2. Shaanxi Rainbow New Material Co., Ltd., Xianyang 712000, Shaanxi, China
As an important transition metal oxide, MnO2 exists in different structural forms. The crystal structure of MnO2 includes three major categories: one-dimensional tunnel structure, two-dimensional layered structure, and three-dimensional network structure. Birnessite MnO2 which is made up of edge-sharing MnO6 octahedron layers belongs to two-dimensional MnO2. Between the MnO6 layers distribute a certain amount of water molecules and charge balance ions[1-2]. Birnessite MnO2 has been widely studied as electrode materials used for the field of energy storage because of its abundant reserves, unique structure, large theoretical specific capacity (1232 mAh/g), and environmental benignity[3-8]. It has been reported that layered birnessite MnO2 can be delaminated into nanosheets[9]. Compared with MnO2 bulks, MnO2 nanosheets have a higher specific surface area, which can provide more location for electrochemical reaction and is in favor of the electrochemical performance[10-11]. Besides, MnO2 nanosheets can be used as components to form nanocomposites[12-15].
It is a traditional and effective method to delaminate birnessite MnO2by insertion of tetramethylammonium (TMA+) ions before water washing[9]. But the above delamination method has a main shortcoming, i.e., the periodic time is longer than a week. Cui et al.[16] reported the delamination of birnessite MnO2 by ultrasonic concussion (300 W, 40 kHz) of layered MnO2 in solution of tetramethylammonium hydroxide (TMAOH). It only took 20 min to transform the bulk materials to the colloidal nanosheets, so the whole period significantly reduced. However, high power ultrasound tends to fragment the MnO2 nanaosheets into smaller chips, which is undesirable because of losing intrinsic properties in the final assemble nanosheet structure.
Ogino et al.[17] successfully delaminated the layered graphite oxide into GO by rapid quenching method without sonication. Chakravarty and Late[18] also delaminated bulk layered transition metal dichalcogenides into nanosheets by rapid cyclic quenching method. The rapid quenching method is time-saving and mild, which consists of rapidly freezing an aqueous solution containing the bulk layered material and subsequently thawing the resultant solution. These reports have inspired us to adopt the rapid quenching method to delaminate birnessite MnO2 into nanosheets. Herein the preparation of MnO2 nanosheets through repetitive freezing and thawing cycles was reported. The electrochemical properties of the prepared MnO2 nanosheets used as an anode material for lithium-ion batteries was also studied.
1 ExperimentalAll chemical reagents used in our experiment were analytically pure and used without further treatment. The water used in the experiment was deionized water.
A solution was prepared by mixing 0.6 M NaOH with 1.1 M H2O2, and then the mixed solution was quickly added into a 0.3 M Mn(NO3)2 solution to form precipitate. The precipitate was collected and washed until it is neutral. The precipitate was transferred to Teflon sealed autoclave and heated at 150 ℃ for 16 h in a 2 M NaOH solution. The Na-birnessite obtained was immersed in 0.1 M HCl solution for three days at room temperature, washed with deionized water, and dried at 70 ℃. The product was designated as H-Birnessite.
0.02 g H-Birnessite and 5 mL aqueous solution of TMAOH (0.35 M) were charged into a propylene tube (inside diameter of 13 mm, length of 128 mm). The tube was immersed in a liquid nitrogen bath for about 30 s to freeze the mixture. Then the tube was immediately moved to a water bath (set at 70 ℃) and stayed there for 30 min to thaw the mixture. After the freezing-thawing cycle was repeated for 50 times, a colloid suspension was obtained. The suspension was washed with water for 4 times through centrifuging at 8000 r·min-1 for 10 min every time to obtain the delaminated birnessite MnO2 slurry. Finally the birnessite MnO2 slurry was freeze-dried.
XRD patterns were collected on a Rigaku D/max-2200PC powder diffractometer equipped with a Cu Kα source (λ=1.5418 Å). The morphology of the products was characterized by employing a scanning electron microscope (S-4800) as well as a transmission electron microscope (Tecnai G2 F20 S-TWIN).
The electrochemical performances of MnO2 nanosheets and Na-birnessite were measured by assembling 2032 coin cells. The mixture of 40% active materials, 50% carbon black, and 10% polyvinylidene difluoride (PVDF) was ground for 30 min, and then moderate N-methyl-2-pyrrolidinone (NMP) was added for grinding for 30 min to form a slurry. The slurry was coated on copper foils and then dried in a vacuum drying oven at 80 ℃ for 24 h. The copper with active material was cut into wafers with a diameter of 16 mm. The mass loading of the active material was about 0.5-0.7 mg/cm2. Lithium plate was selected as the cathode. The electrolyte solution in this test was 1M LiPF6 dissolved in a mixture of ethylene carbonate (EC), ethyl methyl carbonate (EMC), and dimethyl carbonate (DMC) with a 1∶1∶1 volume ratio. The button cells were assembled in a glove box filled with argon. Nyquist plots and cyclic voltammetry were recorded using an electrochemical work station (CHI660e) at selected voltages range of 0.01-3.0 V and frequencies from 0.001 Hz to 100 kHz at room temperature. Galvanostatic charge-discharge was measured by battery system (LANHE CT-2001A) between 0.01 and 3.0 V.
2 Results and DiscussionFig. 1 displays the mechanism diagram of delamination of birnessite MnO2 into nanosheets through rapid quenching method. In this method, water rapidly changed to ice. The volume of water molecules between the MnO6 octahedron layers expanded and contracted repeatedly during rapid freezing and subsequent thawing cycles, which caused fast change in the interlayer distance between layers, and thus weakened the binding force between layers. This way, the intercalation of TMA+ cations into layers became much easier, which enabled a rapid and efficient delamination of birnessite MnO2.
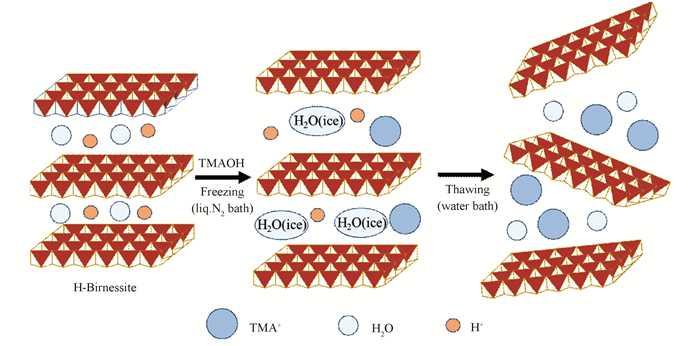
|
Fig.1 The mechanism diagram for the delamination of birnessite MnO2 into nanosheets by rapid quenching method |
Fig. 2 exhibits the XRD patterns of Na-birnessite and delaminated MnO2 slurry. Na-birnessite showed three obvious diffraction peaks at 12.3 ° (001 plane), 24.9 ° (002 plane), and 36.1° (110 plane), suggesting that the Na-birnessite had the typical monoclinic lamellar structure with a basal spacing of 0.7 nm. The delaminated MnO2 slurry displayed only an amorphous halo compared with Na-birnessite, which was similar to the case of other works[9, 11, 19]. The halo indicated the original crystal structure of birnessite MnO2 disappeared, suggesting that the layered H-birnessite was delaminated to scattered MnO2 nanosheets. It follows that H-birnessite can be delaminated to nanosheets effectively by the rapid quenching method.
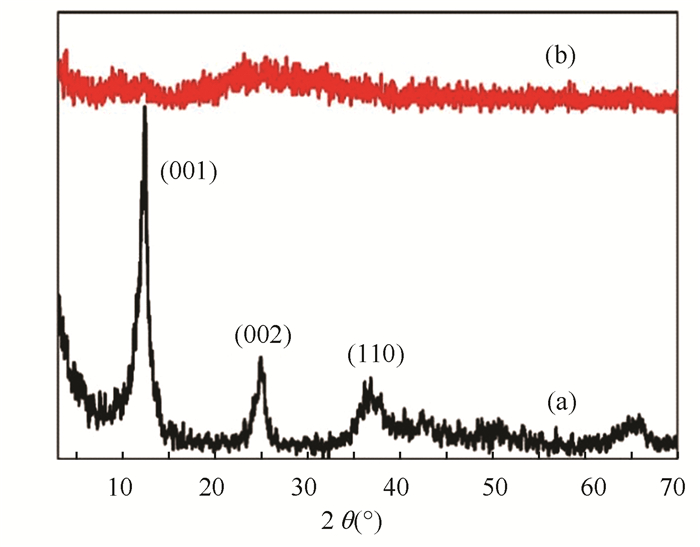
|
Fig.2 XRD patterns of (a) Na-birnessite and (b) delaminated MnO2 slurry |
The SEM image of Na-birnessite is given in Fig. 3(a). The sample showed platelike pattern with a lateral dimension of 1-2 μm. The TEM micrograph (Fig. 3(b)) of the delaminated MnO2 slurry manifested that the targets of observation had homogeneous brightness contrast, which indicated that they were ultrathin and uniform. Besides, it was easily observed that the width of the ultrathin platelets was about 1.5 μm, which was in line with the crystal size of Na-birnessite. The TEM result indicated that the delamination took place without destroying the MnO2 nanoplates. Fig. 3(c) displays the morphology of the delaminated MnO2 slurry followed by freeze-drying. It could be found that the nanosheets were uniform with a lateral dimension of 1-2 μm.

|
Fig.3 (a) SEM image of Na-birnessite, (b) TEM image of the delaminated MnO2 slurry, (c) SEM image of delaminated MnO2 slurry freeze-dried |
The samples of MnO2 nanosheets and Na-birnessite were respectively employed as anodes to assemble lithium ion batteries to test their electrochemical performances. Fig. 4(a) exhibits the cyclic voltammetry (CV) curves of MnO2 nanosheets for the initial three cycles measured between 0.01 and 3 V at a scan rate of 0.1 mV/s. Three main redox peaks were observed at around 0.28, 1.05, and 1.2 V. The CV curves exhibited a high overlap after the first cycle, which meant that the MnO2 nanosheets prepared by rapid quenching method had a good electrochemical reversibility. During the cathodic process, Li+ ions entered into MnO2 crystal lattice and Mn4+ was reduced. The cathodic peak at 1.05 V corresponded to the reduction of MnO2 to Mn2+, and the sharp peak at 0.28 V accounted for the reduction of Mn2+ (MnO) to metallic Mn0[20]. During the anodic process, lithium ions left the crystal lattice of MnO2 and Mn0 was oxidized. The oxidation peaks were at about 1.2 V in all the cycles, which was contributed to the oxidation of Mn0 to Mn4+. Fig. 4(b) shows the charge-discharge curves of MnO2 nanosheets at the 1st, 10th, 50th, and 100th cycles at 100 mA/g from 0.01 to 3.0 V. The initial discharge and charge capacities were 1938.9 and 760 mAh/g respectively, and the coulombic efficiency was about 39%. The large capacity loss in the 1st cycle for MnO2 nanosheets was ascribed to the formation of solid electrolyte interface (SEI) layer, which was common for most electrode materials. The coulombic efficiency reached up to 94% at the 50th cycle and increased to 99% at the 100th cycles.
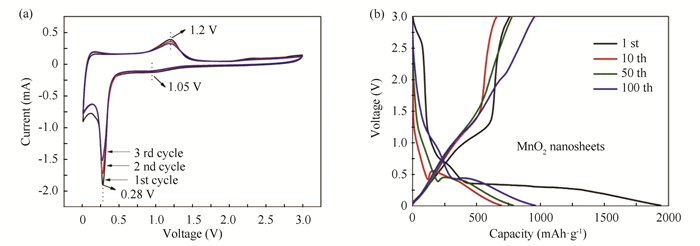
|
Fig.4 (a) Cyclic voltammetry curves of MnO2 nanosheets, (b) The galvanostatic discharge/charge curves at different cycles of MnO2 nanosheets |
The cycle performance of MnO2 nanosheets and Na-birnessite at 100 mA/g are exhibited in Fig. 5(a). Obviously, MnO2 nanosheets showed a clearly increased cyclic capacity. The reversible capacity of the MnO2 nanosheets retained 750 mAh/g after cycling for 20 times, and then increased with cycling and reached 1040.6 mAh/g after 100 cycles, which was much higher than those reported in Refs.[21-23]. However, the Na-birnessite can only reach a lower value of 411.8 mAh/g after 100 cycles. The cycle property at 1000 mA/g was also tested. As can be seen from Fig. 5(b), the specific charge capacity of MnO2 nanosheets tested at 1000 mA/g still stabilized at 438.3 mAh/g after cycling for 100 times. Similarly, after cycling for 100 cycles at 1000 mA/g, the delivered capacity of MnO2 nanosheets was much larger than that of the Na-birnessite. Fig. 5(c) shows the rate performance of MnO2 nanosheets and Na-birnessite. The average specific charge capacities of MnO2 nanosheets were 740, 600, 500, 420, and 320 mAh/gwhen cycling under 100, 200, 500, 1000, and 2000 mA/g, respectively. After the current density returned to 100 mA/g, the average specific charge capacities of MnO2 nanosheets and Na-birnessite recovered to 800 and 500 mAh/g, respectively.
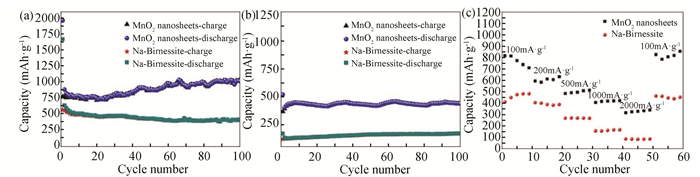
|
Fig.5 Cycling performance of MnO2 nanosheets and Na-birnessite at (a) 100 mA/g and (b)1000 mA/g, (c) rate performance of MnO2 nanosheets and Na-birnessite |
Fig. 6(a) displays the Nyquist plots of MnO2 nanosheets and Na-birnessite from 0.001 Hz to 100 kHz. The impedance data were analyzed using the equivalent circuit inset in Fig. 6(a). The intercept at real axis at high frequency corresponds to ohmic resistance (RΩ). The capacitive loops at high frequencies is put down to the migration resistance (RSEI) resulted from Li+ ions through SEI films. The intercept at mediate-low frequencies corresponds to Rct, representing the charge transfer resistance. The low-frequency oblique line represents the Warburg impedance (Zw), resulting from the lithium ions diffusion in the active materials. The curves of MnO2 nanosheets and Na-birnessite were both composed of a low-frequency oblique line and two high-frequency semicircles. For the MnO2 nanosheets and Na-birnessite samples, RΩ values were 24.31 and 84.36 Ω, RSEI values were 13.69 and 60.4 Ω, Rct values were 19.58 and 122.1 Ω, and the Zw values were 0.0327 and 0.003003 Ω, respectively. The Li+ ion diffusion coefficient was calculated on the basis of the following equation:
| $ {D_{{\rm{L}}{{\rm{i}}^ + }}} = \frac{{{R^2}{T^2}}}{{2{A^2}{n^4}{F^4}{C^2}{\sigma ^2}}} $ | (1) |
| $ {Z'} = {R_\Omega } + {R_{{\rm{ct}}}} + \sigma {\omega ^{ - 1/2}} $ | (2) |

|
Fig.6 (a) Electrochemical impedance spectra of MnO2 nanosheets and Na-birnessite (The inset is the equivalent circuit), (b) The relationship between Z′ and ω-1/2 at the low frequency range |
where R, T, A, n, F, C, and σ denote the gas constant, the absolute temperature, the electrode surface area, the number of reactive electrons during reducing per molecule MnO2, the Faraday constant, the concentration of Li+ ions, and the slope of Z′-ω-1/2 at low-frequency (Fig. 6(b)), respectively. For MnO2 nanosheets and Na-birnessite, Z′ and ω-1/2 presented a good linear relationship at low frequency.
The Li+ ion diffusion coefficients of MnO2 nanosheets and Na-birnessite were calculated according to Eqs. (1) and (2).The records of data are exhibited in Table 1. The obtained Li+ ion diffusion coefficient of the MnO2 nanosheets was 1.3×10-19cm2/S, larger than that of Na-birnessite (1.5×10-21 cm2/S). The smaller Rct and higher Li+ ion diffusion coefficient of as-prepared MnO2 nanosheets indicated the charges and lithium ion transported more easily in them, which was beneficial to lithium ions battery application.
| Table 1 Data of the lithium diffusion coefficients in MnO2 nanosheets and Na-birnessite |
The charging or discharging process of lithium ion battery corresponds to embedding or disembedding lithium ions into or out of anode materials respectively, which is a complex process of multi-step series. It mainly includes 1) the diffusion of Li+ ions in electrolyte, 2) the movement of Li+ ions in SEI membrane, 3) the charge transfer reaction process taking place at the interface between SEI membrane and anode material, and 4) the Li+ ions diffusion in solid phase anode materials[24-25]. It is generally believed that it is difficult for Li+ ions to diffuse in the solid phase than in the liquid phase. Therefore, the diffusion of Li+ ion in solid phase is the dynamic controlling factor in the charging and discharging process[26]. The size of anode particles has direct influence on the length of the solid phase diffusion path of lithium ions, which has an important influence on the electrochemical performance. In our work, the fine electrochemical property of MnO2 nanosheets could be attributed to three aspects. Firstly, when particles were reduced to nanoscale, the migration path of Li+ ions or electrons in solid decreased, thus the Rct and ion diffusion resistance reduced. Besides, the nanosheet structure can increase the contact area between the electrode surface and the electrolyte, thus increasing the active sites. Furthermore, the nanostructure brought larger specific surface area, which then effectively reduced the current density of electrode, thus decreasing the polarization of electrode.
3 ConclusionsMnO2 nanosheets has been successfully synthesized by the freezing and thawing method. This method consisted of many cycles of fast freezing of a mixture solution containing H-birnessite and TMAOH, followed by melting of the resultant solid. This simple method for the delamination of layered birnessite MnO2 was timesaving and environmentally friendly without destroying the initial nanosheet size. Meanwhile, MnO2 nanosheets synthesized by this method exhibited an excellent cycling performance. Cycled for 100 times at 1000 mAh/g, MnO2 nanosheets showed a high charge capacity of 438.3 mAh/g, which is 105% of the initial charge capacity. The fine electrochemical performances was ascribed to the nanosheet structure and increased electrical conductivity.
| [1] |
Pinaud B A, Chen Z B, Abram D N, et al. Thin films of sodium birnessite-type MnO2: optical properties, electronic band structure, and solar photoelectrochemistry. The Journal of Physical Chemistry C, 2011, 115(23): 11830-11838. DOI:10.1021/jp200015p (  0) 0) |
| [2] |
Zhu J B, Li Q Y, Bi W T, et al. Ultra-rapid microwave-assisted synthesis of layered ultrathin birnessite K0.17MnO2 nanosheets for efficient energy storage. Jounal of Materials Chemistry A, 2013, 1: 8154-8159. DOI:10.1039/C3TA11194F (  0) 0) |
| [3] |
Xia A, Yu W R, Yi J, et al. Synthesis of porous δ-MnO2 nanosheets and their supercapacitor performance. Journal of Electroanalytical Chemistry, 2019, 839: 25-31. DOI:10.1016/j.jelechem.2019.02.059 (  0) 0) |
| [4] |
Zhao S Q, Liu T M, Hou D W, et al. Controlled synthesis of hierarchical birnessite-type MnO2 nanoflowers for supercapacitor applications. Applied Surface Science, 2015, 356: 259-265. DOI:10.1016/j.apsusc.2015.08.037 (  0) 0) |
| [5] |
Yan D L, Zhang H, Li S C, et al. Formation of ultrafine three-dimensional hierarchical birnessite-type MnO2 nanoflowers for supercapacitor. Journal of Alloys and Compounds, 2014, 607: 245-250. DOI:10.1016/j.jallcom.2014.04.077 (  0) 0) |
| [6] |
Garcia-Torres J, Roberts A J, Slade R C T, et al. One-step wet-spinning process of CB/CNT/MnO2 nanotubes hybrid flexible fibres as electrodes for wearable supercapacitors. Electrochimica Acta, 2019, 296: 481-490. DOI:10.1016/j.electacta.2018.10.201 (  0) 0) |
| [7] |
Zhang Z R, Xu Z M, Yao Z P, et al. Ultrahigh capacitance of TiO2 nanotube arrays/C/MnO2 electrode for supercapacitor. Journal of Alloys and Compounds, 2019, 805: 396-403. DOI:10.1016/j.jallcom.2019.07.070 (  0) 0) |
| [8] |
Xiong P, Ma R Z, Sakai N, et al. Genuine unilamellar metal oxide nanosheets confined in a superlattice-like structure for superior energy storage. ACS Nano, 2018, 12(2): 1768-1777. DOI:10.1021/acsnano.7b08522 (  0) 0) |
| [9] |
Liu Z H, Ooi K, Kanoh H, et al. Swelling and delamination behaviors of birnessite-type manganese oxide by intercalation of tetraalkylammonium ions. Langmuir, 2000, 16(9): 4154-4164. DOI:10.1021/la9913755 (  0) 0) |
| [10] |
Dang L Y, Wei C Z, Ma H F, et al. Three-dimensional honeycomb-like networks of birnessite manganese oxide assembled by ultrathin two-dimensional nanosheets with enhanced Li-ion battery performances. Nanoscale, 2015, 7: 8101-8109. DOI:10.1039/C5NR00576K (  0) 0) |
| [11] |
Zhang G N, Zheng L, Zhang M, et al. Preparation of Ag-nanoparticle-loaded MnO2 nanosheets and their capacitance behavior. Energy and Fuels, 2012, 26(1): 618-623. DOI:10.1021/ef201446h (  0) 0) |
| [12] |
Jia H, Cai Y, Zheng X, et al. Mesostructured carbon nanotube-on-MnO2 nanosheet composite for high-performance supercapacitors. ACS Applied Materials & Interfaces, 2018, 10(45): 38963-38969. DOI:10.1021/acsami.8b14109 (  0) 0) |
| [13] |
Zeng C, Weng W, Lv T, et al. Low-temperature assembly of ultrathin amorphous MnO2 nanosheets over Fe2O3 spindles for enhanced lithium storage. ACS Applied Materials & Interfaces, 2018, 10(36): 30470-30478. DOI:10.1021/acsami.8b11794 (  0) 0) |
| [14] |
Lin L, Huang M, Ning M, et al. Facile ordered ZnCo2O4@MnO2 nanosheet arrays for superior-performance supercapacitor electrode. Solid State Sciences, 2018, 84: 51-56. DOI:10.1016/j.solidstatesciences.2018.08.007 (  0) 0) |
| [15] |
Li J P, Ren Z H, Wang S G, et al. MnO2 nanosheets grown on internal surface of macroporous carbon with enhanced electrochemical performance for supercapacitors. ACS Sustainable Chemistry & Engineering, 2016, 4: 3641-3648. DOI:10.1021/acssuschemeng.6b00092 (  0) 0) |
| [16] |
Cui Y H, Liu Z H, Wang M Z, et al. New approach to the delamination of layered manganese oxide. Chemisty Letters, 2006, 35(7): 740-741. DOI:10.1246/cl.2006.740 (  0) 0) |
| [17] |
Ogino I, Yokoyama Y, Iwamura S, et al. Exfoliation of graphite oxide in water without sonication: bridging length scales from nanosheets to macroscopic materials. Chemistry of Materials, 2014, 26(10): 3334-3339. DOI:10.1021/cm501305c (  0) 0) |
| [18] |
Chakravarty D, Late D J. Exfoliation of bulk inorganic layered materials into nanosheets by the rapid quenching method and their electrochemical performance. European Journal of Inorganic Chemistry, 2015, 2015(11): 1973-1980. DOI:10.1002/ejic.201500039 (  0) 0) |
| [19] |
Omomo Y, Sasaki T, Wang L Z, et al. Redoxable nanosheet crystallites of MnO2 derived via delamination of a layered manganese oxide. Journal of the American Chemical Society, 2003, 125: 3568-3575. DOI:10.1021/ja021364p (  0) 0) |
| [20] |
Reddy A L M, Shaijumn M M, Gowda S R, et al. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Letters, 2009, 9(3): 1002-1006. DOI:10.1021/nl803081j (  0) 0) |
| [21] |
Xing L, Cui C X, Ma C H, et al. Facile synthesis of α-MnO2/graphene nanocomposites and their high performance as lithium-ion battery anode. Materials Letters, 2011, 65(11): 2104-2106. DOI:10.1016/j.matlet.2011.04.093 (  0) 0) |
| [22] |
Chen W M, Qie L, Shao Q G, et al. Controllable synthesis of hollow bipyramid β-MnO2 and its high electrochemical performance for lithium storage. ACS Applied Materials & Interfaces, 2012, 4(6): 3047-3053. DOI:10.1021/am300410z (  0) 0) |
| [23] |
Zhang B, Wan J Q. Waste utilization method for δ-MnO2 anode composited with MWCNT and graphene by embedding on conductive paper for lithium-ion battery. Nano, 2019, 14(4): 1950051. DOI:10.1142/S1793292019500516 (  0) 0) |
| [24] |
Holzapfel M, Martinent A, Alloin F, et al. First lithiation and charge/discharge cycles of graphite materials, investigated by electrochemical impedance spectroscopy. Journal of Electroanalytical Chemistry, 2003, 546: 41-50. DOI:10.1016/S0022-0728(03)00144-X (  0) 0) |
| [25] |
Zhang S, Shi P F. Electrochemical impedance study of lithium intercalation into MCMB electrode in a gel electrolyte. Electrochimica Acta, 2004, 49(9-10): 1475-1482. DOI:10.1016/j.electacta.2003.10.033 (  0) 0) |
| [26] |
Verbrugge M W, Koch B J, Electrochemistry of intercalation materials. Charge-transfer reaction and intercalate diffusion in porous electrodes. Journal of the Electrochemical Society, 1999, 146(3): 833-839. DOI:10.1149/1.1391689 (  0) 0) |
 2021, Vol. 28
2021, Vol. 28


