Nowadays, Cu, Zn, and Pb are highly demanded heavy metals in the market and it is projected that the supply would reach its maximum in 2030 to 2050[1]. This implies the rise of water pollution by these heavy metals from electronic industry, mining, paints industry, and glass industry in the near future. A recent report published by Kenai Watershed Forum found out that fertiliser, traffic intensification as well as general urbanization contribute to Zn and Cu pollution in Kenai River Watershed[2]. Furthermore, research done by Sankson et al.[3] reported that the combined sewer overflows discharge in Poland consist of 170-1890 μ g/L Zn and 31-590 μ g/L Cu. Moreover, Voghji River was highly polluted with heavy metal consisting of 1.28-82.5 μ g/L Cu and 0.94-105.5 μ g/L Zn due to mining activities[4].
Heavy metals cause deleterious effects to living organisms.Heavy metals could enter human body through different routes, such as inhalation of toxic gas and ingestion of contaminated drinking water and food. High exposure to heavy metal could lead to serious health effects and in extreme situation, it can lead to death. Cu2+ can cause stomach-ache, irritation in eyes, noses, mouths, and headache, while Zn2+ causes stomach cramps, nausea, and respiratory disorder[5]. Additionally, plants which are exposed to high level of Cu2+ will prohibit root elongation as well as root cell membrane[6]. Similarly, Zn2+ also causes growth inhibition in macroalgae, which is economically significant and a fast-growing commodity[7].
In Malaysia, the allowable discharge for Cu2+ according to standard A is 0.2 mg/L and 1 mg/L for standard B[8]. Meanwhile, the allowable discharge for Zn2+ is 2 mg/L for both standard A and B. To ensure good quality of life, it is compulsory to treat the wastewater to the allowable discharge limit. Chemical precipitation, coagulation-flocculation, ion exchange, and membrane filtration are the commonly used method to treat heavy metal polluted water. However, most of these methods have disadvantages including high energy consumption which results in high operating cost, low efficiency of heavy metal removal, and formation of toxic sludge where further treatment is required[9]. Lately, research has been conducted on genetically modified micro-organism to remove heavy metal such as Cd2+ and Pb2+[10]. However, this is a newly introduced feature, which is not suitable for practical application for now.
Adsorption is a well-known separation method, and it is recognized by numerous scientists for wastewater applications in recent years[11]. It is because adsorption shows high efficiency in heavy metals removal at reasonable cost and minimizes the chemical and biological sludge produced[12]. Furthermore, regeneration of adsorbents can be performed in most of the adsorption process, which further reduces the maintenance cost. Isotherm and kinetics studies are commonly conducted in adsorption related research. This is because kinetic study is important to understand the adsorption mechanism and identify the rate of the controlling steps. Pseudo first order (PFO) and pseudo second orders (PSO) are commonly studied to investigate the adsorption mechanism. In PFO, low constant value, K1, indicates slow adsorption process while high constant value, K2, reveals an increase in adsorption rate[13]. Table 1 shows the list of adsorbents used for heavy metals adsorption. Generally, PSO presented the adsorption mechanism well with coefficient of determination, R2, which is approximately 0.99.[13-16] qe represents the absorbed amount at equilibrium.
| Table 1 List of adsorbents used for heavy metals adsorption |
Zeolite is a popular adsorbent due to its inherent high porosity and excellent metal binding capacity. Numerous factors such as type of zeolite, pH, contact time, mass of adsorbent, and concentration of feed solution affect the performance of zeolite in heavy metal removal. Thus, the objective of this study is to identify an ideal operating condition to achieve maximum removal rate by considering the effect of pH and contact time on the performance of zeolite. Besides, kinetic study was also conducted to find out the adsorption mechanism.
1 Materials and Methods 1.1 MaterialsAnalytical grade zeolite crystalline alumiosilicates, which consist of silica and alumina, were supplied by ChemSoln. The zeolite was firstly sieved using 200 μ m × 500 μ m sieves and treated in the oven at 100 ℃ for 24 h before use. CuCl2 and ZnCl2 were used as received.
1.2 Methodology0.5 g zeolite was exposed to 10×10-6 of Cu2+ and Zn2+ solutions at pH 3, 5, 7, 9, and 11. The pH of the solution was adjusted by adding either 0.1 N of NaOH or 0.1 N of HCl into the solutions. Sample was collected and filtered using syringe filter (Thermo Fisher, 0.22 micron) after 60 min to evaluate the adsorptive performance of zeolite. In order to find out the effect of contact time on heavy metal adsorption rate, sample was collected every 30 min within 150 min. The concentration of Cu2+ and Zn2+ before (Ci) and after (Cf) was measured using Atomic Absorption Spectrometer (Agilent Technologies, Model: 240 AA).
The percentage of removed Cu2+ and Zn2+ was calculated by using the following equation:
| $ {\rm{Percentage}}\;{\rm{of}}\;{\rm{removal}} = \frac{{\left( {{C_{\rm{i}}} - {C_{\rm{f}}}} \right)}}{{{C_{\rm{i}}}}} \times 100{\rm{\% }} $ | (1) |
The kinetic of the adsorption process was determined by using PFO (Eq.(2)), PSO (Eq.(3)), Ritchie's equation (Eq.(4)), Boyd's external diffusion equation (Eq.(5)), Weber and Moris (W & M) (Eq.(6)), and Elovich (Eq.(7)). The equations are listed as below:
| $ {\rm{log}}\left( {{q_{\rm{e}}} - {q_t}} \right) = \log {q_{\rm{e}}} - {K_1}t $ | (2) |
| $ \frac{t}{{{q_t}}}{\rm{ = }}\frac{1}{{{K_2}q_{\rm{e}}^2}} + \frac{t}{{{q_{\rm{e}}}}} $ | (3) |
where qe and qt are the amount of adsorbate (solute) adsorbed by the adsorbent (g/mg zeolite) at equilibrium and at time t. K1and K2 are defined as the constant values for PFO and PSO models.
| $ {q_t} = {q_\infty } - {q_\infty }{\left( {1 + \left( {n - 1} \right) \propto t} \right)^{\frac{1}{{n - 1}}}} $ | (4) |
| $ {q_t} = {q_\infty }\left( {1 - {{\rm{e}}^{ - Rt}}} \right) $ | (5) |
| $ {q_t} = {k_{{\rm{W\& M}}}}{t^{0.5}} $ | (6) |
| $ {q_t} = \frac{1}{b}\ln \left( {1 + abt} \right) $ | (7) |
The amount of adsorbate adsorbed on adsorbent at infinity time is labeled as q∞, while n is known as the number of active sites on the adsorbent occupied by adsorbate in Ritchie's equation. R is the rate constants in Boyd's external diffusion equation while kW & M is the intraparticle diffusion coefficient. For Elovich model, a is the initial adsorption rate constant while b is the desorption rate constant.
The following statistic parameters were used to evaluate the validity of the kinetic models.
| $ {R^2} = \frac{{\sum {{{\left( {{q_{{\rm{mean}}}} - {q_{{\rm{cal}}}}} \right)}^2}} }}{{\sum {{{\left( {{q_{{\rm{cal}}}} - {q_{{\rm{mean}}}}} \right)}^2}} + \sum {{{\left( {{q_{{\rm{cal}}}} - {q_{\exp }}} \right)}^2}} }} $ | (8) |
| $ {\chi ^2} = \frac{{\sum {{{\left( {{q_{\exp }} - {q_{{\rm{cal}}}}} \right)}^2}} }}{{q_{{\rm{cal}}}^2}} $ | (9) |
| $ {\rm{SSE}} = \sum {{{\left( {{q_{\exp }} - {q_{{\rm{cal}}}}} \right)}^2}} $ | (10) |
| $ MSE = \frac{1}{{{N_{\exp }}}}{\sum {\left( {{q_{\exp }} - {q_{{\rm{cal}}}}} \right)} ^2} $ | (11) |
R2, Eq.(1), residual sum of square (SSE) and mean square error (MSE) are used.
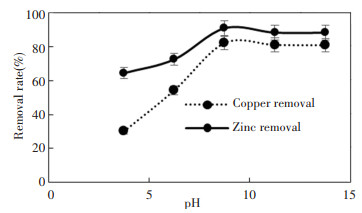
2 Results and Discussion 2.1 Effect of pHFig. 1 shows the adsorptive performance of zeolite in Cu2+ and Zn2+ under varied pH condition. Cu2+ removal increased from 30.16% to 82.15% when the pH of the solution increased from pH 3 to pH 7. However, the removal rate remained constant when the pH value increased from 7 to 11. This is because under acidic condition, the presence of free H+ behaved as the competitor to both Zn2+ and Cu2+. This reduces the number of active sites on the zeolite to adsorb Zn2+ and Cu2+. This finding is similar to recent findings obtained by Refs. [17-18] where the presence of H+ reduced the amount of Pb2+ adsorbed to both natural and Fe (Ⅲ) modified zeolite alginate beads. Similar adsorptive trend was found in Zn2+ solution as shown in Fig. 1, where the percentage of removal rate increased from 64.43% at pH 3 to 90.87% at pH 7 and the removal rate remained constant when the pH is further increased to pH 11.
|
Fig.1 pH effect on the performance of zeolite |
Comparatively, adsorptive performance of zeolite towards Zn2+ was higher compared with Cu2+, where the maximum removal rate was ~90% for Zn2+ and ~80% for Cu2+. This is due to the effect of hydration enthalpy (ΔHhyd), where ΔHhyd=-1955 kJ/mol for Zn2+ and ΔHhyd=-2010 kJ/mol for Cu2+ [19-20]. Hydration enthalpy describes the amount of energy required to detach water molecules from cations. In this case, Cu2+ needs to release higher amount of energy to detach from water compared with Zn2+, thus its interaction with zeolites is lower compared with Zn2+[21]. As a consequence, the percentage of removal for Cu2+ is lower compared with Zn2+ under the same pH condition.
2.2 Effect of Contact TimeFig. 2 shows the effect of contact time on Cu2+ and Zn2+ removal rate. Generally, the removal rate increased from t=30 min to t=120 min. Equilibrium point achieved at t=120 min, where no further increment in terms of removal rate was found after t=150 min. This is because the number of active sites of zeolites is higher at the beginning of the adsorption process. Rapid adsorption process occurred in the first 30 min, where approximately 60% removal was found for both Cu2+ and Zn2+. At t=60 min, the removal rate reduced, where additional 15% and 10% removal rate was recorded for Cu2+ and Zn2+, respectively. This is due to the reduction in the number of active sites, which is available for adsorption. At the saturation point, no active sites are available for either Cu2+ or Zn2+. Hence, the constant removal rate, which was 94% for Cu2+ and 90% for Zn2+, was found after 120 min.

|
Fig.2 Effect of contact time on the adsorptive performance of zeolite |
qe of zeolite calculated in this study was 0.36 mg Cu2+mg/g zeolite and 0.376 mg Zn2+mg/g zeolite when 10×10-6 Cu2+ and Zn2+ were used as the feed. Compared with adsorbents listed in Table 1, the adsorptive capacity of the zeolite is very much lower, although the reported removal rate was comparable which is within 90%-94%. Similar to a study done by Wang et al.[22], the adsorption capacity of SiO2 encapsulated natural zeolite was 12.3 g/mg and 9.0 g/mg for Cu2+ and Zn2+. The removal rate of Cu2+ and Zn2+ were 98% when 10×10-6 Cu2+ and Zn2+ were used as the feed. This suggested that the performance of the zeolite used in this study can be further enhanced by reducing the particle size of zeolite, Fe coating[17], and surfactant modification[23].
Compared with the findings observed in Fig. 1, the adsorptive performance of zeolite was higher for Zn2+ (~90%) compared with Cu2+ (~80%). Without pH adjustment, the effect of hydration radii governs the adsorption process. Hydration radii show the size of ion after it interacts with water molecules. It equals to 4.3 Å and 4.19 Å for Zn2+ and Cu2+, respectively[20]. Thus, Cu2+ with smaller size compared with Zn2+ reached the fine pores in zeolite and higher adsorption rate is achieved.
According to Tuzcu and Atalay[24], the pH of 10×10-6 Zn2+ solution is 6.3 while the pH for 10×10-6 Cu2+ is 6. The pH values reduced from 5.7 to 5.5 when the concentration of Cu2+ increased from 50×10-6 to 100×10-6. Similarly, when the concentration of Zn2+ increased from 50×10-6 to 100×10-6, the pH decreased from 6 to 5.9. It is reasonable to deduce that as the concentration of Zn2+ and Cu2+ reduced, the pH of the solution will go towards neutral. During the adsorption process, pH increased as the concentration of Zn2+ in solution reduced. This explains similar Zn2+ removal rate observed at t=120 min and at pH 7, which is approximately 90%. Meanwhile, this also suggests that by adjusting the pH of Zn2+ solution to minimum pH7, the maximum Zn2+ removal rate 90% can be achieved within 60 min. Similar trend was also observed in Cu2+ solution, where zeolite adsorbed 82.15% of Cu2+ at pH≥7, and without pH adjustment, 75% of Cu2+ was removed at t=60 min. However, this removal rate did not treat the water to the allowable discharge limit in Malaysia, which is 1 mg/L. Thus, the need to increase the contact time from 60 to 120 min was required to achieve 94% of Cu2+ removal, as shown in Fig. 2. At this point, the allowable discharge limit was met, where the discharge concentration was 0.523 mg/L.
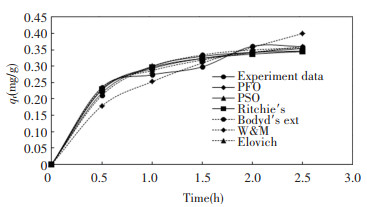
2.3 Kinetic StudyKinetic study for adsorption process is important as it provides valuable insights on the reaction pathways as well as the sorption reaction mechanism. It also describes the adsorbate uptake rate, which affects the residence time of adsorbate update at the solid-liquid interface. Fig. 3 shows that the amount of adsorbed Cu2+ on activated carbon predicted by the 6 kinetic models is very close to the experimental data, except for W & M and Boyd's external diffusion equation. W & M model assumes diffusion of adsorbate is the slowest step while Boyd's external diffusion equation assumes internal diffusion is the rate determining step. Hence, both internal and external diffusion contributed less impact to the overall Cu2+ adsorption process, compared with adsorption reaction on the active site in this study.

|
Fig.3 Predicted and experimental amount of Cu2+ adsorbed on adsorbent at different time interval |
Table 2 presents the constant values of the respective kinetic models. Empirical models such as PFO and PSO are lack of physical meaning[25]. Elovich is commonly used to model chemisorption while Ritchie's equation assumes adsorption is dominated by the reaction at active sites. It is notable that Ritchie's equation and Elovich model predicted the experimental data well with R2 value of 0.9977. Hence, this is difficult to justify which model fitted the data the best based on the R2 value alone. Other statistical parameters such as SSE, MSE, and χ2 are presented in Table 2 to verify the models, and it shows that Ritchie's equation is the best model with the lowest SSE, MSE and χ2 values. This showed that adsorption at active sites is the rate of determining the steps in this study[25].
| Table 2 Kinetic model constants and validity evaluation or Cu2+ adsorption |
Similarly, Zn2+ adsorption data was also predicted by using 6 kinetic models, which covers the external diffusion, internal diffusion, and adsorption on active sites. The results in Fig. 4 and Table 3 show that Elovich predicted Zn2+ adsorption the best with the highest R2 value of 0.9913 and the lowest SSE(0.000783), χ2(0.002473), and MSE (0.000131) values compared with the other models. This indicates that the role of active sites in zeolite played an important role in determining the heavy metals removal rate. This adsorption process can be enhanced by modifying the surface of active site by considering the exchange of metal ion with the functional groups on the surface of adsorbent.
|
Fig.4 Predicted and experimental amount of Zn2+ adsorbed on adsorbent at different time interval |
| Table 3 Kinetic model constants and validity evaluation for Zn2+ adsorption |
3 Conclusions
Results obtained from this study show that zeolite exhibited promising adsorptive performance for Cu2+ and Zn2+ in both neutral and alkali conditions. 82.15% Cu2+ and 90.87% Zn2+ removal rate was found at pH 7. Under the same pH condition, selectivity towards Zn2+ is higher compared with Cu2+, which may be due to the effect of hydration enthalpy. Without pH adjustment, selectivity towards Cu2+ is higher compared with Zn2+ due to smaller hydration radii of Cu2+, which is 0.11 Å smaller than Zn2+. Besides, rapid adsorption was found in the first 30 min of the experiment and saturation point was achieved at 120 min for both Zn2+ and Cu2+. Kinetic study showed that adsorption reaction at the active site on zeolite dominated the heavy metal removal process. Hence, the performance of zeolite could be enhanced by modifying the surface properties such as surfactant coating, where the exchange of metal ion with the functional groups on the surface of zeolite is considered.
| [1] |
Sverdrup H U, Olafsdottir A H, Ragnarsdottir K V. On the long term sustainability of copper, zinc and lead supply, suing a system dynamics model. Resources, Conservation and Recycling, 2019, 4: 100007. DOI:10.1016/j.rcrx.2019.100007 (  0) 0) |
| [2] |
Sires J. A Review of Potential Zinc and Copper Pollution Sources in the Kenai River Watershed. https://dec.alaska.gov/media/5181/zcliteraturereviewfinal.pdf, 2017-04-13.
(  0) 0) |
| [3] |
Sakson G, Brzezinska A, Zawilski M. Emission of heavy metals from an urban catchment into receiving water and possibility of its limitation on the example of Lodz city. Environmental Monitoring and Assessment, 2018, 190: 281. DOI:10.1007/s10661-018-6648-9 (  0) 0) |
| [4] |
Gabrielyan A V, Shahnazaryan G A, Minasyan S H. Distribution and identification of sources of heavy metals in the Voghji River basin impacted by mining activities (Armenia). Journal of Chemistry, 2018, 2018: 7172426. DOI:10.1155/2018/7172426 (  0) 0) |
| [5] |
Uddin M K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chemical Engineering Journal, 2017, 308: 438-462. DOI:10.1016/j.cej.2016.09.029 (  0) 0) |
| [6] |
Azeez O M, Adesanwo O O, Adepetu A J. Effect of Cu2+ (Cu) application on soil available nutrients and uptake. African Journal of Agricultural Research, 2015, 10(5): 359-364. DOI:10.5897/ajar2014.9010 (  0) 0) |
| [7] |
Geddie A W, Hall S G. An introduction to copper and zinc pollution in macroalgae: for use in remediation and nutritional applications. Journal of Applied Phycology, 2019, 31: 691-708. DOI:10.1007/s10811-018-1580-5 (  0) 0) |
| [8] |
Embas D U. P.U.(A) 434. Environmental Quality Act 1974-Environmental Quality (Industrial Effluent) Regulation 2009. Kuala Lumpur: Percetakan Nasioanal Malaysia Berhad, 2009.
(  0) 0) |
| [9] |
Crini G, Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters, 2019, 12: 145-155. DOI:10.1007/s10311-018-0785-9 (  0) 0) |
| [10] |
Zhu N L, Zhang B, Yu Q L. Genetic engineering-facilitated coassembly of synthetic bacterial cells and magnetic nanoparticles for efficient heavy metal removal. ACS Applied Materials and Interfaces, 2020, 12(20): 22399-23654. DOI:10.1021/acsami.0c04512 (  0) 0) |
| [11] |
Schütz T, Dolinská S, Hudec P, et al. Cadmium adsorption on manganese modified bentonite and bentonite-quartz sand blend. International Journal of Mineral Processing, 2016, 150: 32-38. DOI:10.1016/j.minpro.2016.03.003 (  0) 0) |
| [12] |
Renu N A, Madhu A, Singh K. Methodologies for removal of heavy metal ions from wastewater: an overview. Interdisciplinary Environmental Review, 2017, 18(2): 124-142. DOI:10.1504/IER.2017.087915 (  0) 0) |
| [13] |
Mustapha S, Shuaib D T, Ndamitso M M, et al. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb (Ⅱ), Cd (Ⅱ), Zn (Ⅱ) and Cu (Ⅱ) ions from aqueous solutions using Albizia lebbeck pods. Applied Water Science, 2019, 9: 142. DOI:10.1007/s13201-019-1021-x (  0) 0) |
| [14] |
Prabu D, Parthiban R, Kumar P, et al. Adsorption of copper ions onto nano-scale zero-valent iron impregnated cashew nut shell. Desalination and Water Treatment, 2016, 57(14): 6487-6502. DOI:10.1080/19443994.2015.1007488 (  0) 0) |
| [15] |
Neeraj G, Krishnan S, Senthil Kumar P, et al. Performance study on sequestration of copper ions from contaminated water using newly synthesized high effective chitosan coated magnetic nanoparticles. Journal of Molecular Liquids, 2016, 214: 335-346. DOI:10.1016/j.molliq.2015.11.051 (  0) 0) |
| [16] |
Biyikoglu M, Ciftci H. Adsorption of Ag(I) ions from wastewaters using poly(2-aminothiazole): kinetic and isotherm studies. Polymer Bulletin, 2020, 77: 6161-6174. DOI:10.1007/s00289-019-03073-7 (  0) 0) |
| [17] |
Kragovic M, Pasalic S, Markovic M, et al. Natural and modified zeolite - alginate composites. Application for removal of heavy metal cations from contaminated water solutions. Minerals, 2018, 8(1): 11. DOI:10.3390/min8010011 (  0) 0) |
| [18] |
Lee A Y W, Lim S F, Chua S N D, et al. Adsorption equilibrium for heavy metal divalent ions (Cu2+, Zn2+, and Cd2+) into zirconium-based ferromagnetic sorbents. Advances in Materials Science and Engineering, 2017, 2017: 1210673. DOI:10.1155/2017/1210673 (  0) 0) |
| [19] |
Marcus Y. Thermodynamics of solvation of ions. Journal of Chemical Society, Faraday Transactions, 1991, 87(18): 2995-2999. (  0) 0) |
| [20] |
Nightingale E R. Phenomenological theory of ion solvation. Effective radii of hydrated Ions. The Journal of Physical Chemistry, 1959, 63(9): 1381-1387. (  0) 0) |
| [21] |
Amarasinghe B M W P K, Williams R A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chemical Engineering Journal, 2007, 312: 299-309. DOI:10.1016/j.cej.2007.01.016 (  0) 0) |
| [22] |
Wang Z, Tan K Y, Cai J Y, et al. Silica oxide encapsulated natural zeolite for high efficiency removal of low concentration heavy metals in water. Colloids and Surface A: Physicochemical and Engineering Aspects, 2019, 561: 388-394. DOI:10.1016/j.colsurfa.2018.10.065 (  0) 0) |
| [23] |
Jimenez-Castaneda M E, Medina D I. Use of surfactant-modified zeolites and clays for the removal of heavy metals from water. Water, 2017, 9: 235. DOI:10.3390/w9040235 (  0) 0) |
| [24] |
Tuzcu E T, Atalay M U. Material characterization and Cu2+, zinc, and lead removing capacity of Cayirhan fly ash. Turkish Journal of Engineering and Environmental Sciences, 2011, 35(2): 93-105. DOI:10.3906/muh-1005-33 (  0) 0) |
| [25] |
Wang J L, Guo X. Adsorption kinetic models: physical meanings, applications and solving methods. Journal of Hazardous Materials, 2020, 390: 122156. DOI:10.1016/j.jhazmat.2020.122156 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


