, et al. Study on seafood volatile profile characteristics during storage and its potential use for freshness evaluation by headspace solid phase micro-extraction coupled with gas chromatography-mass spectrometry. Analytica Chimica Acta, 2010, 659(1-2): 151-158. DOI:10.1016/j.aca.2009.11.024 (  0) 0) |
| [30] |
Lu F, Zhang J Y, Liu S L, et al. Chemical, microbiological and sensory changes of dried Acetes chinensis during accelerated storage. Food Chemistry, 2011, 127(1): 159-168. DOI:10.1016/j.foodchem.2010.12.120 (  0) 0) |
| [31] |
Domínguez R, Gómez M, Fonseca S, et al. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT - Food Science and Technology, 2014, 58(2): 439-445. DOI:10.1016/j.lwt.2014.04.006 (  0) 0) |
| [32] |
Van Durme J, Nikiforov A, Vandamme J, et al. Accelerated lipid oxidation using non-thermal plasma technology: evaluation of volatile compounds. Food Research International, 2014, 62: 868-876. DOI:10.1016/j.foodres.2014.04.043 (  0) 0) |
 2021, Vol. 28
2021, Vol. 28


