Legumes belong to the Fabaceae family, which includes some of the most important food and feed crops in the world, such as soybean, common bean, pea, chickpea, alfalfa, and peanut. They account for one thirds of global primary crop production and are vital to meet the population demands[1].
White lupin (Lupinus albus L.) is an important high-protein grain crop, which fixes atmospheric nitrogen through the symbiosis with rhizobia and increases phosphorus solubility[2-3].
Genetic variation in main agronomic traits is of great significance for the successful improvement of crops. Plant breeding is a mission of continuously discovering and pyramiding desirable genes of agronomic or end-use interest into breeding lines to produce superior cultivars[4-5].
Since World War I, high-protein crops have been temperately adapted to different conditions due to high demand. Subsequent breeding has concentrated on the introduction of key traits such as early flowering, reduced pod shattering, soft seed, and anthracnose disease resistance[6-7].
The basic lupin breeding method is the typical step-by-step intraspecific hybridization. Multiple, back, reciprocal, diallelic, and polyallelic crossings are used in recurrent schemes of hybridization. Due to reproductive barriers, the interspecific crossing between the Old World and the New World species cannot produce fertile hybrids under natural conditions[8].
Legumes develop symbiotic associations with arbuscular mycorrhizal (AM) fungi, leading to the formation of sites of phosphorus nutrient exchange under conducive environmental conditions[9-10].
Symbiotic selection aims at strengthening the symbiotic interactions of cultural plants with useful soil microorganisms for the mobilization of hard-to-reach elements of nutrition, resistance to pathogens, and adaptation to environmental stresses[11-12].
Genetic variability in the base population plays an important role in any crop-breeding program. The amount of diversity in a crop determines the limits of selection for improvement. Therefore, a large range of variation in the material under investigation is a prerequisite. The important economic characteristics are generally quantitative in nature and exhibit a considerable degree of interaction with the environment[13].
1 Materials and MethodsThe study was conducted between 2014 and 2016 in the experimental field of the Institute of Forage Crops, Pleven, Bulgaria. The crossings among the white lupin varieties PI533704 (Spain), Zuter (France), Lucky801 (France), PI533704×Zuter, PI533704×Lucky801, and their reciprocal combinations were performed (Table 1).
| Table 1 Distinctive features of the investigated genotypes |
Parent forms (P1 and P2) and first and second hybrid generations (F1 and F2) were studied. The sowing of the selection materials was carried out manually under the scheme P1, P2, F1, F2 in optimal time according to the cultivation technology of the crop at two limits of the environment, namely, seeding distances of 50/5 cm (dense sowing) and 50/10 cm (sparse sowing) at a depth of 5 cm. Plant materials from aboveground as well as root biomass of parent and hybrid forms were analyzed. Biometric measurements were carried out on 10 plants of each genotype. The following characteristics were assessed in the beginning of the flowering stage: aboveground mass weight (g/plant), fresh root mass weight (g/plant), nodule number per plant, and nodule weight per plant (g). In the technical maturity of seeds stage, the seed weight per plant (g) was recorded.
For all the characteristics, arithmetical average (х), heterosis effect in F1-(hypothetic and true), inbred depression by Omarov[14], and degree of dominance in F1 (hp1) and in F2 (hp2) by Romero and Frey[15] were studied. Using Sobolev's method[16], the characteristics of transgression (Tn), dominance (D), epistasis (E), coefficient of inheritance in broad sense (H2), coefficient of the mass effectiveness of genotypes by phenotypical performance of the trait (Pp), and cytoplasmatic effect (re) by Reinhold[17] were found. The positive value of re (re > 0) means that the paternal inherited factors have greater influence on the expression of the signs, and the negative (re < 0) indicates the greater influence of maternal cytoplasmic heredity (cytoplasmatic effect). The method of orthogonal regression to identify the phenotype by genotype described by Kramer[18] was applied, which indicates the possibility of the assessment of white lupine hybrids in genetics-physiological systems at different limits of the environment. The coordinate system makes it possible to identify the genotype of the individual organism in the phenotype. Moreover, the relative proportion of the genotype and the environment shall be quantified in a scale of the actual measurements of the attribute.
All experimental data were processed statistically using the computer software SPSS 13 and Excel for Windows XP.
2 ResultsHybridization is one of the main methods for creating new varieties and a correct choice of suitable parent forms which ensures a large degree of selection success[19].
The analysis of the inheriting signs associated with symbiotic processes in the host plant is essential to organize selection-genetic activity with symbiotic systems. In leguminous crops, some of these signs are controlled by ollygogenes, and their alleles determine the qualitative manifestations, such as the ability to form symbiosis and the development of nodules. Other signs are inherited in polygenic way and have quantified manifestation, including the number and weight of the nodules, the intensity of the nitrogen fixation, and the productivity of the symbiotroph developing plants. The greatest interest of breeders is the genes of the second group, which are subject to quantitative genetics[20].
It is evident from the data in Table 2 that the number of nodules per plant is inherited with negative over dominance with greater influence of epistatic gene action.
| Table 2 Biometrical data of the quantitative traits of the investigated crosses dense sowing |
The exception is PI533704×Lucky801, which is characterized by a positive dominance of the attribute. In the same cross, a negative true heterosis(-11.86%) was established, and the heterosis was the highest at its reciprocal, reaching up to 259.45%. In the second hybrid generation, the least depressed plants were obtained from PI533704 and Zuter (62.20%-62.21%), regardless of crossover direction.
It is known that the hybrid effect is greater when the parents differ significantly on the given attribute. The inclusion of the varieties of individual origin in this study implies the existence of differences between them. In the first generation with the highest nodule weight, PI533704×Lucky801 and its reciprocal were distinguished, whereas no statistically significant differences were observed between the individual hybrids and there was a relatively high level of depression. This sign and the four combinations exhibited positive heterosis. As in the case of the reciprocal, the hypothetic heterosis had a significantly higher value (205.88%-276.47%). In the hybrid combination PI533704×Lucky801, the dominant gene effects played a significant role, while the epistasis predominated in all others in the inheritance of the attribute.
Regarding the fresh root mass weight in F1, the hybrids PI533704×Lucky801 and Lucky801×PI533704 prevailed again, and statistically significant differences were established between them. The values of both the hypothetical (147.27%-285.45%) and the true (78.95%-752.94%) heterosis affected Zuter×PI533704 and Lucky801×PI533704, when PI533704 was used in artificial reproductive hybridization as a paternal form. From the values obtained for the depression of plants at F2, it can be assumed that when the varieties of PI533704 and Zuter were crossed, the resulting hybrids were poorly depressed (10.29%-11.32%). The degrees of gene domination in F1 and F2 characterized the heterosis from the perspective of genetic balance theory. It showed that the heterosis in F1 was due to the over dominance, and it had a negative sign only with the Lucky801×PI533704. In the second hybrid generation, the epistatic gene interactions (hp2 > hp1) were significantly greater on the manifestation of the sign in most of the hybrids.
With the sign of fresh aboveground mass weight, the impression made Zuter×PI533704 have high performance in both hybrid generations. However, none of the hybrids exceeded any of the parent forms, which was verified by the negative values of heterosis events. In accordance with these events, PI533704×Lucky801 and its reciprocal might be indicated as the least depressed (≈-35%-36%). This sign was found to be similar with the nodule weight gene interactions in the second generation.
From the biometric data regarding productivity, which is expressed through seed weight per plant, it is evident that the inheritance of the attribute was from positive over dominance in Zuter×PI533704 (2.18) and PI533704×Lucky801 (1.03), positive dominance in PI533704×Zuter (0.85), and negative dominance in Lucky801×PI533704 (-0.53). In the reciprocal crosses, a stronger true heterosis effect was established, ranging from -0.78% in PI533704×Zuter to 22.46% in Lucky801×PI533704. This sign shows that the parent forms were dominant, whose genes determined a lower seed weight. Plants obtained by crossing PI533704 with Zuter were relatively less depressed (7.87%-8.48%), compared with the combination between Lucky801 and PI533704. With the exception of Lucky801×PI533704 in the case of the remaining crosses in the second generation, the epistatic gene effects were predominated, determining the lower values of the sign in the hybrid plants.
Table 3 shows the average values of the F1 and F2 hybrids and the biometric data for the analyzed parameters. For the number of nodules per plant at the dilution of the crop, it was found that in all hybrids, the heterosis (hypothetic and true) effect was negative, which reached 63.58% in Lucky801×PI533704 and up to 74.22% in PI533704 in the level of depression. At different limits of the environment, the plants of the crosses of PI533704×Zuter and its reciprocal were the least depressed. Moreover, in the type of inheritance in the second generation, the epistatic gene interactions (hp2 > hp1) was the significant part in the expression of the sign.
| Table 3 Biometrical data of the quantitative traits of the investigated crosses in sparse sowing |
Changes of environmental conditions influence the manifestations of heterosis and the nodule weight per plant. The true heterosis effect was the most pronounced in PI533704×Lucky801 (233.33%), followed by PI533704×Zuter (83.33%). These hybrids exhibited certain responsiveness to the improvement of growing conditions, while in their reciprocal crossings, the true heterosis was negative. For the nodule weight per plant in PI533704×Zuter and its reciprocal, the inheritance was positively dominant and the other two crosses were negatively dominant. Under favorable conditions of cultivation, genetic control was changing, and there was already predominant gene-epistasis-type interaction in the second generation.
The fresh root mass was characterized by an even stronger expression of the heterosis effect in terms of restrictive cultivation conditions, particularly in reciprocal crosses (805%, 920%). The lowest depression was found in Zuter×PI533704 (-12.15%) and the highest in Lucky801×PI533704 (74.51%). The characteristic of the inheriting attribute in the first generation was preserved as with the dense sowing, but in the second generation in PI533704×Zuter and its reciprocal, Zuter×PI533704 changed, as in the first generation dominant gene effects played a much more important role.
The reciprocal crosses under better growing conditions (sparse sowing) showed their biological capacities and exceeded the parental forms by fresh biomass productivity. The expression of their hybrid strength was the positive value of both the hypothetical (48.55%, 38.34%) and the true heterosis effects (28.21%, 14.48%). In these crosses, the inheritance of aboveground mass weight was positively overdominant. Greater sensitivity to the environmental conditions was found in hybrids with parent components PI533704 and Zuter, lacking of depression only at favorable conditions of cultivation. In a second hybrid generation, interallele interactions predominated in plants of all crosses (hp2 > hp1).
In all the investigated hybrids regarding grain productivity, heterosis events were identified with varying degrees depending on the parent components. In a favorable environment, parents performed better in relation to the first generation hybrids, the seed weight of which was lower. Therefore, the heterosis effect, both hypothetic and true, had a negative sign. The highest true heterosis (-15.20%) was observed in hybrid Lucky801×PI533704 in the direction of the mother variety and the lowest was in the reciprocal (-49.78%). According to Table 3, the inheritance of the attribute in the first generation was positive over dominance for Lucky801×PI533704 and over dominance for others, i.e., a change in the genetic control in the expression of the sign was observed when the environment of cultivation changed. In plants of the second generation reciprocal crosses, the manifestations of epistasis predominated (hp2 > hp1).
Table 4 presents the values of the gene parameters for the tested signs of the studied crosses.
| Table 4 Values of the gene parameters for the quantitative traits of the investigated crosses in F2 generation dense sowing |
The results show that in the conditions of generally accepted density, the positive manifestations of transgrease were found on all signs, the most significant of which were in PI533704×Lucky801 and its reciprocal by the nodule number per plant (11.14-11.21), fresh aboveground biomass (1.24-1.54), and seed weight per plant (1.71-1.87). It indicates that in the hybrid generations of these crossings, homozygous genotypes might be expected, where gene recombination that can cause a quantitative increase of these signs can be anticipated.
The number of genes that differed from parents by number of plant nodules was large. The situation was similar in the case of other indications, while an exception was the seed weight per plant, in which the number of genes controlling the attribute from which the parental forms differ was relatively smaller(12.38-25.31).
On most of the signs, there was a one-way effect of the dominant alleles of genes determining the relevant signs. Their negative value indicates that the action leads to a reduction in the phenotypic manifestation of the signs. To the strongest extent, this was evident in the cross PI533704×Lucky801. Only with Zuter×PI533704 by fresh root mass weight, the allele interactions were positive over dominance(32.62), which justified the formation of a root biomass with greater weight. The data from the hybrid analysis showed that in the second generation of the last cross, epistasis interactions with a negative sign prevailed, and it could be assumed that this would reduce the degree of phenotypic manifestation of the attribute (fresh root mass weight) compared with the full additive inheritance. Positive epistasis interactions occurred with all other hybrids from 1.05 in PI533704×Zuter for nodule weight of 2532.58 in PI533704×Lucky801 for fresh aboveground mass weight. Stronger interallele interactions predominated in PI533704×Lucky801 and its reciprocal in the number of nodules per plant and fresh aboveground biomass weight.
The values of the transgrease indicators made it possible to determine the homozygote genotypes of the samples with a maximum and minimum for the indications that may be obtained from the combination of the parent components involved in the hybrid combinations. The data from the hybrid analysis (Table 5) showed that there was almost the same level of transgression (11.62-12.38) in PI533704×Lucky801 and the reciprocal of the number of nodules per plant, whereas in PI533704×Zuter, the transgression indicator was higher than that in limiting environment. In Zuter×PI533704, plants with positive transgrease could not even be determined on this attribute. A certain similarity was revealed in the hybrids in nodule weight per plant, fresh green mass weight, and seed weight. The negative sign in all hybrids suggested that no plants with increased root biomass weight could be selected.
| Table 5 Values of the gene parameters for the quantitative traits of the investigated crosses in F2 generation in sparse sowing |
The number of genes influences the manifestation of the signs, in which parental forms are different, both in terms of the limit of the environment and the direction of crossover. In cross PI533704×Zuter, the difference was small (2-3), while it was significant in its reciprocal (4227). With a relatively small number of genes, different varieties of PI533704 and Lucky801 were obtained by nodule weight per plant (8). The same varieties differed significantly in the number of genes, determining the expression of the root and aboveground biomass weights serving a certain analogy in the case of dense sowing. It is similar with the seed weight, where the number of genes controlling the sign with different parental forms is relatively small (7.79-62.55).
From the values for allele interactions, a positive dominance and over dominance of the dominant alleles were observed, determining the expression of all signs in Zuter×PI533704 and the nodule number per plant and fresh aboveground mass weight in PI533704×Lucky801. Dominant alleles resulted in a better numerical expression of these signs in plants than those hybrids.
When analyzing the interallele interactions, it can be found that the significant part of the signs of epistasis was negative. In the case of crosses between PI533704 and Lucky801, the positive epistasis was maintained in respect of the nodule weight, fresh root mass weight, and seed weight, which is an indication of a relatively strong influence on the phenotypic manifestation of signs. The values of fresh root mass (310.55) and nodule number (106.95) in Lucky801×PI533704 were particularly high.
For the genetic part in the overall phenotypic manifestation of the investigated indicators, which is expressed by the coefficient of inheritance in a broad sense, it was high in crosses PI533704×Zuter and Lucky801×PI533704 for number of nodules (0.51, 0.92), weight of nodules (0.71, 0.79), and seed weight per plant (0.93, 0.96). In the case of crosses between PI533704 and Lucky801 by nodule weight, significantly higher coefficient of inheritance of the attribute (0.83, 0.79) was found when the plants were cultivated under more favorable conditions.
From the values which show the effect of selection, a positive difference compared with the baseline formulations was reflected in crosses PI533704×Zuter by number of nodules (0.57) and Zuter×PI533704 by seed weight (0.70). Taking into account the values of the coefficients of inheritance, it can be assumed that in these combinations, the mass selection would be effective in the earlier hybrid offspring.
Fig. 1 presents the influence of the mother cytoplasm in inheriting the signs. In the hybrid combination PI533704×Zuter, the influence of the mother cytoplasm on the inheritance of the number of nodules (-6.40, -3.93) and weight of nodules (-0.15, -0.07) both in dense and sparse sowing was found. In the same cross, the mother cytoplasm had an influence on the inheritance of seed weight per plant when the crop was diluted.
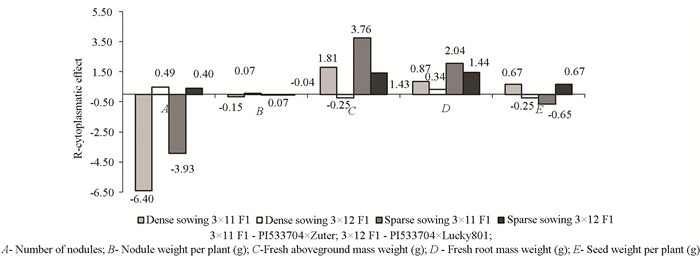
|
Fig.1 Cytoplasmatic effect (re) in inheritance of signs tested in the first hybrid generation |
A comparatively weaker cytoplasmatic effect occurred when PI533704 and Lucky801 were in normal seed rate on the signs of fresh aboveground mass weight and seed weight (-0.25), as well as the weight of nodules when the crop was diluted (-0.04).
Fig. 2 shows the test results of the sixteen F1 hybrids at the limit of the environment expressed by two different sowing methods (dense and sparse sowing) for the signs of aboveground mass weight and seed weight per plant. It can be found that under the deterioration of the environment (dense sowing, Fig. 2(b)), the most valuable hybrids were obtained at the crossing of the varieties Lucky801 and PI533704 (4). This hybrid exhibited a good combination of genes for adaptability and attraction (rapid removal of plastic substances) in worsening conditions. When diluting the crop (Fig. 2(a)), such a combination was found only in hybrids Zuter×PI533704 (3) and PI533704×Lucky801 (2) with positive values of attraction and adaptability.

|
Fig.2 Distribution of mean values of varieties |
There were no hybrids which, in both limits of the environment, retained their place in the same quadrant, suggesting that the holder of strong genes for green mass productivity should be demanded in more advanced hybrid generations.
Hybrid plants from crosses PI533704×Zuter and PI533704×Lucky801 (without PI533704×Lucky801 (4)) in dense sowing fell into the quadrant, and were determined by negative adaptability and attraction, which indicates that genetic control over adaptability was redefined adversely to a higher density of the crop for these hybrids. The hybrids Lucky801×PI533704 and Zuter×PI533704 were characterized by positive attractiveness, but differed greatly in their adaptive capacities, occupying diametrically opposed places along the line of orthogonal regression. When diluting the crop, the scattering around the regression line was significantly decreased.
Similarly, when a donor cannot be accepted, the seed weight per plant was obtained combining the high values of the root mass and plant seed weight. The graphical analysis showed that in both limits of environment, the breadth of the variability of adaptability and attraction is essential.
On the genetic change of the attraction of the hybrids of white lupin, the presence of strong polymorphism could be established. Some of the hybrids changed their positions in the coordinate system when the growing conditions were changed, either in the positive or negative part of the line of regression, which suggests a good prospect of selection improvement in this culture.
The assessment of the initial material allows the management of the valuable genotypes selection based on the studied signs with a high probability and speeds up the process of creating new varieties of white lupin.
3 DiscussionIt is revealed that in addition to the main genes determinants symbiosis, plants have many genes that affect the quantitative side of the nitrogen fixing process. These include, for example, the genes that control photosynthesis, the duration of the vegetation period, the reaction of plants to the photoperiod and mineral nitrogen, and many others[21].
The intraspecific (inter-varietal) hybridization in our research is an effective method of creating the initial material for the selection of peas, with the selection of parental forms on the contrasting manifestations of signs. In the process of using this method, valuable hybrid populations are obtained[22].
When investigating hybrid combinations in pea for plant height, Zolotaryova[23] established all types of inheritance from negative over dominance(31.8%) to positive over dominance (24.7%).
The study of the offspring of the selected plants revealed that the material presented in the trial is dynamically heterosis populations where the hybrid forms are constantly formed and individually selected, restoring the average level of the population, which does not ensure the success of breeding[24].
With the number of seeds in pod, different types of inheritance were obtained, including full dominance, incomplete and complete dominance. The inheritance type of as many as 1000 seeds was found to be incomplete dominance (hp=0.27-0.40)[22].
Heterosis on the symbiotic activity of peas plants is manifested discretely and depends on a combination of crossing, which can be expressed on the majority or only on separate signs. Generally, hybrids are superior to parental forms by the number of nodules, dry mass weight, and seed productivity, while less frequently by nitrogenase activity[25].
Cross-breeding shows that in hybridization of different legume varieties, the inheritance in a broad sense for the nitrogen-fixing activity and the number of nodules could reach 0.8-0.9, and the inheritance in the narrow sense could reach -0.6-0.8. For alfalfa, soybeans, and clover in the selection to increase symbiotic signs, high inheritance was realized (up to 0.8-0.9)[11].
According to Ref. [26], heritability was high for all the analyzed traits (H2>50%), especially for seed yield, seed protein yield, and thousand seed weight. Accordingly, the examined traits were under relatively strong genetic control, indicating the potential for genetic progress in improving seed quality and yield traits. Similar results were reported by other researchers[27]. It has been established that the heterosis effect is manifested in the hybrid combinations of yellow lupine by the number of pods per plant in 78.3% of combinations, number of seeds of 69.6, seed weight per plant at 56.5%, percentage of seeds in pod at 17.4%, and 1 000 seeds weight in 4.3% of combinations yellow lupine.
4 ConclusionsThe hybrid analysis provides an idea to understand the genetic properties of the studied hybrid populations and the following conclusions are drawn.
The change in environmental conditions influences the manifestations of heterosis, indicating that it is the result of the interaction of the genotype with the environment. A positive true heterosis was found in crosses PI533704×Zuter and PI533704×Lucky801 by nodule weight and fresh root mass weight at both limits of the environment.
The signs of weight of the nodules per plant and fresh root mass weight in PI533704×Zuter and Zuter were inherited with a pronounced positive dominance and over dominance. The dominance of Lucky801×PI533704 by nodule weight and root mass weight per plant was with a negative sign.
Under more favorable growing conditions, epistatnite gene interactions played a greater role in inheriting the number of nodules, weight of nodules, and fresh aboveground mass weight, while dominant gene action was more important to inherit the seed weight in dense sowing.
With a high coefficient of inheritance in both environments, the hybrids of PI533704×Zuter and Lucky801×PI533704 were characterized by the number and weight of nodules, Zuter×PI533704 by fresh root and aboveground mass weight, and almost all hybrids by seed weight per plant. The total phenotypic manifestation of the seed weight per plant in Zuter×PI533704 was highly genotypically determined and a greater effect could be expected from the holding of a mass selection team in the earlier hybrid generations (F2-F3).
| [1] |
Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Reviews Microbiology, 2008, 6(10): 763-775. DOI:10.1038/nrmicro1987 (  0) 0) |
| [2] |
Massonneau A, Langlade N, Léon S, et al. Metabolic changes associated with cluster root development in white lupin (Lupinus albus L.): relationship between organic acid excretion, sucrose metabolism and energy status. Planta, 2001, 213(4): 534-542.. DOI:10.1007/s004250100529 (  0) 0) |
| [3] |
Neumann G, Martinoia E. Cluster roots-An underground adaptation for survival in extreme environments. Trends in Plant Science, 2002, 7(4): 162-167. DOI:10.1016/s1360-1385(02)02241-0 (  0) 0) |
| [4] |
Yang H, Tao Y, Zheng Z, et al. Application of next-generation sequencing for rapid marker development in molecular plant breeding: a case study on anthracnose disease resistance in Lupinus angustifolius L. BMC Genomics, 2012(13): 318. DOI:10.1186/1471-2164-13-318 (  0) 0) |
| [5] |
Raman R, Vipin C, Luckett D J, et al. Localisation of loci involved in resistance to Diaporthe toxica and Pleiochaeta setosa in white lupin (Lupinus albus L.). Open Journal of Genetics, 2014, 4(3): 210-226. DOI:10.4236/ojgen.2014.43022 (  0) 0) |
| [6] |
Phan H T T, Ellwood S R, Adhikari K, et al. The first genetic and comparative map of white lupin (Lupinus albus L.): identification of QTLS for anthracnose resistance and flowering time, and a locus for alkaloid content. DNA Research, 2007, 14(2): 59-70. DOI:10.1093/dnares/dsm009 (  0) 0) |
| [7] |
Simioniuc D, Burlacu-Arsene M, Simioniuc V, et al. A genetic linkage map for white lupin (Lupinus albus L.). Agronomy Series of Scientific Research, 2011, 54(2): 96-98. DOI:10.1046/j.1439-0523.2002.733320.x (  0) 0) |
| [8] |
Kurlovich B S, Kartuzova L T. Lupin Breeding. Kurlovich B S. Lupins (Geography, Classification, Genetic Resources and Breeding). St. Petersburg, Russia: Publishing House "Intan", 2002. 351-374.
(  0) 0) |
| [9] |
Schulze J, Temple G, Temple S J, et al. Nitrogen fixation by white lupin under phosphorus deficiency. Annals of Botany, 2006, 98(4): 731-740. DOI:10.1093/aob/mcl154 (  0) 0) |
| [10] |
Mantri N, Basker N, Ford R, et al. The role of miRNAs in legumes with a focus on abiotic stress response. The Plant Genome, 2013, 6(3): 1-14. DOI:10.3835/plantgenome2013.05.0013 (  0) 0) |
| [11] |
Provorov N A, Tikhonovich I A. Genetic resources for improving nitrogen fixation in legume-rhizobia symbiosis. Genetic Resources and Crop Evolution, 2003, 1(50): 89-99. DOI:10.1023/A:1022957429160 (  0) 0) |
| [12] |
Vishnyakova M A. Grain legumes gene pool and adaptive breeding as factors of biologization and ecologization of plant industry (review). Agricultural Biology, 2008, 3: 3-23. (  0) 0) |
| [13] |
Hefny M M. Use of genetic variability estimates and interrelationships of agronomic and biochemical characters for selection of lupin genotypes under different irrigation regimes. African Crop Science Journal, 2013, 21(1): 97-108. (  0) 0) |
| [14] |
Omarov D S. Towards the methods of plant heterosis recording and assessment. Agricultural Biology, 1975, 10: 123-127. (  0) 0) |
| [15] |
Romero G E, Frey K J. Inheritance of semidwarfness in several wheat crosses. Crop Science, 1973(3): 334-337. DOI:10.2135/cropsci1973.0011183x01300030015x (  0) 0) |
| [16] |
Sobolev N A. Hybridological analysis of polygenic traits. Cytology and Genetics, 1976, 10(5): 424-436. (  0) 0) |
| [17] |
Reinhold K. Maternal effects and the evolution of behavioural and morphological characters: a literature review indicates importance of extended maternal care. Journal of Heredity, 2002, 93(6): 400-405. DOI:10.1093/jhered/93.6.400 (  0) 0) |
| [18] |
Dragavcev V A. New approaches to the evaluation of the genetical structure of plant populations for in situ and ex situ conservation of plant genetic resources. Proceedings of an International Symposium on Plant Genetic Resources in Europe. Gatersleben, Germany: 1993. 200-202.
(  0) 0) |
| [19] |
Levko G D. Theoretical Substantiation and Practical Use of Methods of Selection and Seed Production of Flower Crops. http://earthpapers.net/teoreticheskoe-obosnovanie-i-prakticheskoe-ispolzovanie-metodov-selektsii-i-semenovodstva-tsvetochnyh-kultur, 2019-12-24.
(  0) 0) |
| [20] |
Provorov N A, Tikhonovich I A. Ecological-genetic principles of plant breeding for increasing the efficiency of interaction with microorganisms S-Kh. Biol, 2003, 3: 11-25. (  0) 0) |
| [21] |
Kadermas I G. Formation of Photosynthetic and Symbiotic Apparatuses of Plants and Their Contribution to the Increase in the Productivity of Pea Sowing Agrocenoses (Pisum sativum L. ). http://earthpapers.net/formirovanie-fotosinteticheskogo-i-simbioticheskogo-apparatov-rasteniy-i-ih-vklad-v-povyshenie-produktivnosti-agrotsenozo, 2019-12-24.
(  0) 0) |
| [22] |
Ashiev A R. Initial Material of Peas (Pisum sativum L. ) and Its Selective Use in the Conditions of the Pre-rural Steppe of the Republic of Bashkortostan. http://earthpapers.net/ishodnyy-material-goroha-pisum-sativum-l-i-ego-selektsionnoe-ispolzovanie-v-usloviyah-preduralskoy-stepi-respubliki-bashk, 2019-12-24.
(  0) 0) |
| [23] |
Zolotaryova S V. Evaluation and Creation of the Initial Material for the Selection of Vegetable Pea in the Central Region of the Nonchernozem Zone of Russia. https://www.dissercat.com/content/otsenka-i-sozdanie-iskhodnogo-materiala-dlya-selektsii-gorokha-ovoshchnogo-v-tsentralnom-rai, 2019-12-24.
(  0) 0) |
| [24] |
Tyurin Yu S, Zolotaryov V N. Biological, breeding and technological foundations for potential productivity realization of hairy winter vetch (Vicia willosa Roth. ), variety Lugovskaya 2. Scientific-practical International On-line Journal Adaptive Fodder Production, 2013, 13 (1): 31-43.
(  0) 0) |
| [25] |
Solovov I I. Study of the Initial Pea Material (Pisum sativum L. ) and Its Use in Breeding for Increasing Symbiotic Activity in the Northern Part of the Central Black Earth Region of Russia. https://www.dissercat.com/content/izuchenie-iskhodnogo-materiala-gorokha-pisum-sativum-l-i-ispolzovanie-ego-v-selektsii-na-pov, 2019-12-24.
(  0) 0) |
| [26] |
Beyer H, Schmalenberg A K, Jansen G, et al. Evaluation of variability, heritability and environmental stability of seed quality and yield parameters of L. angustifolius. Field Crops Research, 2015, 174: 40-47. DOI:10.1016/j.fcr.2014.12.009 (  0) 0) |
| [27] |
Vitko I, Taranukho G I. Signs of heterosis in hybrids of yellow lupine. Bulletin of the Belarusian State Agricultural Academy: The Guidance Journal, 2011, 2: 64-70. (  0) 0) |
 2021, Vol. 28
2021, Vol. 28


