2. School of Environment, Harbin Institute of Technology, Harbin 150090, China
Bioflocculant is a large biomacromolecule[1] produced during microbial growth, mainly including saccharides, protein, cellulose and DNA. It can sediment the suspended particles, somatic cell and colloid particles efficiently in wastewater[2]. Although nowadays synthetic organic and inorganic flocculants are widely applied in various industrial fields for their efficient flocculation and cheapness, the usage of them has started to be limited with the higher environmental demands due to some secondary pollution and healthy issues caused by them. Therefore, environment-firendly flocculants have received increasing concerns in the past decades. Bioflocculants, with safe, nontoxic, biodegradability, no secondary pollution and other positive characteristics, have been researched and reported continually in recent years[3]. Currently, more than 60 species of bioflocculant producing bacteria have been found[4], including Vagococcus sp.[5], Halomonas sp.[6], Pseudomonas sp.[7-8], Kocuria rosea[9], Bacillus megaterium[10], Bacillus salmalaya[11], Bacillus licheniformis[12]. With further researches, the conception of compound bioflocculant (BFC) was firstly put forward by Ma et al.[13], who found that the efficiency of flocculation could be promoted after mixed culture of bioflocculant producing bacteria. This phenomenon can be boiled down to the synergistic symbiosis of different bacterial strains, which allows their extracellular polymeric substances (EPS) to be more suitable for the complex and ever-changing ecological environment. However, the application of bioflocculant in practical engineering cases is rarely reported because of its low yield, unstable flocculation capacity and high cost of production[14]. Therefore, in order to break down these barriers to pursue the best flocculation properties of various bioflocculant producing bacteria, carrying out massive studies to explore their chemical consistence, mechanization of flocculation and the best culture and application conditions is quite essential[15-17].
In terms of the production of bioflocculant, it is believed that the development of conception of compound bioflocculant is relative complex, affected by substantial internal and external factors[18] For instance, the significance of nitrogen source and carbon source in a culture medium has been highlighted by massive researches and the function of them typically defers for various bacterial strains[19-20]. Moreover, a culture medium's pH may also play a key role as it would affect the electric charge of the cells and redox potential, thus influencing the nutrient absorption and the rates of enzymatic reactions[19]. Massive studies indicated that the neutral pH is always advantageous to produce high active bioflocculant[21-23]. Nevertheless, former researchers paid more attention to single factor optimization, requiring prodigious experiments but neglecting the interactive effects among these factors[20, 24, 25]. In order to settle this issue, the mathematics statistic method of response surface methodology (RSM), grounding on the combination of a polynomial equation to the data from experiments, attracted increasing attention recently. It can not only reflect the relation between various dependent variants but also evaluate the levels of them, so that the prime performance of this system can be reached[25].
Accordingly, the objective of this project is to obtain the optimal culture condition for a compound bioflocculant, produced by two bacterial strains which were screened from coastal zone sediments with relative great flocculation performance. Then single factor experiments and Plackett-Burman design were carried out to identify the significant factors in the mixed culture medium, followed by RSM to determine the ideal levels of these factors, obtaining the optimal culture medium of this compound bioflocculant while predicting the best flocculation active.
1 Materials and Methods 1.1 Sediment Samples Collection and Isolation of StrainsSediment samples were taken from the offshore sewage outlets of the Golden Bay tourist area in Weihai, China, and mixed with sterile saline at 150 r/min for 1 h for isolation. The pretreated sediment samples were plated on sterilized M2 agar plate (peptone 5.0 g, yeast extract 0.5 g, CH3COONa 5.0 g, nutrient broth 0.5 g, glucose 0.5 g, sucrose 0.5 g, soluble starch 0.5 g, Na3C6H5O7 · 2H2O 0.05 g, malic acid 0.05 g, seignette salt 0.05 g, NH4NO3 1.0 g, NH4Cl 0.2 g, seawater 1.0 L, agar 16 g, pH 7.5-7.6), F agar plate (beef extract 4 g, yeast extract 1 g, peptone 4 g, glucose 1 g, agar 15 g, seawater 1.0 L, pH 7.0), R2A agar plate (Yeast extract 0.50 g, peptone 0.50 g, glucose 0.50 g, soluble starch 0.50 g, Casein hydrolysate 0.50 g, K2HPO4 0.30 g, C3H3NaO3 0.3g, MgSO4 0.05g, agar 16 g, seawater 1.0 L) and G agar plate (K2HPO4 2 g, peptone 10 g, Na2HPO4 8 g, agar 16 g, seawater 1.0 L, pH 7.1-7.3), using a tenfold dilution.
1.2 Production and Purification of BioflocculantPlates were placed in a 28 ℃ incubator for 3-6 days. Cross colonies were selected and recultivated until the separation of purebred single colonies appeared. Then these single colonies were inoculated in beef extract peptone medium (beef extract 5.0 g, peptone 10.0 g and NaCl 33.0 g, distilled water 1.0 L, pH 7.2-7.5), placed in 28 ℃, 120 r/min shaker activation for 24 h, after which 4 bacterial strains were cultivated into 50 mL shake flasks containing 20 mL fermentation medium (glucose 10.0 g, K2HPO4 5.0 g, MgSO4 0.2 g, KH2PO4 2.0 g, NaCl 33.0 g, urea 0.5 g, yeast extract 0.5 g, distilled water 1.0 L, pH 7.2-7.5) under the same cultivation condition for 72 h, to gain viscous fermentation broth. Finally, the extraction of bioflocculant was obtained as described by Liu et al.[26] with some minor modifications. After 72 h fermentation, the culture was centrifuged at 6000 r/min for 15 min at 4 ℃, followed by the addition of two volumes of cold ethanol and overnight store at 4 ℃ to make bioflocculant precipitate. The resulting sediment then was collected by centrifugation at 10000 r/min for 10 min at 4 ℃, washed twice with ethanol, dissolved in deionised water and lyophilized to get pure bioflocculant.
1.3 Flocculation Activity TestThe measurement of flocculation rate was designed based on the approach of Kurane et al[27]. Kaolin clay was used as the standard substrate for the flocculation activity test in this experiment. 0.3 mL of 10% CaCl2 (w/v) and 2 mL of 1 g/L bioflocculant were added to 200 mL of 4 g/L Kaolin clay suspension in a 250 mL glass beaker, after which the initial pH was adjusted to 7.30 before the mixture was stirred at 700 r/min for 2 min and 80 r/min for 10 min step by step, followed by a 2-min settle. A spectrophotometer (WFJ2000) was utilized to measure the absorbance of the supernatant at 550 nm while compared with the controlled data of kaolin suspension only containing 10 CaCl2. The flocculation activity was calculated according to the following formula:
| $ \text { Flocculation rate }(\%)=(A-B) / A \times 100 \% $ | (1) |
where A and B are optical densities of the control and sample at 550 nm, respectively.
1.4 Bacterial Strain IdentificationIndividual strain was identified by analyzing 16S rDNA sequencing[28]. PCR amplification was utilized to amplify 16S rDNA with aid of bacterial universal primers 27F (5'-AGA GTT TGA TCC TGG CTC AG-3') and 1522R (5'-AAG GAG GTG ATC CAG CCG CA-3). The PCR product was then sequenced by Shanghai Sangon Biotech company and their sequences were contrasted to available sequences in the ezbiocloud database and GenBank database to find model strains with the highest proportion of similarity and completeness. A phylogenetic tree was constructed by the neighbor-joining algorithm using MEGA software (6.0).
1.5 Construction of Compound BioflocculantAccording to flocculation activity test, four strains with the best flocculation performance were selected and they were mixed and co-cultured at the volume ratios of 1:1, 1:1:1 and 1:1:1:1 in the same fermentation medium with 4 of total inocula. After 3-day cultivation in a shaker at 28 ℃, 120 r/min, the best combination of strains was obtained by contrasting their final bioflocculation rates, and the optimal ratio of this combination was investigated in the same way as the former.
1.6 Culture Medium Optimization Experiment DesignCentral composite design-response surface methodology was used for the experiments to investigate the effects of the significant compositions from culture media on the flocculating activity and attain the optimal culture medium composition.
1.6.1 Screening of variables and Plackett-Burman designThe response of the system might be affected by numerous variables, such as carbon sources (lactose, soluble starch, sucrose, maltose, glucose, fructose), organic nitrogen sources (peptone, yeast extract, beef extract), inorganic nitrogen sources (urea, NaNO3, (NH4)2SO4, NH4Cl, (NH4)2C2O4) and salt (K2HPO4, KH2PO4, MgSO4). But it was practically unfeasible to identify and control the little contribution of every factor[25]. Thus, single-factor experiments were designed to select two kinds of variables with key effects from carbon sources, organic nitrogen sources and inorganic nitrogen sources separately. Plackett-Burman design was then carried out by minitab 16.0 software to screen which of these variables and their interactions had more significant effects with the minimum number of experiments[29]. Table 1 and Table 2 respectively show the two coded levels of every variable and the experimental design with 12 trials for screening 4 variables. After screening a significant factor from these four groups, addition single-factor experiments were carried out to find out the region with the best response.
| Table 1 Coded levels of factors employed in Plackett-Burman design |
| Table 2 Plackett-Burman design of experiments and experimental results (Every data is the average of three parallel repeats) |
1.6.2 Response surface analysis
The Minitab software was utilized to design a four-variable central composite design and its analytical model quality was assessed according to the analysis of variance (ANOVA). Coded levels of four key factors are shown in Table 3 and the experimental design is shown in Table 4.
| Table 3 Coded levels of key factors employed in central compound experiment design(CCD) |
| Table 4 Design of experiments (CCDs) and experimental results of the response values (Every data is the average of three parallel repeats) |
A quadratic polynomial equation was used to fit the response variable (Y):
| $ Y=\beta_{0}+\sum\limits_{i=1}^{m} \beta_{i} x_{i}+\sum\limits_{i < j}^{m} \beta_{i j} x_{i} x_{j}+\sum\limits_{i=1}^{m} \beta_{i i} x_{i}^{2} $ | (2) |
where Y is the response variable to be modeled, xi and xj are independent variables, β0, βi and βii are the offset term, i linear coefficient, and quadratic coefficient, respectively, and βij is the term that reflects the interaction between xi and xj[15].
2 Results and Discussion 2.1 Strain Screening and IdentificationTotally, 57 strains were isolated from G, R2A, M2 and F culture medium and 9 strains among of them were identified as bioflocculant producing bacteria. The amounts of these strains screened from the media of F, R2A, M2 and G were 3, 3, 2 and 2, respectively. This indicated that oligotrophic medium (F and R2A medium) could screen microbial flocculant producing bacteria more. The strains of A9, F2, F5 and M5 were selected for further study with the highest flocculation ability of 69.67, 78.67, 65.40, 78.20.
After sequencing the 16S rRNA gene of the above four strains by Shanghai Sangon Biotech company, their sequences were contrasted in the EzBioCloud database (http://www.ezbiocloud.net/). The nucleotide sequences of 16S rRNA genes of the four strains were input into NCBI GenBank database with the accession number of MH362712, MH362719, MH362720 and MH362722. The results showed that the similarities between F2 and Pseudoalteromonas marina, F5 and Pseudoalteromonas haloplanktis, M5 and Pseudoalteromonas hodoensis, A9 and Pseudoalteromonas marina were 99, 99, 100 and 99, respectively.
2.2 Construction of Compound StrainsBioflocculant mainly consists of polysaccharides, proteins, nucleic acids and other cellular components, which are produced by strains and named as bacterial extracellular polymeric substances[30]. The flocculation activity of it is largely influenced by culture environment[31]. Mixed culture can increase the microbial diversity in a culture medium which may promote the adaptive capacity of EPS[32]. Therefore, it is vital to investigate the symbiotic combination of various microbes to gain the optimal combination of mixed culture with ideal bioflocculant production and activity[33].
The flocculation rate of the individual strain and compound strains are presented in Fig. 1. After 72 h of culture, the flocculation rates of F5-A9, F5-M5-F2 and F5-M5-A9 were all lower than that of the single strain with the lowest flocculation rate. It was possible that the antagonism or mutual competition affected the growth and metabolism of strains[34].

|
Fig.1 Comparison of the flocculation rate between single strain and different compound strains |
The highest flocculation performance of bioflocculant was obtained by co-culture of F5-M5 and M5-A9, both reaching 82.91, which might result from the mutual promotion between the two groups of compound strains. Numerous researches have been reported that some strains had the ability of synthesizing the nutrients for other microbes and secreting some enzymes to help themselves to meet their nutrient demands in a complex culture medium, leading to produce more flocculable ingredients than single strain[33].
Since the flocculation rates of the combinations of F5 and M5 were both higher than those of the combinations of A9 and M5 at the 24th and 48th hour of culturing, F5 and M5 were finally determined to be researched in the further study. The optimal ratio of these compound strains was explored (Fig. 2). It was found that the differences between various percentages of M5 and F5 were similar and the best flocculation ratio was 2:1 of M5:F5.
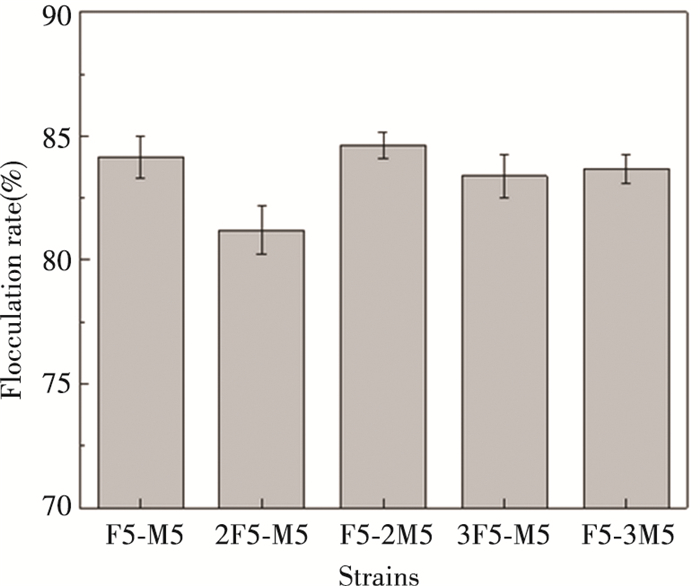
|
Fig.2 Effect of different proportions on bioflocculant production of compound strains F5 and M5 |
2.3 Effect of Carbon and Nitrogen Sources on Bioflocculant Activity
Carbon and nitrogen sources are quite essential to bioflocculant production as microbial metabolism is influenced by the C/N ratio largely. Bioflocculant with the best performance within the shortest incubation time can be obtained in the culture medium containing the suitable carbon and nitrogen sources[35].
In this experiment, same-mass lactose, soluble starch, sucrose, maltose, glucose and fructose were used as carbon sources to explore the effect of different carbon sources on the flocculant produced by the compound strains. It was found that sucrose and glucose were advantageous to compound bioflocculant production, followed by fructose, maltose and lactose, while the flocculantion rate was relatively low in soluble starch medium (Fig. 3). The highest flocculation rate of 85.65% was obtained when sucrose was used. Starch, sucrose and glucose have been found as optimal carbon sources for various bioflocculant producing strains by massive learners, respectively[36-38], which was related to the composition of the flocculant, types of enzymes and sites of action when the strain utilized different saccharides. Sucrose was composed of 1 molecule of glucose and 1 molecule of fructose and compound strains might be more suitable for growing and secreting flocculant substances by using this sucrose as a carbon source.

|
Fig.3 Effect of different carbon sources on bioflocculant production of compound strains |
The effects of organic nitrogen sources and inorganic nitrogen sources on the production of microbial flocculants by compound strains were researched. The experimental results showed that optimal flocculation activity of organic nitrogen sources was 81.29 in the yeast extract medium and 83.48 in the peptone medium respectively, while (NH4)2SO4 and NH4Cl were the prime inorganic nitrogen sources with flocculation efficiency of 80.34 and 77.68 separately (Fig. 4). Due to the different function and certain synergy of organic and inorganic nitrogen sources[39], the combination of these two kinds of nitrogen sources should be considered in the following study.
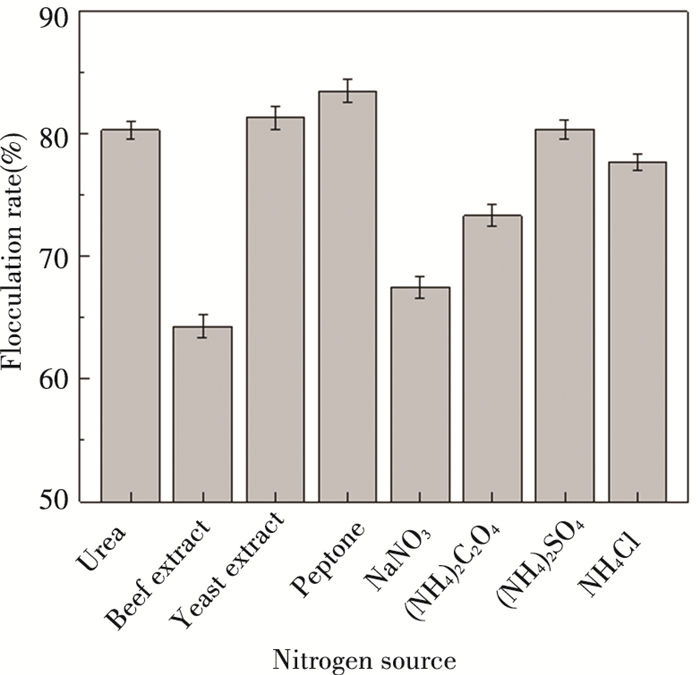
|
Fig.4 Effect of different nitrogen sources on bioflocculant production of compound strains |
2.4 Plackett-Burman Design to Screen Significant Factors
Based on the single factor experiments, two factors affecting the yield of flocculant were selected from the carbon sources (sucrose and glucose), the organic nitrogen sources (peptone and yeast extract) and the inorganic nitrogen sources ((NH4)2SO4 and NH4Cl), respectively. Therefore, the relative significance of key factors of carbon and nitrogen sources and three sulphites of the original medium was investigated by Plackett-Burman design while regarding the flocculation rate as an indicator. The model's adequacy was investigated and the factors with significant effects were found out by the regression analysis (Table 5 and Table 6). As shown in Table 6, the coefficient of determination of the model R-Sq=99.5, indicating that 99.5% of the variability in this model can be explained by this model. Variables with p-values of less than 0.05 were regarded as having notable effects on the response so that they would be selected for the following optimization researches[40]. Therefore, peptone (0.015), yeast extract (0.019), (NH4)2SO4 (0.025) and K2HPO4 (0.012) were determined to be the significant factors of the medium. The p-value of MgSO4 was less than 0.01 corresponding to 99% confidence interval, indicating that MgSO4 was the most significant factor affecting the yield of flocculant in the medium. NH4Cl, KH2PO4 and MgSO4 showed positive effects while other components all had negative effects.
| Table 5 Statistical analysis of Plackett-Burman design |
| Table 6 Regression analysis and the variance analysis of Plackett-Burman design |
Finally, the medium was divided into four parts: carbon source, organic nitrogen source, inorganic nitrogen source and sulphites, and their corresponding significant factors were sucrose, peptone, (NH4)2SO4 and MgSO4, respectively, which would be investigated in the next addition test and response surface test research. K2HPO4 and KH2PO4 contained in the origin medium were only chosen in the high level (4 g/L) and the low level (3 g/L) respectively for further optimization.
2.5 Influence Factor Addition TestIn the response surface design, in order to establish an effective response surface fitting equation, the levels of each factor must be close to the maximum flocculation rate region, so the amount of influencing factors should be investigated based on the Plackett-Burman design experiment. The effects of sucrose, peptone, (NH4)2SO4 and MgSO4 on the flocculation activity were investigated separately while other variables remained the same (Fig. 5).

|
Fig.5 Effect of sucrose, MgSO4, peptone and (NH4)2SO4 concentration on compound strain producing flocculant |
The flocculation rate gradually increased with an increase of the amount of sucrose, reaching the peak of 78.90 when the amount of sucrose is 14 g/L, after which it started to descend with the continual increase of sucrose. When the concentration of MgSO4 was 3.0 g/L, the flocculation activity peaked at 87.46, before dropping down with the rising dosage of MgSO4. Peptone, as a significant factor, did not affect the flocculation rate strongly within the range of 0.1-1.5 g/L, with the maximum rate of 88.79 at 1 g/L, presumably due to the existence of sucrose and MgSO4 in the medium, which was enough to meet the needs of microbial growth so that the utilization of the composite strains F5 and M5 to the available nitrogen source (peptone) was reduced. However, the flocculation rate dipped suddenly at 2 g/L, perhaps because the production of flocculants was inhibited. The flocculation rate also increased with the increase in the concentration of (NH4)2SO4, amounting to 81.24 at 0.5 g/L, followed by a downward tendency. Due to the difference of laboratory orders, kaolin B was used into the imitate water in the addition amount of (NH4)2SO4 and subsequent experiments, so that under the same condition, the absorbance of the blank control was 0.717, compared with the previous one (Kaolin A with 0.351 of blank control), suggesting that the particles of Kaolin B were smaller and the sedimentation performance was worse. Therefore, the flocculation rate was lower than the previous experiments.
Finally, the CCD test level ranges of sucrose, MgSO4, peptone and (NH4)2SO4 were 10-16 g/L, 2.0-3.5 g/L, 0.5-1.5 g/L and 0.1-1.0 g/L, respectively.
2.6 Central Compound Experiment Design (CCD)Based on the former experiments, RSM using CCD was applied to screen out the ideal level of the four selected factors (sucrose, (NH4)2SO4, peptone and MgSO4) which had significant contribution to the flocculation activity of the compound bioflocculant. With concentration of four factors as independent variables and flocculation rate as the response surface, CCD of four factors and two levels were carried out. Other components in the fermentation medium were K2HPO4 4 g/L, KH2PO4 3 g/L and NaCl 33 g/L. Table 3 shows coded levels of key factors employed in CCD design and Table 4 illustrates the design matrix and the corresponding results of RSM experiments to determine the effects of four independent factors.
The significance of each coefficient can be investigated according to the p-values, which contributes to reflect the interaction pattern between these factors (Table 7). The significance of the coefficient will be greater as p-value is smaller. Therefore, there was a direct relationship between sucrose, MgSO4, peptone, (NH4)2SO4 and flocculation rate. The quadratic polynomial regression model was constructed by using minitab 16 software. The model equation was
| $ \begin{aligned} Y=& 0.79-0.01 A-0.03 B+0.03 C+0.03 D-\\ & 0.03 A^{2}-0.003 B^{2}-0.03 C^{2}-0.02 D^{2}-\\ & 0.01 A * B-0.02 A * C-0.01 A * D-\\ & 0.02 B * C+0.01 B * D-0.01 C * D \end{aligned} $ | (3) |
| Table 7 The statistical analysis of the quadratic polynomial regression model |
where A, B, C and D were the coded factors of sucrose, MgSO4, peptone and (NH4)2SO4, respectively.
The appropriateness of the model was verified by ANOVA of the regression model and the regression correlation coefficient R-Sq[41]. p=0.000 of the ANOVA of the regression model showed that the regression model was statistically significant at a 99 probability level, while p=0.408>0.05 of the imputation term, indicating that the model could work with the data accurate fit (Table 7). R-Sq=96.55 indicated that 96.55 of the data in the model could be explained by this model. R-Sq (adjusted)=93.54, which was higher than R-Sq, indicating that the model has good significance.
The response surface curves were shown in Fig. 6 to visualize the effects of four key factors on the flocculation rate and to illustrate the interaction of them. Each figure demonstrated the effect of two factors while the other factors were fixed at zero level. The degree of influence of the interaction of any two factors on the flocculation rate and the optimal range of each factor could be analyzed and evaluated by the response surface in Fig. 6. The magnitude of interaction between various factors could be seen from the shape of a contour in Fig. 7, which meant that the interaction was more significant as the shape was closer to a circle, and vice versa. The closeness of the elliptical arrangement could reflect the degree of influence of factors on the change of response. From the following response surface maps, the extreme conditions appeared at the center of the contour line. The effects of the interaction of NH4(SO4)2 and MgSO4, peptone and MgSO4, sucrose and MgSO4 on the flocculation rate reached an extremely significant level, while the other interactions among these key factors had a significant impact on the flocculation rate.
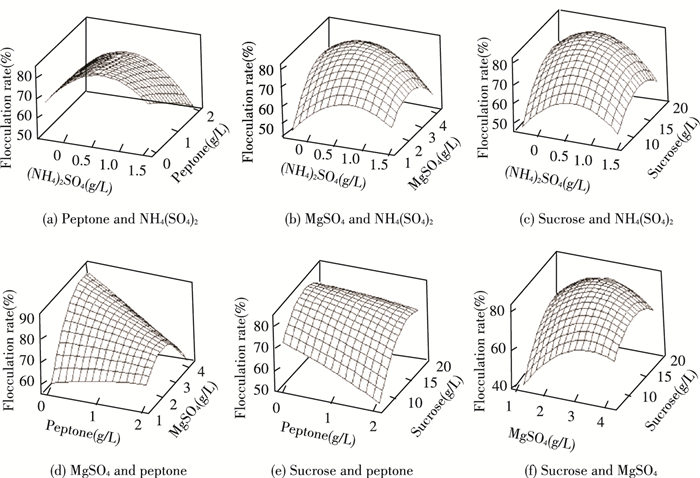
|
Fig.6 Response surface of various factors interaction on flocculation rate |
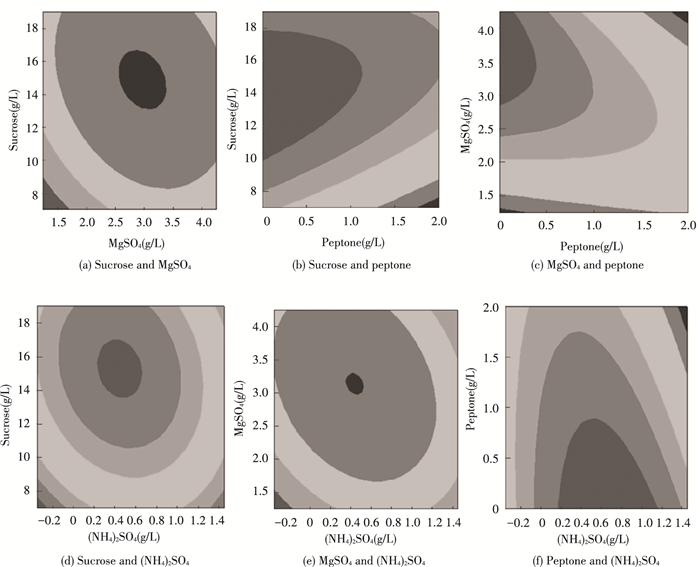
|
Fig.7 Contour map of various factors interaction on flocculation rate |
To obtain the highest level of floccuation rate, the factor levels were set at the values given by MINITAB's Multiple Response Optimizer under global solution, and the predictive culture medium component ((NH4)2SO4 0.523 g/L, peptone 0.00 g/L, MgSO4 3.70 g/L and sucrose 12.82 g/L) as well as the predictive maximum flocculation rate (88.47) were gained. From the prediction method and the response surface pattern, it was found that the flocculation effect of compound strains' medium without adding peptone was relatively good, which might result from the interaction of the four factors and the other components within a certain range, allowing the demands of early growth of microorganisms to be met. Thus, compound strains eliminated the need for a readily available organic nitrogen source, and the delayed nitrogen (NH4(SO4)2) alone was able to provide enough nitrogen for the compound strain, which might facilitate the synthesis of metabolites and the production of flocculatable materials. This result further validated the conjecture on the results of single factor experiments on the peptone addition.
To verify the accuracy and validity of the model and compare the flocculation rate of present compound bioflocculant producing bacterial strains with F5*2M5 after fermentation formula optimization, the optimal fermentation conditions were tested in shake flask fermentation experiments with parallel experiments for 5 times, obtaining the experimental results shown in Table 8.
| Table 8 Comparison of flocculation rate on compound strain producing flocculant before and after optimization of culture medium component |
The predicted value of the maximum yield obtained by the regression equation was close to the average value of the validation test, indicating that the model could accurately reflect the various selected factors on the flocculation rate, while it also proved that application of RSM to find the optimal medium components was feasible.
3 ConclusionsThe objective of the present study was to construct a compound biofloccualnt produced by bacterial strains screened from coastal sediments and optimized its culture medium by using Plackett-Burman design and RSM to obtain the maximum flocculation efficiency. The experiments found that when the ratio of the new strain combination of F5 and M5, both of which were determined as Pseudomonas sp. by 16S rRNA sequencing, was 1:2, the flocculation rate could increase by 6 higher than the single strain (use kaolin A). The ideal carbon and nitrogen sources were also investigated, and the significant factors that affected the flocculation rate were selected. Based on the previous experiments, the multivariate binomial equation and the optimal point of the response were determined over a range of factors. The best medium components were obtained: (NH4)2SO4 0.523 g, MgSO4 3.7 g, sucrose 12.82 g, K2HPO4 4 g, KH2PO4 3 g and NaCl 33 g. Under these conditions, five verification tests were carried out and the test results were in good accordance with the predicted values. After fermentation formula optimization, the flocculation rate of the composite flocculant producing bacteria F5 and M5 was 88.92%, which was 16.60% higher than that using the unoptimizable medium. The result showed that optimization of the medium's formula by using the response surface method was reasonable and reliable. Additionally, as the total amount and cost of the new formula's nitrogen source were less than the original formula's, the experimental cost was also reduced after optimization of the culture media.
| [1] |
Pang C L, Li A, Cui D, et al. Complete genome sequence of Klebsiella pneumoniae J1, a protein-based microbial flocculant-producing bacterium. Journal of Biotechnology, 2016, 220: 90-91. DOI:10.1016/j.jbiotec.2016.01.020 (  0) 0) |
| [2] |
Liu J W, Ma J W, Liu Y Z, et al. Optimized production of a novel bioflocculant M-C11 by Klebsiella sp. and its application in sludge dewatering. Journal of Environmental Sciences, 2014, 26(10): 2076-2083. DOI:10.1016/j.jes.2014.08.007 (  0) 0) |
| [3] |
Zhao C Q, Yang Q H, Zhang H. Optimization of microbial flocculant-producing medium for Bacillus subtilis. Indian Journal of Microbiology, 2017, 57(1): 83-91. DOI:10.1007/s12088-016-0631-3 (  0) 0) |
| [4] |
Wei W, Fang M, Yue X L, et al. Purification and characterization of compound bioflocculant. Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering. Piscataway: IEEE, 2008. DOI:10.1109/ICBBE.2008.275 (  0) 0) |
| [5] |
Gao J, Bao H Y, Xin M X, et al. Characterization of a bioflocculant from a newly isolated Vagococcus sp. W31. Journal of Zhejiang University. Science. B, 2006, 7(3): 186-192. DOI:10.1631/jzus.2006.B0186 (  0) 0) |
| [6] |
Okaiyeto K, Nwodo U U, Mabinya L V, et al. Characterization of a bioflocculant produced by a consortium of Halomonas sp. Okoh and Micrococcus sp. Leo. International Journal of Environmental Research and Public Health, 2013, 10(10): 5097-5110. DOI:10.3390/ijerph10105097 (  0) 0) |
| [7] |
Guo H P, Hong C T, Zhang C, et al. Bioflocculants' production from a cellulase-free xylanase-producing Pseudomonas boreopolis G22 by degrading biomass and its application in cost-effective harvest of microalgae. Bioresource Technology, 2018, 255: 171-179. DOI:10.1016/j.biortech.2018.01.082 (  0) 0) |
| [8] |
Qi Z Y, Zhu Y Y, Guo H P, et al. Production of glycoprotein bioflocculant from untreated rice straw by a CAZyme-rich bacterium, Pseudomonas sp. HP2. Journal of Biotechnology, 2019, 306: 185-192. DOI:10.1016/j.jbiotec.2019.10.011 (  0) 0) |
| [9] |
Chouchane H, Mahjoubi M, Fttoumi B, et al. A novel thermally stable heteropolysaccharide-based bioflocculant from hydrocarbonoclastic strain Kocuria rosea BU22S and its application in dye removal. Environmental Technology, 2018, 39(7): 859-872. DOI:10.1080/09593330.2017.1313886 (  0) 0) |
| [10] |
Pu L, Zeng Y J, Xu P, et al. Using a novel polysaccharide BM2 produced by Bacillus megaterium strain PL8 as an efficient bioflocculant for wastewater treatment. International Journal of Biological Macromolecules, 2020, 162: 374-384. DOI:10.1016/j.ijbiomac.2020.06.167 (  0) 0) |
| [11] |
Abu Tawila Z M, Ismail S, Dadrasnia A, et al. Production and characterization of a bioflocculant produced by Bacillus salmalaya 139SI-7 and its applications in wastewater treatment. Molecules, 2018, 23(10): 2689. DOI:10.3390/molecules23102689 (  0) 0) |
| [12] |
Wang Z, Shen L, Zhuang X L, et al. Flocculation characterization of a bioflocculant from Bacillus licheniformis. Industrial & Engineering Chemistry Research, 2015, 54(11): 2894-2891. DOI:10.1021/ie5050204 (  0) 0) |
| [13] |
Ma F, Liu J L, Li S G, et al. Development of complex microbial flocculant. China Water & Wastewater, 2003, 19(4): 1-4. DOI:10.3321/j.issn:1000-4602.2003.04.001 (  0) 0) |
| [14] |
Zhao Y X, Gao B Y, Shon H K, et al. Anionic polymer compound bioflocculant as a coagulant aid with aluminum sulfate and titanium tetrachloride. Bioresource Technology, 2012, 108(3): 45-54. DOI:10.1016/j.biortech.2012.01.012 (  0) 0) |
| [15] |
Salehizadeh H, Yan N, Farnood R. Recent advances in polysaccharide bio-based flocculants. Biotechnology Advances, 2018, 36(1): 92-119. DOI:10.1016/j.biotechadv.2017.10.002 (  0) 0) |
| [16] |
Kim D G, La H J, Ahn C Y, et al. Harvest of Scenedesmus sp. with bioflocculant and reuse of culture medium for subsequent high-density cultures. Bioresource Technology, 2011, 102(3): 3163-3168. DOI:10.1016/j.biortech.2010.10.108 (  0) 0) |
| [17] |
Ben Rebah F, Mnif W, Siddeeg S M. Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: A Review. Symmetry-Basel, 2018, 10(11): 556. DOI:10.3390/sym10110556 (  0) 0) |
| [18] |
Yu L, Tang Q W, Zhang Y J, et al. A novel Fe(Ⅲ) dependent bioflocculant from Klebsiella oxytoca GS-4-08: culture conditions optimization and flocculation mechanism. Scientific Reports, 2016, 6: 34980. DOI:10.1038/srep34980 (  0) 0) |
| [19] |
Xia S Q, Zhang Z Q, Wang X J, et al. Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Bioresource Technology, 2008, 99(14): 6520-6527. DOI:10.1016/j.biortech.2007.11.031 (  0) 0) |
| [20] |
Giri S S, Harshiny M, Sen S S, et al. Production and characterization of a thermostable bioflocculant from Bacillus subtilis F9, isolated from wastewater sludge. Ecotoxicology & Environmental Safety, 2015, 121: 45-50. DOI:10.1016/j.ecoenv.2015.06.010 (  0) 0) |
| [21] |
Agunbiade M O, Pohl C, Van Heerden E, et al. Evaluation of fresh water actinomycete bioflocculant and its biotechnological applications in wastewaters treatment and removal of heavy metals. International Journal of Environmental Research and Public Health, 2019, 16(18): 3337. DOI:10.3390/ijerph16183337 (  0) 0) |
| [22] |
Aljuboori A H R, Idris A, Abdullah N, et al. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresource Technology, 2013, 127(1): 489-493. DOI:10.1016/j.biortech.2012.09.016 (  0) 0) |
| [23] |
Aljuboori A H R, Uemura Y, Osman N B, et al. Production of a bioflocculant from Aspergillus niger using palm oil mill effluent as carbon source. Bioresource Technology, 2014, 171: 66-70. DOI:10.1016/j.biortech.2014.08.038 (  0) 0) |
| [24] |
Xiong Y Y, Wang Y P, Yu Y, et al. Production and characterization of a novel bioflocculant from Bacillus licheniformis. Applied & Environmental Microbiology, 2010, 76(9): 2778-2782. DOI:10.1128/AEM.02558-09 (  0) 0) |
| [25] |
Bezerra M A, Santelli R E, Oliveria E P, et al. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta, 2008, 76(5): 965-977. DOI:10.1016/j.talanta.2008.05.019 (  0) 0) |
| [26] |
Liu W J, Wang K, Li B Z, et al. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresource Technology, 2010, 101(3): 1044-1048. DOI:10.1016/j.biortech.2009.08.108 (  0) 0) |
| [27] |
Kurane R, Hatamochi K, Kakuno T, et al. Production of a bioflocculant by Rhodococcus erythropolis S-1 grown on alcohols. Journal of the Agricultural Chemical Society of Japan, 1994, 58(2): 428-429. DOI:10.1271/bbb.58.428 (  0) 0) |
| [28] |
Güssow D, Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Research, 1989, 17(10): 4000. DOI:10.1093/nar/17.10.4000 (  0) 0) |
| [29] |
Cui F J, Zhao L M. Optimization of Xylanase production from Penicillium sp.WX-Z1 by a two-step statistical strategy: Plackett-Burman and Box-Behnken experimental design. International Journal of Molecular Sciences, 2012, 13(8): 10630-10646. DOI:10.3390/ijms130810630 (  0) 0) |
| [30] |
Nouha K, Kumar R S, Balasubramanian S, et al. Critical review of EPS production, synthesis and composition for sludge flocculation. Journal of Environmental Sciences, 2018, 66: 225-245. DOI:10.1016/j.jes.2017.05.020 (  0) 0) |
| [31] |
Siddeeg S M, Tahooh M A, Ben Rebah F. Agro-industrial waste materials and wastewater as growth media for microbial bioflocculants production: a review. Materials Research Express, 2020, 7(1): 012001. DOI:10.1088/2053-1591/ab5980 (  0) 0) |
| [32] |
Kleerebezem R, van Loosdrecht M C M. Mixed culture biotechnology for bioenergy production. Current Opinion in Biotechnology, 2007, 18(3): 207-212. DOI:10.1016/j.copbio.2007.05.001 (  0) 0) |
| [33] |
More T T, Yadav J S S, Yan S, et al. Extracellular polymeric substances of bacteria and their potential environmental applications. Journal of Environmental Management, 2014, 144: 1-25. DOI:10.1016/j.jenvman.2014.05.010 (  0) 0) |
| [34] |
Salehizadeh H, Yan N, Farnood R. Recent advances in polysaccharide bio-based flocculants. Biotechnology Advances, 2018, 36(1): 92-119. DOI:10.1016/j.biotechadv.2017.10.002 (  0) 0) |
| [35] |
He N. Production and characterization of a novel bioflocculant produced by Bacillus licheniformis. New Biotechnology, 2009, 25(6): S230-S231. DOI:10.1016/j.nbt.2009.06.207 (  0) 0) |
| [36] |
Aljuboori A H R, Idris A, Abdullah N, et al. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresource Technology, 2013, 127(1): 489-493. DOI:10.1016/j.biortech.2012.09.016 (  0) 0) |
| [37] |
Elkady M F, Farag S, Zaki S, et al. Bacillus mojavensis strain 32A, a bioflocculant-producing bacterium isolated from an Egyptian salt production pond. Bioresource Technology, 2011, 102(17): 8143-8151. DOI:10.1016/j.biortech.2011.05.090 (  0) 0) |
| [38] |
Zhong L, Shan Z, Lei H Y, et al. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresource Technology, 2009, 100(14): 3650-3656. DOI:10.1016/j.biortech.2009.02.029 (  0) 0) |
| [39] |
Wang K. Research on Screening and Identification of Biological Control Bacteria Against Aflatoxins and Mechanism of Strain JPP1 for Biocontrol. Harbin: Harbin Institute of Technology, 2013. (  0) 0) |
| [40] |
Reddy L V A, Wee Y J, Yun J S, et al. Optimization of alkaline protease production by batch culture of Bacillus sp. RKY3 through Plackett-Burman and response surface methodological approaches. Bioresource Technology, 2008, 99(7): 2242-2249. DOI:10.1016/j.biortech.2007.05.006 (  0) 0) |
| [41] |
Chen X S, Tang L, Li S, et al. Optimization of medium for enhancement of ε-Poly-L-Lysine production by Streptomyces sp. M-Z18 with glycerol as carbon source. Bioresource Technology, 2011, 102(2): 1727-1732. DOI:10.1016/j.biortech.2010.08.071 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


