2. School of Energy Science and Engineering, Harbin Institute of Technology, Harbin 150001, China;
3. Department of Energy and Power Engineering, Tsinghua University, Beijing 100084, China
With the development of the economy, heavy production and use of coal lead to a prominent environmental problem. Washed products are used according to different qualities, with coal slime being a poor-quality by-product of the coal washing process[1-2]. Due to the particularity of the process of coal washing, coal slime has the characteristics of high moisture content and water holding, high ash and low heat value, fine grain size, and paste shape[3-4].
When coal slime is utilized as pulverized coal, the main disadvantage will be extending ignition delay time, which will lead to ignition difficulty. Kurose et al.[5] studied the influence of coal moisture content on the combustion characteristics of pulverized coal by numerical simulation. The results showed that the increase in moisture will cause the decrease in flame temperature and NOx and that the peak value will move to downstream. The increase in moisture content will increase the proportion of unburned carbon and reduce the NOx conversion rate at the outlet of the furnace. Li et al.[6] used a homemade thermogravimetric device to test the effect of moisture content on the pulverized coal combustion characteristics. It was found that the use of pulverized coal containing water can lead to a decrease in the temperature of the combustor and cause an increase in the burnout time. Zhao et al.[7] conducted two types of coal pulverized coal combustion experiments in a 250 kW pilot-scale offset combustion simulator. The influence of equivalent moisture on the combustion characteristics of pulverized coal under reducing atmosphere was studied. The results showed that the reduction of moisture will reduce the convection distance, increase the stability of the flame, increase the burnout rate, and improve the ignition characteristics. The study also found that moisture inhibits ignition at an early stage, whereas it has a positive effect in the subsequent combustion. With the increase in water equivalent, the sub-bituminous coal presents a homogeneous-heterogeneous combination ignition model. Binner et al.[8] studied the difference in combustion characteristics of Victoria lignite coal between dry coal and wet pulverized coal using a drop tube furnace and found that the vaporization and evolution of water during the combustion of wet coal led to the delay of ignition, whereas the coal after preheat drying had no ignition delay.
Utilization of coal slime as it is could possibly be a benefit of CFB boilers, which means less work on controlling of water content and fuel size. Moisture plays a decisive role in a series of coal slime properties, e.g., thermal explosion and agglomeration will occur during the combustion of coal slime in circulating fluidized bed (CFB) boilers[9-10]. These two combustion characteristics will play a key role in the wet use of coal slime in the CFB boiler. Yin et al.[11] used numerical simulation to study the drying and movement processes of large-diameter slime during the falling process. Wang et al.[4] studied the combustion characteristics of different types of coal slime with different diameters under different furnace temperatures. It was found that the diameter and furnace temperature significantly affect the ignition characteristics of coal slime, and the ignition time and burnout time of large diameter coal slime are significantly longer. A lower furnace temperature will also lead to this result, as different types of coal slime have different volatiles and ash contents, resulting in different combustion characteristics. Furthermore, significant changes in the macrostructure of coal slime have been discovered during the combustion process[3]. Thus, there will be significant emissions for which the main components is amorphous carbon, as analyzed by XRD, in the homogeneous combustion stage, indicating that the structure of the coal slime has undergone changes from a simple structure to a complex structure and then back to a simple structure in the process.
Moisture also showed some effects in controlling NOx and SOx emission. Binner et al.[8] also found that the low temperature during the combustion of wet coal and the presence of water was found to promote the reduction of NO to N2 and N2O[12]. Glarborg et al.[13] found that the presence of water vapor has an important effect on the oxidation of CO, which in turn influences the reduction of NO. It has been found that the reduction of NO is mainly related to H and CO[14], and the concentration of H and OH radicals in the atmosphere has an important effect on the reduction of NOx[15]. Wei et al.[16] studied the combustion and emission characteristics of high-moisture coal in a bench-scale fluidized bed and found that bed temperature and the emission SOx decreased with increasing water content. Stewart et al.[17] found that the addition of water vapor can significantly reduce SOx emissions.
Previous research studies have been performed on the combustion characteristics and utilization of coal slime[3, 4, 11]; however, few studies have considered the effect of moisture on the combustion characteristics of coal slime. Most scholars have studied coal, especially young lignite, with high moisture content[18-20]. In this paper, how the moisture in big size coal slime spheres influences its combustion characteristics under CFB conditions has been investigated, and its controlling mechanism of NOx and SOx has been explored.
1 Experiment 1.1 Experimental SystemThis experiment was conducted using the bench-scale fluidized bed experiment system shown in Fig. 1. The experimental system is mainly composed of a reaction furnace, a preheating furnace, a gas analysis system, a temperature acquisition system, a gas supply system. The reactor quartz tube has an inner diameter of 50 mm and a length of 1200 mm. The inner diameter of the quartz tube in the reactor is 50 mm, and the length is 1200 mm. Coal slime with bigger size will be more favorable for this study, but due to limitation of the reactor size and considering collection of samples, 10 mm diameter sphere was chosen. The bed material is quartz sand with a particle size range of 150 μ m to 280 μ m, and the static bed height is 30 mm. The reactor temperature was set to 850 ℃, the fluidizing air velocity was 15 L/min and heated through the preheating furnace under 400 ℃. It is to be noted that the reactor temperature will be inevitably influenced by heat release from the combustible coal slime samples due to the big size, so it can only be taken as a nominal temperature. The Finland GASMET Dx4000 portable FTIR gas analyzer was used in this experiment. The fuel used in the experiment is the flotation tailings of the Shanxi Linxian County coal preparation plant. The ultimate and proximate analyses of coal slime are shown in Table 1.
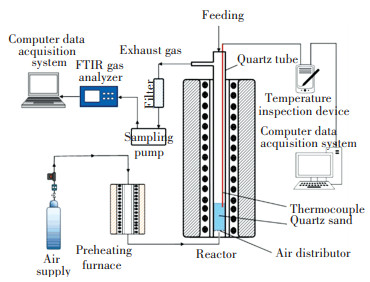
|
Fig.1 Schematic diagram of the experimental system |
| Table 1 Ultimate and proximate analyses of coal slime |
1.2 Experimental Methods and Procedures
Coal slime was made into a spherical shape with a diameter of 10 mm through the mold before being fed into the furnace, and then the moisture content was dried to 24.80%, 16.80%, 9.06%, and 0.92% in a drying oven at 105 ℃. Each sample of prepared slime with a different moisture content was fed from the top of the furnace; the amount of feed per time was 5 g. The prepared coal slime was quickly fed from the furnace top feeding port after the temperature of the reactor and preheating furnace were heated to the set temperature and the fluidizing velocity was set. The bed temperature changes were recorded by a K-type thermocouple. The generated flue gas was filtered twice by filter and then sent to the FTIR flue gas analyzer through a sampling pump; the flue gas composition was recorded by computer CALCMET software.
2 Results and Discussion 2.1 Effect of Moisture on the Bed Temperature of the Fluidized BedThe effect of moisture on the bed temperature was studied on a bench-scale fluidized bed reactor; the variation in the bed temperature is shown in Fig. 2. The bed temperature goes through an initial descent and then slowly descends to the furnace temperature when the coal slime is placed into the furnace. After the coal slime was placed into the furnace and reached the bed, the heat absorption of the material itself and the large amount of evaporation and heat absorption of the moisture occurred first. See reaction formulas from (r1) to (r6), where s, l, g represent soild, liguid and gas state, respectively. Moreover, the water gas reaction (r2) probably occurred due to the presence of moisture, with the reaction being an endothermic reaction, resulting in a decrease in the temperature of the bed. After the water evaporated to a certain extent, the coal slime began to burn and release heat. CO, H2, and other small molecules of combustible gas will burn and release heat, causing the rate of heat release to gradually exceed the rate of heat absorption, which in turn will cause the temperature of the furnace to rise again and start a recovery section. When the maximum temperature was reached, the furnace temperature began to decline with the burnout of the slime.
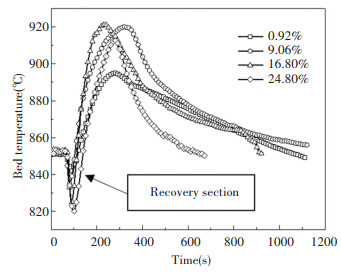
|
Fig.2 Changes in bed temperature with 15:45:46 |
After the different moisture coal slimes were fed into the furnace, the overall variation in the bed temperature was similar. The bed temperature changed more drastically and the slope of the temperature curve changed more steeply at lower moisture. The most obvious characteristic of the temperature curve is the effect of moisture on the minimum bed temperature and the maximum bed temperature, as shown in Fig. 3; the minimum bed temperature will decrease as the moisture increases[16] because under the same amount of feed the moisture content is high, and the combustible component is reduced. More heat is required to evaporate the water, and the curve shows a nearly linear downward trend as the water changes. The existence of an optimal moisture content or range makes the exothermic heat of the coal slime combustion process more efficient, and thus the maximum temperature reached by the combustion and the high or low moisture content will affect the combustion process.
| $ {{\rm{H}}_2}{\rm{O}}\left( {\rm{l}} \right) \to {{\rm{H}}_2}{\rm{O}}\left( {\rm{g}} \right) $ | (r1) |
| $ {\rm{C}}\left( {\rm{s}} \right) + {{\rm{H}}_2}{\rm{O}}\left( {\rm{g}} \right) \to {\rm{CO}}\left( {\rm{g}} \right) + {{\rm{H}}_2}\left( {\rm{g}} \right) $ | (r2) |
| $ {\rm{C}}\left( {\rm{s}} \right) + {{\rm{O}}_2}\left( {\rm{g}} \right) \to {\rm{CO}}\left( {\rm{g}} \right) $ | (r3) |
| $ {\rm{C}}\left( {\rm{s}} \right) + {{\rm{O}}_2}\left( {\rm{g}} \right) \to {\rm{C}}{{\rm{O}}_{\rm{2}}}\left( {\rm{g}} \right) $ | (r4) |
| $ 2{{\rm{H}}_2}\left( {\rm{g}} \right) + {{\rm{O}}_2}\left( {\rm{g}} \right) \to 2{{\rm{H}}_2}{\rm{O}}\left( {\rm{g}} \right) $ | (r5) |
| $ 2{\rm{CO}}\left( {\rm{g}} \right) + {{\rm{O}}_2}\left( {\rm{g}} \right) \to 2{\rm{C}}{{\rm{O}}_2}\left( {\rm{g}} \right) $ | (r6) |

|
Fig.3 Changes in the minimum and highest bed temperatures |
2.2 Effect of Moisture on the Combustion Efficiency
The conversion rate of carbon in slime was used to define the combustion efficiency, as shown in Eq.(1), where Ec, inst is the instantaneous combustion efficiency[21-23], the numerator is the instantaneous concentration of CO2, and the denominator is the concentration of the carbon containing components. However, because tar and carbon black are liquids and solids respectively, they are not easy to measure, and the amounts produced are also small. Therefore, Eq.(1) can be simplified as Eq.(2), and the instantaneous combustion efficiency of coal slime can be approximated by Eq.(2); the calculated instantaneous combustion efficiency will also be large.
| $ \begin{array}{l} {E_{{\rm{c,inst}}}}\left( t \right) = \\ \frac{{y{\rm{C}}{{\rm{O}}_2}\left( t \right)}}{{y{\rm{C}}{{\rm{O}}_2}\left( t \right) + y{\rm{CO}}\left( t \right) + y{\rm{C}}{{\rm{H}}_4}\left( t \right) + y\left( {{\rm{Carbon}}\;{\rm{black}} + {\rm{Tar}}} \right)\left( t \right)}} \end{array} $ | (1) |
| $ {E_{{\rm{c,inst}}}}\left( t \right) = \frac{{y{\rm{C}}{{\rm{O}}_2}\left( t \right)}}{{y{\rm{C}}{{\rm{O}}_2}\left( t \right) + y{\rm{C}}{{\rm{O}}_2}\left( t \right) + y{\rm{C}}{{\rm{H}}_4}\left( t \right)}} $ | (2) |
Eq.(2) is integrated to obtain Eq.(3), and Eq.(3) is used to calculate the coal slime combustion efficiency.
| $ {E_{\rm{c}}}{\rm{ = }}\int_0^t {{E_{{\rm{c,inst}}}}\left( t \right){\rm{d}}t} = \frac{{\int_0^t {y{\rm{C}}{{\rm{O}}_2}\left( t \right){\rm{d}}t} }}{{\int_0^t {\left[ {y{\rm{C}}{{\rm{O}}_2}\left( t \right) + y{\rm{CO}}\left( t \right) + y{\rm{C}}{{\rm{H}}_4}\left( t \right)} \right]} {\rm{d}}t}} $ | (3) |
Fig. 4 shows the change in CO concentration with time in the combustion process of different moisture coal slimes; from the diagram, it can be seen that the slime will produce CO in a short time after entering the furnace. The coal slime with different moisture contents has experienced an instant increase in CO concentration after being fed into the furnace that reaches the peak and then decreases. It can be seen from the diagram that the presence of water has a direct effect on the formation of CO in slime. The effect of water on the formation of CO is reflected in the following aspects: 1) According to Eq.(2), the presence of water will lead to the reaction of water gas, which leads to the production of CO; 2) High temperature leads to moisture vaporization, which causes instantaneous increase in internal pressure, resulting in thermal explosion of coal slime, which in turn promotes evaporation and vaporization of moisture in coal slime. A large amount of water vapor generated in a short period of time isolates the oxygen around the coal slime, resulting in the occurrence of coal slime complete combustion reaction (r3); 3) The presence of water causes the furnace temperature to decrease rapidly, causing the degree of incomplete combustion of the coal slime in the furnace to increase.

|
Fig.4 Variation in CO concentration with time in the process of coal combustion with different moisture contents |
There are obvious differences in the production of CO when coal slime moisture varies. In Fig. 5 an increasing tendency can be seen according to moisture contents. It can be seen from the figure that the CO concentration curve produced by high-moisture slime is always above the CO concentration curve produced by low-moist coal slime, proving that the higher moisture content of the coal slime, the higher concentration and production of CO produced. From the aspect of chemical reaction, the increase in water content will result in a higher concentration of water vapor in the combustion process of the coal slime; for reaction (r2)[8, 24], the increase in the reactant concentration can increase the chemical reaction rate, thus causing an increase in CO production. The increase in water content leads to an increase in the concentration of surrounding water vapor during combustion of the coal slurry, causing further sequestration of air around the slime[25] that promotes the conduct of reaction (r3), and then increases the concentration of CO. In addition, the increase in water vapor causes the decrease in the temperature of the furnace; this reduced temperature is not conducive to the combustion of coal slime, resulting in an increase in CO production. The instantaneous concentration of CO and CO2 can be integral to time, as shown in Fig. 5. Fig. 5 reveals that with the increase in water content, the total amount of CO2 produced shows a downward trend, and the total amount of CO shows an upward trend; increased water content leads to incomplete combustion of coal slime, thereby increasing the total amount of CO. During the experiment, the presence of CH4 was almost undetectable; thus, the amount of CH4 could be ignored when using the combustion efficiency of the coal slime calculated by Eq.(3). The combustion efficiency of slime calculated by Eq.(3) is shown in Fig. 6. It can be seen from the diagram that the combustion efficiency of different samples is all above 90%. This value is actually too large because in the calculation process some simplifications were made for the convenience of calculation, i.e., ignoring the unburned components in carbon black, tar, and residues. It can be clearly seen from the figure that the combustion efficiency decreases almost linearly with the increase in moisture within the experimentally tested moisture range[26]. It is obvious that the effect of water on combustion efficiency is very obvious. It is necessary to comprehensively consider the problem of decreased combustion efficiency caused by moisture in the process of utilization in a CFB boiler.

|
Fig.5 Total amount of CO2 and CO with the change in water |
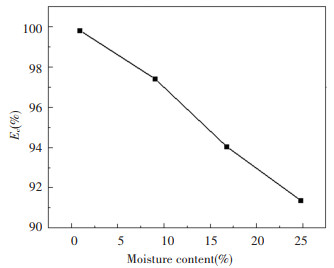
|
Fig.6 Combustion efficiency with the change in moisture |
2.3 Effect of Water on NO x and SO2 Emission
The nitrogen oxides produced during the combustion of the coal slime are mainly NO, with a small amount of N2O generated, in agreement with the fact in Ref.[27] that H2O can reduce NO to N2O. The reason why NO2 was not detected may be that a large amount of moisture evaporates during the combustion process, resulting in the isolation of the water around the coal slime particles and preventing the oxidation of NO. In addition, a certain amount of NO2 will react with water vapor to generate HNO3 and NO. Fig. 7 shows the NOx production over time. The figure shows that the coal slime produced a large amount of NOx in the feed furnace in a short period of time. Through the integration of NOx roduction, the total amount of NOx generated during the combustion process of coal slime with different moisture contents is shown in Fig. 8. The diagram shows that, with the increase in moisture, the NOx produced during combustion will decrease because the increase in moisture content of the same quality coal slime leads to the reduction of combustible components and fuel nitrogen in coal slime, thereby reducing the production of NOx in the process of combustion. Moreover, a large amount of water vapor is generated after the coal slime is placed into the furnace, generating H and OH radicals under high temperature conditions. The generated H radicals will reduce NO through CO; the specific restoration path is shown in Table 2[28]. The high-moisture coal slime mentioned above will produce high concentrations of CO, produce H2, etc., all of which will undoubtedly improve the ability of NO to be reduced. In addition, NO will react with oxygen and water to generate HNO3. The increase in water content in the slime increases the concentration of water vapor, thus increasing the rate of chemical reaction and causing more NO to convert into HNO3 through the reaction. Simultaneously, water vapor will hinder the propagation of flame and reduce the temperature of the reaction zone, thereby reducing the generation of NOx[29].

|
Fig.7 NO x changes over time |

|
Fig.8 Total NO x produced by coal slime with different moisture contents |
| Table 2 The path of the NO to be restored[28] |
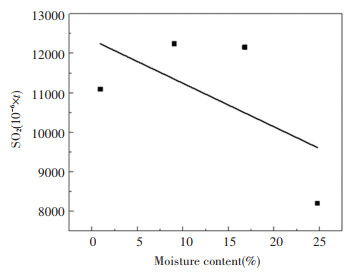
The concentration of SO2 produced over time is shown in Fig. 9; there are two obvious peaks in the distribution of SO2: a lower peak between 10-15 s after being put into the furnace, and a larger peak afterwards. The NOx change in Fig. 7 also shows such a rule; however, the SO2 changes are more delayed than the NOx changes: NOx almost reached the first peak in a few seconds after being put into the furnace, and it almost disappeared after 20 s; SO2 will continue to be produced to 75 s. The cause of these two peaks may be due to the existence of water, leading to the existence of two combustion stages after the coal slime is placed into the furnace. The first stage is the massive evaporation of water and the incomplete combustion of fuel, and the second stage is intense combustion after evaporation of water. The total amount of SO2 generated in Fig. 10 is obtained by integrating SO2 in Fig. 9. Overall, with the increase in moisture content, the total amount of SO2 production tends to decrease[16-17]. On the one hand, the increase in moisture content in the coal slime leads to a decrease in the flammable components, which in turn leads to a decrease in the sulfur in the flammable components. On the other hand, the presence of water will probably cause water and sulfur dioxide to react separately in accordance with reaction (r7) and (r8) to produce sulfurous acid and sulfuric acid. Therefore, the higher the moisture content, possibly the greater the amount of SO2 converted to sulfite and sulfuric acid. At the same time, the increase in water content leads to the increase in water vapor concentration, which can increase the rate of chemical reaction and facilitate the chemical reaction.
| $ {\rm{S}}{{\rm{O}}_2} + {{\rm{H}}_2}{\rm{O}} \to {{\rm{H}}_2}{\rm{S}}{{\rm{O}}_3} $ | (r7) |
| $ 2{\rm{S}}{{\rm{O}}_2} + {{\rm{O}}_2} + 2{{\rm{H}}_2}{\rm{O}} \to 2{{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4} $ | (r8) |
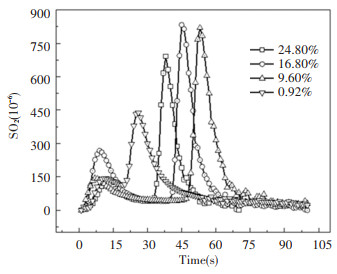
|
Fig.9 SO2 changes over time |
|
Fig.10 Total amount of SO2 produced by coal slimes with different moisture contents |
It can be seen that water can reduce NOx and promote NOx and SO2 conversion to corresponding acids, thereby reducing pollutant emissions. However, when pollutants are reduced, acidic substances, such as nitric acid and sulfuric acid, are generated. Once these acids are condensed, they cause corrosion in boilers, pipes, and other parts. For some high-sulfur and high-nitrogen coal slime, the impact of moisture content on the boiler must be comprehensively considered when coal slime is used in the combustion process of a CFB boiler.
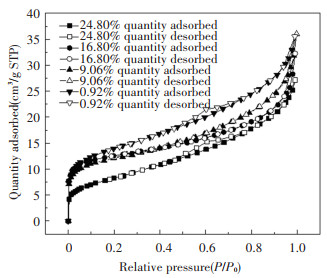
2.4 Changes of Pore Structure and Micromorphology of Coal Slime with Different Moisture ContentThe change of pore structure during coal slime combustion will affect the combustion process, especially the coal slime with high moisture content. In order to facilitate the study of the change of pore structure of coal slime with different moisture content, the 10 mm-diameter spherical particles of coal slime with the same four moisture content were placed in a muffle furnace in air atmosphere at 850 ℃ for 2 min and then taken out. The JW-BK132F high-performance specific surface area and pore size analyzer was used for the N2 adsorption analysis of the burned coal slime particles. The heating degassing treatment was carried out before the test, and then the liquid nitrogen adsorption was carried out at 150 ℃. The isothermal adsorption and desorption curve of different moisture content of coal slime is shown in Fig. 11. It can be seen from the characteristics of the curve trend that the type of adsorption curve is non-porous, and the curve of this type is generally dominated by non-pores and macropores. In the low pressure area (0 < P/P0 < 0.1), it can be seen that the slope of the curve increases obviously and rises almost vertically, indicating that there is a certain amount of micropores in the sample, which has a stronger effect on nitrogen adsorption. In the medium pressure area (0.2 < P/P0 < 0.8), the slope of the curve decreases, the surface gradually forms a multi-molecular layer adsorption, and the number of adsorption layers increases. The smaller hysteresis loop appears when the moisture content is 24.80% and 0.92%. That is the phenomenon of capillary condensation, which shows that there are a small number of mesoporous existence under this condition. In the high pressure area (P/P0>0.8), the slope of the curve increases and shows an upward trend, indicating that the accumulation of particles is uneven. The isothermal adsorption and desorption curve of coal slime decreases with the increase of moisture content. The isothermal desorption curve of high moisture content coal slime is located below the low moisture content coal slime, indicating that the moisture has a retardation effect on the formation of pore structure of coal slime. At the same time, the coal slime with higher moisture content takes more time to evaporate and lose water, so the time to form pores in the later combustion process is reduced, and the results also show that the pores formed by the vigorous evaporation of water are not mainly under such conditions, the pores formed by the late volatilization and coke combustion are the main ones. The specific surface area and specific pore volume decrease with the increase of moisture content, as show in Fig. 12, indicating that low moisture is more likely to form larger specific surface area and more pores under the same burning time. Since the water in the coal slime is mostly external moisture, the water vapor formed during the evaporation of water mostly passes through the gap between the particles, and it is difficult to form pores from the inside of the particle. However, in the later combustion process, the precipitation of volatiles is from the inside of the particles, and it is easy to form pores, and the combustion of combustible components in the heterogeneous combustion process also leads to the formation of pores. Therefore, water is not the main factor for forming pores under such conditions.
|
Fig.11 Isothermal adsorption and desorption curves of different moisture content of coal slime |

|
Fig.12 BET Specific surface area and specific pore volume change with moisture content |
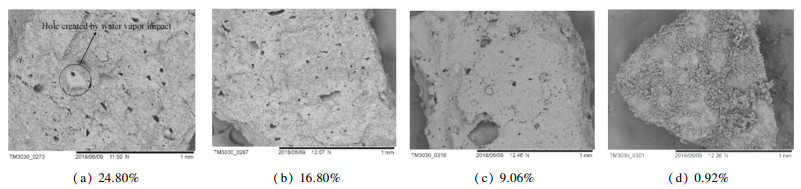
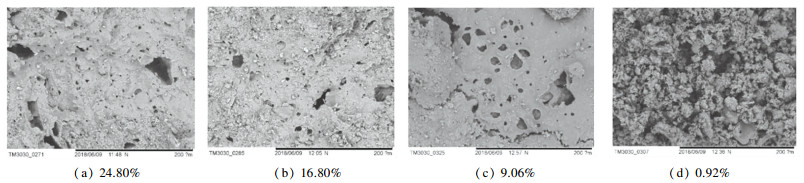
The scanning electron microscope (SEM) images of the pore structure of the coal with different moisture contents after two minutes of combustion at 100, 500, and 2000 times are shown in Figs. 13, 14, and 15. Obviously larger holes can be observed under different moisture conditions in 100 times SEM image, and some even exceed 1 mm. When the moisture content is 24.80%, it can be observed that there is a significant silt accumulation formed by the impact of water vapor around the hole. This is because under such conditions, the moisture content is high, and the water vapor formed by the high temperature impacts to form larger hole, and the internal coal slime is carried out by the water vapor and accumulated in the orifice. There are different degrees of large holes under other moisture content conditions. As the moisture content decreases, the large holes decrease significantly. This is because the water vapor is reduced due to the decrease of moisture content, and the holes formed by water vapor impact are reduced. More hundred-nanometer-level pores can still be observed in the 500-time SEM images, and it is obvious that the pores formed by water are mostly large holes. These holes can be called water vapor channels. Fig. 15 shows the nano-scale pores on the surface of the particles observed under 2000 times. It is difficult to observe the change in the number of pores with moisture content changer. However, with the analysis of the nitrogen adsorption data above, it is found that the small pores gradually increase with the decrease of water, and the smaller nano-scale pores formed are mostly caused by the combustion process of volatilization and the formation of coke after the evaporation of water. In a comprehensive analysis, the effect of water on the combustion process of slime is mostly the impact of the severe vaporization process in the early stage to form a large water vapor channel, which is beneficial to the evaporation and loss of water, conducive to the reaction of water and coal combustion products, such as the formation of CO, reduction of NO x, SO2, etc. The large holes formed by the impact of water vapor decrease with the decrease of moisture content, and the nanoscale pores are mostly caused by the combustion process of volatilization and coke formation after the end of vaporization, and increase with the decrease of moisture content.
|
Fig.13 SEM images at 100 times of the pore structure after burning for 2 min with different moisture contents |
|
Fig.14 SEM images at 500 times of the pore structure after burning for 2 min with different moisture contents |

|
Fig.15 SEM images at 2000 times of the pore structure after burning for 2 min with different moisture contents |
3 Conclusions
The moisture in coal slime determines the key properties of the coal slime during the process of combustion, such as thermal explosion, and agglomeration, as well as important effects on the combustion efficiency, pollutant emissions, and other characteristics that are critical to the utilization of coal slime in CFB boiler.
1) In the process of fluidized bed combustion, the higher the moisture content of the slime, the greater the degree of decrease in bed temperature. The presence of moisture can cause thermal explosion of the slime, and the maximum bed temperature achieved is significantly different; the intermediate moisture makes the maximum bed temperature larger, indicating that the thermal explosion degree of that case is more intense.
2) Coal slime will undergo chemical reactions, such as water gas reactions during the combustion process; in addition, water vapor will make the combustion of coal slime isolated from air and reduce the bed temperature of the fluidized bed. All of these phenomena will lead to incomplete reaction products, such as CO during the combustion. The higher the moisture content, the more CO will be generated, leading to a linear decrease in the combustion efficiency.
3) CO and H2 produced by reaction and H radicals generated by water decomposition will cause NOx to be reduced, and the reduction in temperature of the reaction zone caused by water vapor will lead to a decrease in NOx emissions. In addition, water vapor and NOx and SO2 generated during the combustion process are probably converted into corresponding acids through a series of reactions. As the water content increases, the amount of NOx and SO2 involved in the reaction conversion increases, leading to a downward trend.
4) The effect of water on the combustion process of slime is mostly the impact of the severe vaporization process in the early stage to form a large water vapor channel, which is beneficial to the evaporation and loss of water, conducive to the reaction of water and coal combustion products, such as the formation of CO, reduction of NOx, SO2, etc. The large holes formed by the impact of water vapor decrease with the decrease of moisture content, and the nanoscale pores are mostly caused by the combustion process of volatilization and coke formation after the end of vaporization, and increase with the decrease of moisture content.
| [1] |
Gui X H, Liu J T, Cao Y J, et al. Coal preparation technology: status and development in China. Energy and Environment, 2015, 26(6-7): 997-1013. DOI:10.1260/0958-305X.26.6-7.997 (  0) 0) |
| [2] |
Zheng J H, Xu C Y, Hu P F. Study on flotation process of coal slime. Advanced Materials Research, 2013, 734-737: 1093-1096. DOI:10.4028/www.scientific.net/AMR.734-737.1093 (  0) 0) |
| [3] |
Wang H, Liu S L, Li X T, et al. Morphological and structural evolution of bituminous coal slime particles during the process of combustion. Fuel, 2018, 218: 49-58. DOI:10.1016/j.fuel.2018.01.022 (  0) 0) |
| [4] |
Wang H, Liu S, Wang X Y, et al. Ignition and combustion behaviors of coal slime in air. Energy and Fuels, 2017, 31(10): 11439-11447. DOI:10.1021/acs.energyfuels.7b01960 (  0) 0) |
| [5] |
Kurose R, Tsuji H, Makino H. Effects of moisture in coal on pulverized coal combustion characteristics. Fuel, 2001, 80(10): 1457-1465. DOI:10.1016/S0016-2361(01)00019-9 (  0) 0) |
| [6] |
Li C, Feng T, Wang C B. Study on the influence of moisture in coal on combustion characteristics of pulverized coal. Electric Power Science & Engineering, 2013, 29(10): 70-73. DOI:10.3969/j.issn.1672-0792.2013.10.013 (  0) 0) |
| [7] |
Zhao Y J, Zeng G, Zhang L Y, et al. Effects of fuel properties on ignition characteristics of parallel-bias pulverized-coal jets. Energy and Fuels, 2017, 31(11): 12804-12814. DOI:10.1021/acs.energyfuels.7b02055 (  0) 0) |
| [8] |
Binner E, Zhang L, Li C Z, et al. In-situ observation of the combustion of air-dried and wet Victorian brown coal. Proceedings of the Combustion Institute, 2011, 33(2): 1739-1746. DOI:10.1016/j.proci.2010.07.076 (  0) 0) |
| [9] |
Cen K F, Huang G Q, Luo Z Y, et al. Improving fluidized bed combustion effieieney of coal by agglomeration phenom enon of CWM. Journal of Zhejiang University, 1986, 20(6): 132-141. (  0) 0) |
| [10] |
Ni M J, Luo W H, Huang G Q, et al. Agglomeration of CWM in fluidized bed combustor. Journal of Zhejiang University, 1986, 6(20): 38-45. (  0) 0) |
| [11] |
Yin W D, Li B, Wu Y X, et al. Model of coal slime combustion behavior in CFB boiler. Meitan Xuebao/Journal of the China Coal Society, 2015, 40(7): 1628-1633. DOI:10.13225/j.cnki.jccs.2014.1092 (  0) 0) |
| [12] |
Hayashi J-i, Hirama T, Okawa R, et al. Kinetic relationship between NO/N2O reduction and O2 consumption during flue-gas recycling coal combustion in a bubbling fluidized-bed. Fuel, 2002, 81(9): 1179-1188. DOI:10.1016/S0016-2361(02)00016-9 (  0) 0) |
| [13] |
Glarborg P, Kubel D, Kristensen P G, et al. Interactions of CO, NOx and H2O under post-flame conditions. Combustion Science and Technology, 1995, 110-111(1): 461-485. DOI:10.1080/00102209508951936 (  0) 0) |
| [14] |
Li S, Wei X L, Guo X F. Effect of H2O vapor on NO reduction by CO: experimental and kinetic modeling study. Energy & Fuels, 2012, 26(7): 4277-4283. DOI:10.1021/ef300580y (  0) 0) |
| [15] |
Li S, Wei X L. Behavior of alkali metal hydroxides/chlorides for NO reduction in a biomass reburning process. Energy & Fuels, 2011, 25(8): 3465-3475. DOI:10.1021/ef200661x (  0) 0) |
| [16] |
Wei X L, Sheng H Z, Sun W C, et al. The combustion and emission characteristics of high moisture coal in FBC. Ranshao Kexue Yu Jishu/Journal of Combustion Science and Technology, 1997, 3(3): 264-269. (  0) 0) |
| [17] |
Stewart M C, Symonds R T, Manovic V, et al. Effects of steam on the sulfation of limestone and NOx formation in an air- and oxy-fired pilot-scale circulating fluidized bed combustor. Fuel, 2012, 92(1): 107-115. DOI:10.1016/j.fuel.2011.06.054 (  0) 0) |
| [18] |
Prationo W, Zhang J, Cui J F, et al. Clarifying the influence of moisture on the ignition and combustion of wet Victorian brown coal in air-firing and oxy-fuel modes: Part 2: Contribution of gasification reaction to char oxidation rate. Fuel Processing Technology, 2015, 138: 680-686. DOI:10.1016/j.fuproc.2015.07.009 (  0) 0) |
| [19] |
Arima K, Tsuchiyama Y, Sawatsubashi T, et al. Drying of wet brown coal particles by a steam-fluidized bed dryer. Drying Technology: An International Journal, 2018, 36(6): 1-9. DOI:10.1080/07373937.2017.1323337 (  0) 0) |
| [20] |
Yu Y J, Jiang H L, Mi Y T, et al. Effect of hydrothermal dewatering on the moisture content of brown coal. Energy Sources, Part A: Recovery, Utilization and Environmental Effects, 2018, 40(3): 358-363. DOI:10.1080/15567036.2017.1419516 (  0) 0) |
| [21] |
Kumar A, Eskridge K, Jones D D, et al. Steam-air fluidized bed gasification of distillers grains: effects of steam to biomass ratio, equivalence ratio and gasification temperature. Bioresource Technology, 2009, 100(6): 2062-2068. DOI:10.1016/j.biortech.2008.10.011 (  0) 0) |
| [22] |
Lee D H, Yan R, Shao J G, et al. Combustion characteristics of sewage sludge in a bench-scale fluidized bed reactor. Energy and Fuels, 2008, 22(1): 2-8. DOI:10.1021/ef700266q (  0) 0) |
| [23] |
Wang P N, Leion H, Yang H R. Oxygen-carrier-aided combustion in a bench-scale fluidized bed. Energy and Fuels, 2017, 31(6): 6463-6471. DOI:10.1021/acs.energyfuels.7b00197 (  0) 0) |
| [24] |
Zhang Y G, Li Q H, Meng A H, et al. Carbon monoxide formation and emissions during waste incineration in a grate-circulating fluidized bed incinerator. Waste Management and Research, 2011, 29(3): 294-308. DOI:10.1177/0734242X10368581 (  0) 0) |
| [25] |
Dobrosz-Gomez I, Kocemba I, Rynkowski J M. Carbon monoxide oxidation over Au/Ce1-xZrxO2 catalysts: effects of moisture content in the reactant gas and catalyst pretreatment. Catalysis Letters, 2009, 128(3-4): 297-306. DOI:10.1007/s10562-008-9749-1 (  0) 0) |
| [26] |
Dzurenda L, Banski A. Influence of moisture content of combusted wood on the thermal efficiency of a boiler. Archives of Thermodynamics, 2017, 38(1): 63-74. DOI:10.1515/aoter-2017-0004 (  0) 0) |
| [27] |
Wu J Z, Wang B F, Cheng F Q. Thermal and kinetic characteristics of combustion of coal sludge. Journal of Thermal Analysis and Calorimetry, 2017, 129(3): 1899-1909. DOI:10.1007/s10973-017-6341-1 (  0) 0) |
| [28] |
Zhu C Q, Liu S Y, Liu H, et al. NOx emission characteristics of fluidized bed combustion in atmospheres rich in oxygen and water vapor for high-nitrogen fuel. Fuel, 2015, 139: 346-355. DOI:10.1016/j.fuel.2014.08.058 (  0) 0) |
| [29] |
Yue P J, Zhang Z X, Zhang J, et al. NOx reduction by urea solution in fuel-rich pulverized coal combustion. Energy Sources, Part A: Recovery, Utilization and Environmental Effects, 2017, 39(22): 2090-2097. DOI:10.1080/15567036.2017.1403507 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


