2. State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China
Sludge bulking is a frequent phenomenon in the activated sludge system with severe environmental and economic consequences [1]. It is mainly associated with excessive growth of filamentous bacteria. The commonest factors to induce "filamentous bulking" are long sludge retention time (SRT), low dissolved oxygen (DO), low organic load, and so on[2-3]. Except for filamentous bulking, there is another type of sludge bulking termed "viscous bulking", which is determined by sludge bulking without filamentous bacteria. Viscous bulking is usually formed by excessive extracellular polymeric substances (EPS)[4], and often occurs when the nutrients is deficient or the organic load is high[5]. When viscous bulking occurs, the viscosity of sludge flocs increases significantly[6]. Unlike filamentous bulking, viscous bulking does not occur frequently, and adding flocculant is considered to be an effective method to prevent viscous bulking [7].
So far, most research about sludge bulking is focused on how to eliminate it but not to make use of it. Recently, Guo et al.[8] established a low energy-saving wastewater treatment system. In the system, limited or slight filamentous bacteria were stimulated by the low DO. By utilizing the characteristics of filamentous bacteria such as larger specific surface area and stronger ability to degrade low concentration substrates, this system can improve treatment performance while saving energy consumption. It presented a novel pathway to treat filamentous sludge bulking. In some special cases, sludge bulking can be utilized. However, few researches have been reported in this new area, especially for viscous bulking.
The aim of this research was to investigate the characteristics of nutrients removal performance in viscous bulking state. The kinetics of nitrification between normal sludge and viscous bulking sludge were compared. It is also hoped this research can provide a new perspective on viscous bulking.
1 Material and Methods 1.1 Experimental DesignThe experiments were operated using synthetic wastewater which contained (per liter)CH3COONa·3H2O (663.80 mg), NH4Cl (166.90 mg), KH2PO4 (18.80 mg), CaCl2·2H2O (40 mg), NaHCO3 (375 mg), MgSO4 (80 mg), and micro nutrients solution[9] (0.30 mL). The corresponding water quality was 330 mg/L COD, 45 mg/L NH4+-N, and 4.30 mg/L PO43--P.
The study was carried out using one sequencing batch reactor (SBR) with a height of 700 mm, diameter of 200 mm, and working volume of 12 L. Sampling ports with a 10 cm interval were set in the vertical direction of the reactor, as shown in Fig. 1. Each cycle contained feed period, anaerobic period, aerobic period, and settle/discharge/decant period (Table 1). At the end of each aerobic period, excess sludge was discharged to control SRT. At the end of settle period, supernatant was discharged to control hydraulic retention time (HRT). Temperature was controlled at 21 ± 1 ℃ by temperature controller. Inoculated sludge was obtained from an anaerobic/ anoxic/aerobic (AAO) reactor in our laboratory (SVI and MLSS were around 90 mL/g and 2000 mg/L, respectively), and synthetic wastewater was treated with the same components as above. This research consisted of a sequence of three experimental phases. In the first phase, inoculated sludge was acclimated to the synthetic wastewater under low COD load; in the second phase, COD load increased with the increase of volume exchange ratio to induce viscous sludge bulking; in the third phase, activated sludge under viscous bulking was operated under low COD load. Operation procedures are highlighted in Table 1.
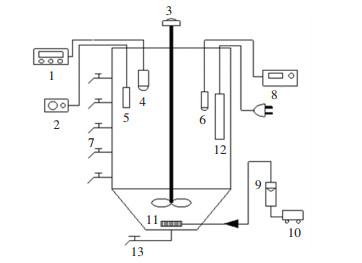
|
Fig.1 Schematic diagram of experimental equipment |
| Table 1 Operation procedures in each experimental phase |
1.2 Kinetic Analysis
The nitrification rates are fitted by the Monod equation[10]:
| $ V=-\frac{\mathrm{d} C}{\mathrm{~d} t}=V_{m} X \frac{C}{K_{s}+C} $ | (1) |
where V is the nitrification rates of NH4+-N and NO2--N, mg/(L·h); X is the MLSS, mg/L; Vm is the maximum specific nitrification rates of NH4+-N and NO2--N, mg/(g·h); Ks is the half-saturation constant, mg/L.
Otherwise, the nitrification rate could also be expressed in the following form:
| $ V=-\frac{\mathrm{d} C}{\mathrm{~d} t}=k C^{n} $ | (2) |
where n is the reaction order; k is the reaction rate constant. Actually, many nitrification kinetics occurring in biological nitrogen removal systems is close to a first-order model (n=1)[10].
1.3 Calculatory ProceduresThe SND efficiency and NO2--N accumulation ratio (NAR) were calculated using Eq.(3) and Eq.(4)[11]:
| $ \mathrm{SND}_{\text {efficiency }}=\frac{\mathrm{NH}_{4}^{+}-\mathrm{N}_{\text {(oxidized) }}-\mathrm{NO}_{x}^{-}-\mathrm{N}_{\text {(produced) }}}{\mathrm{NH}_{4}^{+}-\mathrm{N}_{\text {(oxidized) }}} \times 100 \% $ | (3) |
| $ \mathrm{NAR}=\frac{\mathrm{C}_{\mathrm{NO}_{2}{ }^{-}-\mathrm{N}}}{\mathrm{C}_{\mathrm{NO}_{2}{ }^{-}-\mathrm{N}}+\mathrm{C}_{\mathrm{NO}_{3}{ }^{-}-\mathrm{N}}} \times 100 \% $ | (4) |
where NH4+-N(oxidized) is the amount of oxidized NH4+- N, mg; NOx--N(produced) is the amount of produced NOx--N, mg;
Liquid samples were filtered with a 0.45 μ m filter. The analyses of COD, PO43--P, NH4+-N, NO2--N, NO3--N, MLSS, SVI, and MLVSS were measured according to the standard methods [12]. Each water sample was tested three times. Microorganism morphology was observed with an OLYPUS BX51 optical microscope. Viscosity was tested using an NDJ-79 viscosimeter. EPS were extracted by heat extraction method[13]. Polysaccharides were analyzed by the Dubois method[14]. Protein was analyzed by the modified Lowry method[15]. Distribution of particle size was tested using HIAC Royco 9703 particle size analyzer. Temperature, pH, DO, and ORP were recorded by WTW inoLab Oxi level 2. Correlation between different variables was analyzed by SPSS software (11.0).
2 Results and Discussion 2.1 Variations of Activated Sludge CharacteristicsEPS, MLSS, and sludge settleability fluctuated during the experiment. In this experiment, EPS mainly consisted of polysaccharides and proteins. The ratio of EPS to MLSS can be used to measure the amount of EPS secreted per unit mass of activated sludge. It correlated well with SVI (r2=0.92), as shown in Fig. 2. In the first experimental phase, the average SVI and EPS/MLSS were 98.30 mL/g and 18.68 mg/g, respectively. In the third experimental phase, they changed to 345.36 mL/g and 27.76 mg/g, respectively. Meanwhile, the average viscosity and MLVSS/MLSS increased from 1.21 mPa/s and 0.82 to 1.51 mPa/s and 0.88, respectively. The morphology of activated sludge in each experimental phase was observed by microscope. Only little filamentous bacteria were present. Therefore, the viscosity sludge bulking was confirmed[16]. Particle size distribution was tested in the 100th and the 250th cycle, and the results are shown in Fig. 3. Compared with normal sludge, the viscous bulking sludge had a larger particle size. The polysaccharides and proteins contained in EPS are all hydrophilic substances. Researches have demonstrated that EPS could contribute to aggregate sludge flocs, so the viscous bulking sludge with more EPS are prone to form larger particle size[17].
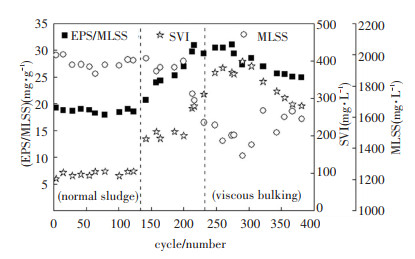
|
Fig.2 Variations in sludge characteristics |
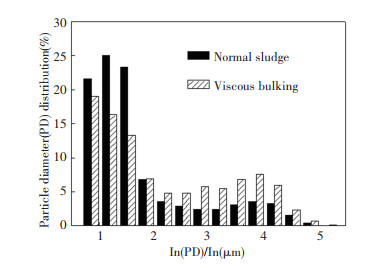
|
Fig.3 Particle diameter distribution |
2.2 Nutrients Removal Performance 2.2.1 Variations in nutrient concentrations
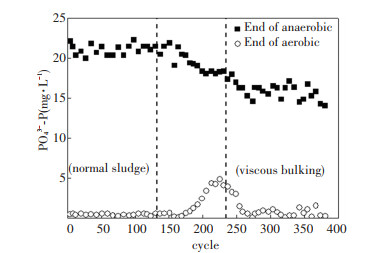
The concentration variations of nitrogen- containing compounds are displayed in Fig. 4. In the first phase, the average NO3--N and NH4+-N were 9.05 and 0.91 mg/L, respectively, and no NO2--N was accumulated. In the second phase, NH4+-N increased greatly with a decrease in NO3--N. In the third phase, the average NH4+-N reduced to 0.87 mg/L with significant NO2--N accumulation. It should be noted that at the first several cycles of the third phase, NH4+-N dropped down to lower than 2 mg/L immediately. In such a short time, ammonia-oxidizing bacteria (AOB) could not proliferate significantly. It seemed AOB was fallow in high COD loading state. Similar phenomenon was also reported by van den Akker[18]. Theoretically, biological phosphorus removal is divided into two processes: anaerobic phosphorus release and aerobic phosphorus uptake. Compared with the PO43--P concentrations at each experimental phase, it was found that the amount of released PO43--P and uptaken PO43--P both decreased during viscous bulking formation process, as shown in Fig. 5. Mass balance calculations for both N and P were carried out. The results showed that in first phase (normal sludge) 24.17% of TN and 10.20% of TP from the influent existed in the effluent, while in the third phase (viscous bulking sludge), 14.38% of TN and 17.50% of TP were residual.
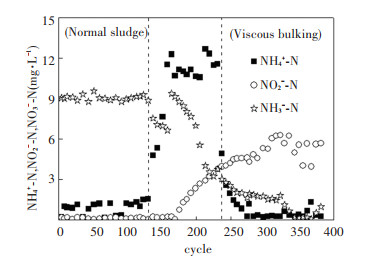
|
Fig.4 Nitrogen removal performance |
|
Fig.5 Phosphorus removal performance |
2.2.2 Cyclic analysis
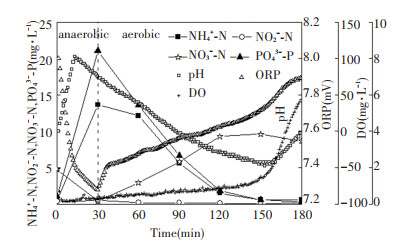
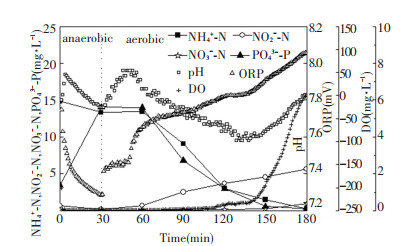
Cyclic analysis of pH, DO, ORP, and nutrients was conducted at the 123th cycle and 381th cycle (Fig. 6 and Fig. 7). In the anaerobic period, NO2--N, NO3--N, and ORP decreased, while pH did not drop until the denitrification was completed. The turning point was called "nitrate knee", which could exactly indicate the end of denitrification[19]. Compared with normal sludge, nitrate knee of viscous bulking sludge occurred earlier. During the anaerobic period, PO43--P increased from 1.06 and 3.45 mg/L to 21.04 and 14.04 mg/L, respectively.
|
Fig.6 Variations in concentrations of nutrients during a typical cycle (the 123th cycle) |
|
Fig.7 Variations in concentrations of nutrients during a typical cycle (the 381th cycle) |
During the aerobic period, because nitrification produced acid, pH decreased gradually in normal sludge system (Fig. 6). When NH4+-N was depleted, pH switched from decreasing to increasing. The turning point on the pH profile was called "ammonia valley". After the ammonia valley, DO rapidly increased. By the end, NH4+-N was basically nitrified to NO3--N with no accumulation of NO2--N, and PO43--P had been uptaken to 0.24 mg/L. In contrast, in viscous bulking system, because the sludge adsorbed large amount of organics, pH gradually increased with the conversion of organics to CO2 at the beginning of aerobic period, and the acidity decreased when CO2 was blown off[19]. As the nitrification process began at the 55th min, NH4+-N and pH decreased gradually just as those occurred in normal sludge. The difference was obvious accumulation of NO2--N occurred. At the end, PO43--P had been uptaken to 0.25 mg/L.
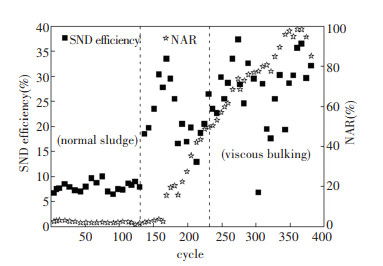
2.2.3 Nutrients removal characteristicsThe ideal COD/TN to sustain conventional biological nitrogen removal was considered to be 6.00-8.00[20]. In this experiment, the COD/TN ratio was 7.67. In addition to denitrification, assimilation, phosphorus release, and respiration also utilized COD. In the first and third phases, the effluent NH4+-N concentrations were limited. The NOx--N was mainly NO3--N in the first phase, while it changed to NO2--N in the third phase. The average nitrite accumulation ratios were 2.26% and 78.02%, respectively, as shown in Fig. 8. It has been reported that the sludge flocs with bigger particle size were prone to form low DO micro-environment inside [21], and the AOB is more adapted to low DO [19], so the growth advantage of AOB over nitrite oxidation bacteria (NOB) may be the cause of nitrite accumulation. In addition to NO2--N accumulation, obvious SND phenomenon occurred in viscous bulking state. The average efficiency of SND increased from 7.92% in the first phase to 27.31% in the third phase. The reason may be that compared with NO3--N, denitrifying NO2--N to N2 can save 40% of carbon source[22-23].
|
Fig.8 Nitrogen removal characteristics |
EPS acted as a determining factor in the transmission and accumulation of PO43--P between phosphorus-accumulating bacteria (PAO) and bulk liquid[24]. It was found that EPS/MLSS correlated well (r2 = -0.90) with the mass of released PO4-P3-(mp), as shown in Fig. 9. This indicated that EPS secreted in viscous bulking sludge could reduce the amount of released PO43--P. The reason may be that the Ca2+ in EPS could crystallize with PO43--P[25]. Zhou et al.[26] found that 15.70% of the phosphorus in biological phosphorus removal process was removed by EPS storage. In this experiment, the discrepancy between specific phosphorus uptake (Vmpu) and specific phosphorus release (Vmpr) showed obvious positive correlation with EPS/MLSS (r2=0.71), which confirmed that EPS played the role of container in phosphorus removal process.
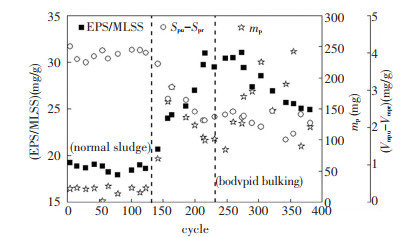
|
Fig.9 Phosphorus removal characteristics |
2.3 Nitrification Kinetics
The oxidation rate of NH4+-N (NO2--N) was measured through observing the change in NH4+-N (NO3--N) over time. The measured values were fitted by Eq.(1) to determine the parameters of Vm and Ks[8]. The parameter values of nitrification kinetics in different sludge states are shown in Table 2.
| Table 2 Parameter values of nitrification kinetics in different activated sludge states |
The R2 of Monod equation in each state is close to 1.00, which means that Monod equation is basically followed. For normal sludge, the First-order equation can well describe nitrification kinetics. The reason was that under the dilution effect of feed period, the nitrogen concentration was in low level[27]. In contrast, for viscous bulking sludge, R2 of the First-order equation were only around 0.70. This might be because diffusion in viscous bulking flocs could also affect nitrification rate [28]. It was found the Vm of NH4+-N between normal sludge and viscous bulking sludge were similar, which agreed well with the nitrification performance (Fig. 4). But the Vm of NO2--N dropped from 24.69 to 1.20 mg/(g·h), and the lower Vm of NO2--N correlated well with NO2--N accumulation in the third phase (Fig. 4).
2.4 Evaluation of Nutrients Removal Characteristics in Viscous BulkingIn general, the main determining factors of the above results might be floc particular size and EPS. For example, research by Han et al.[21] showed at the internal center of flocs, DO concentration would decrease by 10%-55% with the floc size increasing 2.50 times. Same phenomenon was also observed in the transmission of NH4+-N and NOx--N. Similarly, Li et al.[29] showed that DO only partially penetrated from the flocs surface. Therefore, low DO concentration in the floc was the main cause of NO2--N accumulation in viscous bulking state. In this experiment, not only the viscous bulking sludge secreted more EPS, but also the average percentages of polysaccharides in EPS increased by 22.36% compared with normal sludge. Lin et al.[30] found that struvite could be accumulated in EPS. In the synthetic wastewater, NH4Cl and MgSO4 were both added, so all the necessary components to synthesize struvite existed. It should be noted that COD load and C/N ratio are very important factors determining nutrients removal performance. In this experiment, during the first and third experimental phases, the two factors were similar. So their effects on nutrients removal could be negligible.
3 ConclusionsThis research explored the characteristics of nutrients removal in viscous sludge bulking state. In terms of nitrogen removal, the maximum specific oxidation rate of NH4+-N was similar to normal sludge, while obvious accumulation of NO2--N occurred during the aerobic period. The impact of bigger particle size and more EPS on transfer resistance was considered as the main cause of NO2--N accumulation. In terms of phosphorus removal, the storage effect of EPS was more obvious than normal sludge. Its mechanism needs to be further researched.
| [1] |
Fan N S, Wang R F, Qi R, et al. Control strategy for filamentous sludge bulking: bench-scale test and full-scale application. Chemosphere, 2018, 210: 709-716. DOI:10.1016/j.chemosphere.2018.07.028 (  0) 0) |
| [2] |
Keelan M F, Batstone D J, van Loosdrecht M C M, et al. A mathematical model for electrochemically active filamentous sulfide-oxidising bacteria. Bioelectrochemistry, 2015, 102: 10-20. DOI:10.1016/j.bioelechem.2014.11.002 (  0) 0) |
| [3] |
Ramin E, Sin G, Mikkelson P S, et al. Significance of settling model structures and parameter subsets in modelling WWTPs under wet-weather flow and filamentous bulking conditions. Water Research, 2014, 63: 209-221. DOI:10.1016/j.watres.2014.05.054 (  0) 0) |
| [4] |
Kaewpipat K, Grady Jr C P L. Population dynamics in laboratory-scale activated sludge reactors. Water Science & Technology, 2002, 46(1-2): 19-27. DOI:10.2166/wst.2002.0450 (  0) 0) |
| [5] |
Shao Y X, Yang X, Mohammed A, et al. Impacts of ammonium loading on nitritation stability and microbial community dynamics in the integrated fixed-film activated sludge sequencing batch reactor (IFAS-SBR). International Biodeterioration & Biodegradation, 2018, 133: 63-69. DOI:10.1016/j.ibiod.2018.06.002 (  0) 0) |
| [6] |
Yang Q X, Zhao H L, Du B B. Bacteria and bacteriophage communities in bulking and non-bulking activated sludge in full-scale municipal wastewater treatment systems. Biochemical Engineering Journal, 2017, 119: 101-111. DOI:10.1016/j.bej.2016.12.017 (  0) 0) |
| [7] |
Martins A M P, Pagilla K, Heijnen J J, et al. Filamentous bulking sludge-a critical review. Water Research, 2004, 38(4): 793-817. DOI:10.1016/j.watres.2003.11.005 (  0) 0) |
| [8] |
Guo J H, Peng Y Z, Peng C Y, et al. Energy saving achieved by limited filamentous bulking sludge under low dissolved oxygen. Bioresource Techology, 2010, 101(4): 1120-1126. DOI:10.1016/j.biortech.2009.09.051 (  0) 0) |
| [9] |
Ma H Y, Zhang Y L, Xue Y, et al. A new process for simultaneous nitrogen removal and phosphorus recovery using an anammox expanded bed reactor. Bioresource Technology, 2018, 267: 201-208. DOI:10.1016/j.biortech.2018.07.044 (  0) 0) |
| [10] |
Zhang S F, Wang Y Y, He W T, et al. Impacts of temperature and nitrifying community on nitrification kinetics in a moving-bed biofilm reactor treating polluted raw water. Chemical Engineering Journal, 2014, 236: 242-250. DOI:10.1016/j.cej.2013.09.086 (  0) 0) |
| [11] |
Third K A, Burnett N, Cord-Ruwisch R. Simultaneous nitrification and denitrification using stored substrate (PHB) as the electron donor in an SBR. Biotechnology and Bioengineering, 2003, 83(6): 706-720. DOI:10.1002/bit.10708 (  0) 0) |
| [12] |
State Environmental Protection Administration of China. Water and Waste Water Monitoring Analysis Methods, 4th ed. Beijing: Chinese Environmental Science Publishing House, 2002.
(  0) 0) |
| [13] |
Sheng G P, Yu H Q, Yu Z. Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila. Applied Microbiology and Biotechnology, 2005, 67(1): 125-130. DOI:10.1007/s00253-004-1704-5 (  0) 0) |
| [14] |
Jabariyan S, Zanjanchi A M, Arvand M, et al. Colorimetric detection of glucose using lanthanum-incorporated MCM-41. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2018, 203: 294-300. DOI:10.1016/j.saa.2018.04.044 (  0) 0) |
| [15] |
Hong P-N, Honda R, Noguchi M, et al. Optimum selection of extraction methods of extracellular polymeric substances in activated sludge for effective extraction of the target components. Biochemical Engineering Journal, 2017, 127: 136-146. DOI:10.1016/j.bej.2017.08.002 (  0) 0) |
| [16] |
William A S K, Mancell E, David J K, et al. Limit of stokesian settling concentration characterizes sludge settling velocity. Water Research, 2016, 90: 100-110. DOI:10.1016/j.watres.2015.12.007 (  0) 0) |
| [17] |
Basuvaraj M, Fein J, Liss S N. Protein and polysaccharide content of tightly and loosely bound extracellular polymeric substances and the development of a granular activated sludge floc. Water Research, 2015, 82: 104-117. DOI:10.1016/j.watres.2015.05.014 (  0) 0) |
| [18] |
van den Akker B, Beard H, Kaeding U, et al. Exploring the relationship between viscous bulking and ammonia-oxidiser abundance in activated sludge: a comparison of conventional and IFAS systems. Water Research, 2010, 44(9): 2919-2929. DOI:10.1016/j.watres.2010.02.016 (  0) 0) |
| [19] |
Yang Q, Peng Y Z, Liu X H, et al. Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities. Environmental Science and Technology, 2007, 41(23): 8159-8164. DOI:10.1021/es070850f (  0) 0) |
| [20] |
Chiu Y C, Lee L L, Chang C N, et al. Control of carbon and ammonium ratio for simultaneous nitrification and denitrification in a sequencing batch bioreactor. International Biodeterioration & Biodegradation, 2007, 59(1): 1-7. DOI:10.1016/j.ibiod.2006.08.001 (  0) 0) |
| [21] |
Han Y P, Liu J X, Guo X S, et al. Micro-environment characteristics and microbial communities in activated sludge flocs of different particle size. Bioresource Technology, 2012, 124: 252-258. DOI:10.1016/j.biortech.2012.08.008 (  0) 0) |
| [22] |
Krasnits E, Beliavsky M, Tarre S, et al. PHA based denitrification: municipal wastewater vs. acetate. Bioresource Technology, 2013, 132: 28-37. DOI:10.1016/j.biortech.2012.11.074 (  0) 0) |
| [23] |
Peng Z X, Peng Y Z, Su X Y, et al. Investigation of the characteristics of nitrogen and phosphorus removal under viscosity sludge bulking. Acta Scientiarum Naturalium Universitatis Pekinensis, 2010, 46(3): 417-421. (in Chinese) DOI:10.13209/j.0479-8023.2010.061 (  0) 0) |
| [24] |
Wang Y Y, Jiang X X, Wang H, et al. Comparison of performance, microorganism populations, and biophysiochemical properties of granular and flocculent sludge from denitrifying phosphorus removal reactors. Chemical Engineering Journal, 2015, 262: 49-58. DOI:10.1016/j.cej.2014.09.065 (  0) 0) |
| [25] |
Wang R D, Peng Y Z, Cheng Z L, et al. Understanding the role of extracellular polymeric substances in an enhanced biological phosphorus removal granular sludge system. Bioresource Technology, 2014, 169: 307-312. DOI:10.1016/j.biortech.2014.06.040 (  0) 0) |
| [26] |
Zhou J, Li J J, Long T R, et al. Study on the action of extracellular polymeric substances (EPS) in biological phosphorus removal from wastewater. Acta Scientiae Circumstantiae, 2008, 28(9): 1758-1762. (in Chinese) DOI:10.13671/j.hjkxxb.2008.09.020 (  0) 0) |
| [27] |
Watten B J, Sibrell P L. Comparative performance of fixed-film biological filters: application of reactor theory. Aquacultural Engineering, 2006, 34(3): 198-213. DOI:10.1016/j.aquaeng.2005.03.006 (  0) 0) |
| [28] |
Chen S L, Ling J, Blancheton J-P. Nitrification kinetics of biofilm as affected by water quality factors. Aquacultural Engineering, 2006, 34(3): 179-197. DOI:10.1016/j.aquaeng.2005.09.004 (  0) 0) |
| [29] |
Li Y, Liu Y, Shen L, et al. DO diffusion profile in aerobic granule and its microbiological implications. Enzyme and Microbial Technology, 2008, 43(4-5): 349-354. DOI:10.1016/j.enzmictec.2008.04.005 (  0) 0) |
| [30] |
Lin Y M, Bassin J P, van Loosdrecht M C M. The contribution of exopolysaccharides induced struvites accumulation to ammonium adsorption in aerobic granular sludge. Water Research, 2012, 46: 986-992. DOI:10.1016/j.watres.2011.11.072 (  0) 0) |
 2021, Vol. 28
2021, Vol. 28


