2. Department of Civil and Environmental Engineering, Harbin University of Science and Technology, Harbin 150000, China
Phosphorus plays an important role in the growth of all organisms[1-2]. However, with the development of urbanization, excessive phosphorus emissions may result in the eutrophication of aquatic environments. Thus, water quality is deteriorated and the aquatic ecosystem is destroyed, which ultimately endangers human health[3-6]. Therefore, strict regulations for the concentration of phosphorus containing wastewater entering the natural water system have been enacted. For instance, the Water Framework Directive in EU has reduced the allowable phosphorous concentration in wastewater treatment plant effluents from 1-2 mg P/L to 0.1 mg P/L[5, 7]. To meet the ever-increasing strict emission standards, many efforts have been devoted to develop high-efficiency and environmentally friendly methods for removing phosphorus from wastewater.
Up till now, there are many kinds of technologies for phosphorus removal, such as biological treatment[8-9], physic-chemical precipitation[10], membrane separation[11], electrochemical process[12], crystallization[13], and adsorption[14-15]. Among these technologies, adsorption was considered to be the most widely used method to remove phosphorus from wastewater due to its simple design, low cost, high selectivity and easy operation[1, 5, 16]. Currently, metal hydroxide[17], modified zeolite[18], activated carbon[19], furnace slag[20] and so on have been widely applied in treatment of phosphorus-containing wastewater and displayed prospective for adsorption of phosphorus. Recently, researchers pay much more attention to biochar due to their excellent adsorption ability and broad raw material source. The biochar can be prepared from abundant waste materials, such as rice straw, wheat straw, corn cob, vegetable leaves, wood waste and waste activated sludge[1]. Waste activated sludge is the byproduct of wastewater treatment plants, and it is quite difficult to be properly treated due to its complex composition and large quantity. It has been demonstrated that the waste activated sludge is full of organic materials and can be used to prepare biochar through anoxic pyrolysis[1, 21-22]. However, the adsorption capacity of virgin biochar for phosphorus is unfavorable[23]. In order to enhance the adsorption ability of biochar, modified methods of using sulfuric acid, lanthanum or metals have been applied[21-22].
Recently, metals such as manganese, aluminum and iron have been employed to modify sludge biochar to improve its adsorption for phosphorus. For example, Peng et al.[1] prepared an Mn/Al double oxygen sludge biochar (MA-SBC), and found that the maximum adsorption amount of MA-SBC for phosphorus can reach 28.20 mg/g. Yang et al.[22] used FeCl3 impregnation to modify sludge biochar, and found that the phosphate adsorption capacity amounts to 111.0 mg/g. Akaganeite (β-FeOOH) is one type of iron oxydroxide with a tunnel structure[24], which exhibits its potential to remove various pollutants including phosphate by adsorption[24]. It was also reported that akaganeite has a high selectivity for phosphate ions in mixed anion solutions at pH 8[25]. Harijan and Chandra[24] synthesized β-FeOOH nanorods/graphene oxide sheets nanocomposite, and its maximum adsorption capacity for phosphate was 45.2 mg/g at pH 7. Therefore, β-FeOOH modified sludge biochar (FSBC) was prepared in this study to enhance phosphorus removal performance.
In this study, FSBC was prepared using dewatered waste activated sludge, and the physicochemical characteristics of FSBC were investigated by Brunauer-Emmett-Teller (BET) and X-ray diffraction (XRD) analysis. The adsorption capacity, regeneration performance, effects of pH and coexisting species were assessed and compared with that of virgin sludge biochar (SBC). The adsorption isotherm and kinetics were also investigated to reveal the mechanism of adsorption process.
1 Materials and Methods 1.1 Sludge and ChemicalsDewatered sludge (water content was 82%) used in this experiment was taken from a municipal sewage treatment plant in Tianjin, China, and dried to a constant weight at 105 ℃. Then the sludge was sieved through a 200-mesh sieve and saved in a desiccator for further use. The chemical composition of the raw sludge (RS) is shown in Table 1. The chemicals reagents used in this study include zinc chloride (ZnCl2), sulfuric acid (H2SO4), hydrochloric acid (HCl), ferric chloride (FeCl3) and sodium dihydrogen phosphate (NaH2PO4). The above reagents were analytically pure.
| Table 1 Chemical composition of raw sludge, SBC and FSBC(%) |
1.2 Preparation and Modification of Sludge Biochar
Several grams of sludge were activated by mixture solution of ZnCl2 (1 mol/L) and H2SO4 (1 mol/L) with a volume ratio of 3∶1 in 80 ℃ water bath for 24 h. The activated sludge was centrifuged at 3000 r/min for 10 min for solid-liquid separation. Subsequently, the obtained solid product was incinerated for 120 min in N2 atmosphere at a stable temperature of 700 ℃ in tubular furnace. After being washed by HCl solution (1 mol/L) to remove impurities from biochar holes, the biochar was rinsed with deionized water until neutral, and then dried to constant weight at 105 ℃, and thus the sludge biochar (SBC) was finally obtained.
The FSBC was prepared as follows: several grams of SBC were added to 0.05 mol/L FeCl3 solution at a solid-liquid ration of 1 g∶30 mL with the pH value adjusted to 6 by NaOH solution (1 mol/L) and HCl solution (1 mol/L). The mixed solution was stirred for 6 h at the temperature of 40 ℃, aged for 24 h, and then cooled down to room temperature (23±1 ℃). Subsequently, free β-FeOOH was removed by solid-liquid separation. The obtained solid was washed with deionized water until Fe3+ was not detected in the supernatant, then filtered by a 0.45 μm membrane. The solid retained by the filter was dried at 60 ℃ to obtain FSBC. The chemical composition of SBC and FSBC is shown in Table 1.
1.3 Adsorption ExperimentA 1000 mg/L phosphate solution was prepared using KH2PO4 and diluted to the required concentration. For each batch adsorption experiment, 100 mL phosphate solution with initial concentration of 10 mg/L was added to a 250 mL conical flask, and the pH was adjusted to 5 using NaOH solution (1 mol/L) and HCl solution (1 mol/L), and then FSBC or SBC was added. The conical flask was placed in a shock box and reacted for 24 h at a temperature of 25 ℃. After adsorption, samples were filtered instantly through 0.45 μm membranes to separate FSBC/SBC from the phosphate solution. The phosphate adsorption capacity was calculated according to Eq.(1):
| $ q=\frac{\left(c_{0}-c_{1}\right) V}{m} $ | (1) |
where q is the equilibrium adsorption capacity (mg/g), c0 is the initial phosphate concentration, c1 is the phosphate concentration in the equilibrium solution after adsorption (mg/g), m is the mass of added adsorbent, and V is the volume of the solution.
1.4 Adsorption Models 1.4.1 Isotherm modelsThe mechanism of phosphate adsorption can be revealed by adsorption isotherm, which was carried out under 1g/L FSBC, 10 mg/L-500 mg/L phosphate and pH 5. The Langmuir isotherm (Eq.(2)) and Freundlich isotherm (Eq. (4)) were used to fit the adsorption equilibrium data.
Langmuir:
| $ \frac{C_{\mathrm{e}}}{q_{\mathrm{e}}}=\frac{C_{\mathrm{e}}}{q_{\max }}+\frac{1}{K_{1} q_{\max }} $ | (2) |
| $ R_{\mathrm{L}}=\frac{1}{1+K_{1} C_{0}} $ | (3) |
Freundlich:
| $ \ln {q_{\rm{e}}} = \frac{1}{n}\ln {C_{\rm{e}}} + \ln {K_2} $ | (4) |
where qe (mg/g) is the adsorption capacity at equilibrium; Ce (mg/L) is the phosphate concentration at equilibrium; qmax (mg/g) denotes the Langmuir maximum capacity; K1 (L/mg) denotes the Langmuir constant; K2 (mg/g) and n are the Freundlich adsorption capacity and adsorption intensity, respectively. RL is the separation factor.
1.4.2 Kinetic modelsA pseudo-first-order model (Eq. (5)), a pseudo-second-order model (Eq. (6)), and anintra-particle diffusion model (Eq. (7))[26] were applied to fit the kinetics to investigate the efficiency of phosphate adsorption by FSBC. The detailed equations are as follows:
| $ \ln \left(q_{1}-q_{t}\right)=\ln q_{1}-k_{1} t $ | (5) |
| $ \frac{t}{q_{t}}=\frac{1}{k_{2} q_{2}^{2}}+\frac{1}{q_{2}} t $ | (6) |
where q1(mg/g) means the adsorbed amount of phosphate at equilibrium for pseudo-first-order model, q2(mg/g) means the adsorbed amount of phosphate at equilibrium for pseudo-second-order model, qt (mg/g) denotes the adsorbed amount of phosphate at time t, k1 and k2 are the kinetic constants.
| $ q_{t}=k_{\mathrm{id}} t^{0.5}+C $ | (7) |
where kid (mg/(g·h0.5)) is diffusion rate constant of intra-particle diffusion and C represents the thickness of boundary layer.
1.5 Regeneration PerformanceThe 1 g adsorbent was mixed with 100 mL 50 mg/L KH2PO4 solution in a 250 mL conical flask, reacted at 150 r/min for 24 h at 25 ℃ and pH 5. Afterwards, the SBC or FSBC was collected and washed using deionized water several times. Then SBC was dried at 105 ℃ for 2 h, FSBC was dried at 60 ℃ for 4 h. After the separation of SBC or FSBC, the phosphate concentration in the filtrate was measured. For the regeneration of SBC or FSBC, the adsorbent was immersed in 1% NaOH solution, shaking with a mixing speed of 150 r/min for 12 h at 25 ℃. After that, the adsorbent was separated and washed using deionized water until pH value was neutral. The regenerated adsorbent was used for the next adsorption cycle. Four adsorption-desorption cycles were conducted. The adsorption capacity of the regenerated adsorbent was calculated according to Eq.(1).
1.6 Analytical MethodsThe microstructure and specific surface area of SBC and FSBC were investigated by the specific surface area and pore size analyzer (BET; ASAP2020M+C, USA). The mineralogical composition and crystal phase of FSBC and SBC were analyzed by X-ray diffraction (XRD; DaVinci, Germany).The micro-morphology of adsorbent was observed by scanning electron microscope (SEM; JEOL 7610F, Japan). The composition of RS, SBC and FSBC were detected by an X-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi, United State). The point of zero charge (pHzpc) was measured using a zeta potential analyzer (Zetasizer Nano ZS, England). The concentration of phosphate was detected by a UV-vis spectrophotometer (UV-5800PC, China) using a method of molybdenum antimony spectrophotometry[27]. All experiments were conducted in triplicate, and the date was represented by mean of the measured values.
2 Results and Discussion 2.1 Physicochemical Characteristics of AdsorbentsXRD was employed to investigate the mineralogical composition and crystal structure on the surface of sludge biochar. XRD patterns of FSBC in the range from 15° to 65° were characterized and the results are shown in Fig. 1. It could be seen that there are a large number of irregular peaks on the surface of FSBC, indicating that the mineral crystals of sludge biochar were very complex and contained a variety of metal oxides (SiO2, Al2O3, Fe2O3). Compared with SBC, FSBC exhibited characteristic peaks of β-FeOOH at 2θ=23.8°, 33.9° and 48.8°[24], which means FSBC was successfully loaded on β-FeOOH. The peaks of 31.4° and 38.7° were due to the presence of FeCl3, which was not successfully converted into β-FeOOH during the loading process. The peaks of 47.5° and 51.7° were due to the residual zinc chloride in the preparation process. Besides iron compounds, the other diffraction peaks did not change significantly before and after β-FeOOH loading.
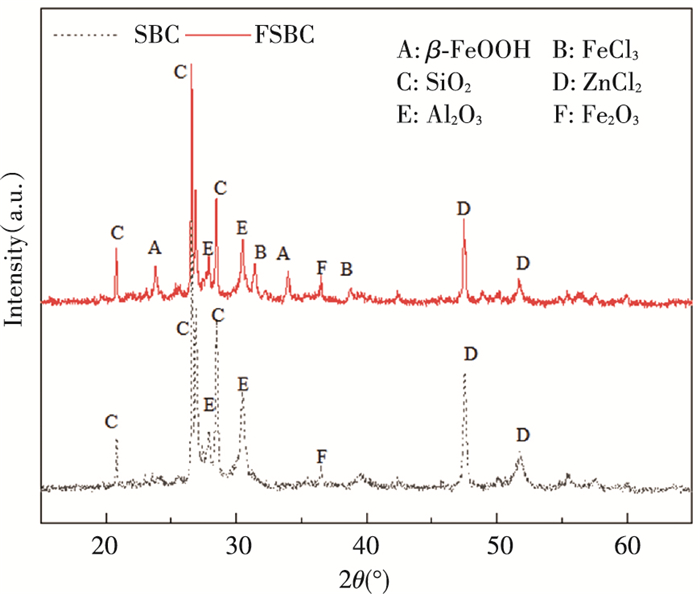
|
Fig.1 XRD patterns of SBC and FSBC |
The SEM images of SBC and FSBC are shown in Fig. 2. It was shown that there are obvious porosities and plenty of pores of different sizes on the surface of SBC. As for FSBC, its surface became rough, and the pore size and number were smaller than those of SBC. Due to the loading of β-FeOOH, some pores of SCB were blocked and the pore size and number were reduced to some extent. The result was coincided with the BET analysis of FSBC.
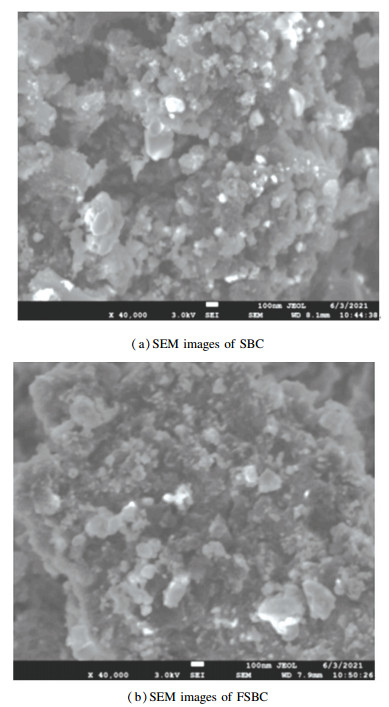
|
Fig.2 SEM images of SBC and FSBC at 40000 magnification |
The specific surface area and pore properties of adsorbents were analyzed by BET (Table 2).It was shown that the specific surface area were 189.2 m2/g and 171.2 m2/g for SBC and FSBC, respectively. Both SBC and FSBC had a mesoporous structure (2 nm-50 nm) with the pores diameter between 2 nm and 3 nm. Compared with SBC, the specific surface area, the total pore volume and pore diameter of FSBC were all reduced, but the changes were not significant.
| Table 2 Surface and pore structure parameters of FSBC and SBC |
2.2 Adsorption and Regeneration Capacity of FSBC
The adsorption and regeneration capacity of FSBC was studied (compared with SBC and CAC (commercial activated carbon)), and the results are shown in Fig. 3. As shown in Fig. 3(a), when the FSBC dosage was 10 mg/L, the adsorption of FSBC reached equilibrium with the adsorption capacity of 0.83 mg/g, and the maximum phosphate removal rate was 85.6%.While for SBC and CAC, the maximum phosphate adsorption capacity of 0.65 mg/g and 0.35 mg/g were obtained at the dosage of 12 mg/L, with the corresponding phosphate removal rate of 78.5% and 42.6%, respectively. The specific adsorption capacity of FSBC was 21.7% higher than that of SBC, and 57.8% higher than that of CAC.

|
Fig.3 Adsorption and regeneration capacity of FSBC and FTIR and XRD spectra of FSBC before and after adsorption |
FT-IR and XRD for the FSBC before and after adsorption were characterized (Fig. 3(b-c)). For FSBC, the band appearing at 3400 cm-1 was attributed to the asymmetric stretching vibration of the O-H and adsorbed water[28]. The band appearing at 1610 cm-1 contributed to the stretching vibration peak of C=C[29]. The double-peak appearing around 1100 cm-1 and 1050 cm-1 could be attributed to stretching vibration of C-OH. 670 cm-1 was the bend vibration of =CH[30]. 557 cm-1 indicated the existence of Fe-O[31-32]. After adsorption, the strength of the peaks for FSBC was weakened. This may be due to the influence of phosphate which was adsorbed on the surface of FSBC. It was reported that the hydroxyl could react with phosphate through hydrogen bonding[33]. Furthermore, Harijan and Chandra[34] reported that the hydroxyl could exchange with phosphate. These functions caused the decrease of the peak strength at 3400 cm-1. The XRD (Fig. 3(c)) results showed that the AlP (2θ=26.88° (100)) was detected on the surface of FSBC after adsorption. It indicated that the Al2O3 on the surface of FSBC could react with phosphate to form the precipitation of aluminum phosphate compounds. Additionally, β-FeOOH could increase the positive charge on FSBC (Fig. 3(c)), which enhanced the electrostatic adsorption of phosphate.
The regeneration and reusability of FSBC was evaluated, and the result was compared with that of SBC (Fig. 3(d)). The results showed that after four adsorption/desorption cycles, the adsorption capacity of FSBC was 1.36 mg/g, which decreased by 36.4% compared with the original FSBC. As for SBC, the adsorption capacity decreased by 57.5%. The decrease in adsorption capacity was because some phosphate was closely bound to the adsorbent, or entered the deep part of the adsorbent, leading to the difficulty to be replaced by hydroxyl. The higher regeneration ability of FSBC indicates FSBC had promising potential to be used as a recovery/recycle platform.
2.3 Effect of pH on Adsorption Capacity of FSBCThe effect of the initial pH on the adsorption performance of FSBC for phosphate was investigated by changing pH value from 3.0 to 11.0 at 1g/L FSBC and 10 mg/L phosphate. As shown in Fig. 4(a), the phosphate adsorption performance of FSBC was obviously superior when compared with that of SBC.
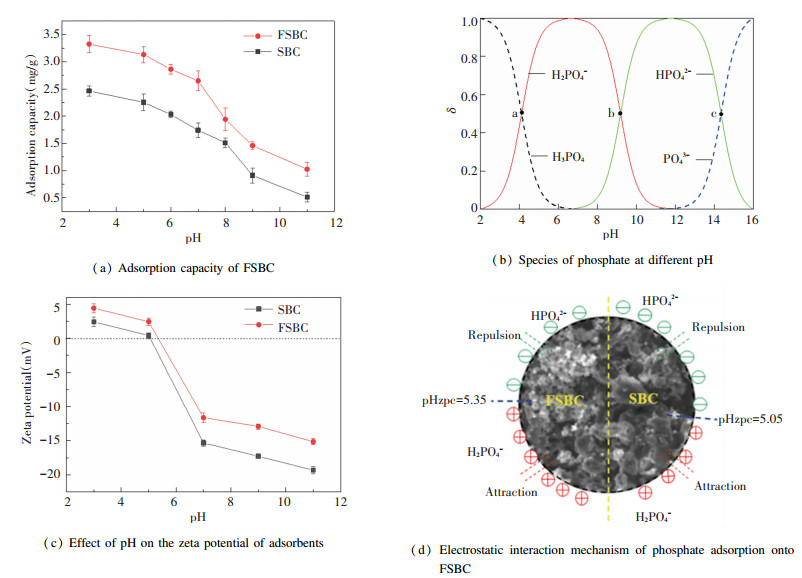
|
Fig.4 Effect of initial pH on the adsorption capacity, zeta potential and adsorption mechanism of FSBC and species of phosphate |
The adsorption ability for phosphate was related to the surface charge of FSBC and the species of phosphate. Phosphate has three values pKa, pKb and pKc, thus within the pH range of 3-11, the predominant species of phosphate exist: (Ⅰ) H2PO4- at solution pKa (pH=2.12) < pH < pKb (pH=7.20); (Ⅱ) HPO42- at pKb (pH=7.20) < pH < pKc (pH=12.36); (Ⅲ) PO43- at pH>pKc (pH=12.36) (Fig. 4(b)). The pHzpc of FSBC was 5.35, which was higher than that of SBC (which is 5.05) (Fig. 4(c)). This indicated that the positive charge of FSBC increased after β-FeOOH is loaded. When the pH was less than 5.35, the surface of FSBC was protonated to make the biochar positively charged[1], thereby strengthening the electrostatic attraction between the biochar and phosphate which almost existed in the forms of H2PO4-. When the pH value was greater than 5.35, phosphate gradually changed from H2PO4- to HPO42-. HPO42- is not easy to be adsorbed because its adsorption free energy is higher than that of H2PO4-[35]. At this time, the surface of the FSBC was deprotonated[1], and the surface charge of FSBC was changed to negative. Electrostatic repulsion existed between FSBC and the phosphate, and the repulsive force was enhanced with the increase of pH value. As a result, the adsorption ability of FSBC for phosphate was reduced. In addition, the weaker adsorption performance of FSBC at higher pH could also be attributed to the presence of excessive OH-. The negatively charged OH- could compete with phosphate anion for active sites on FSBC. Furthermore, the adsorbed OH- on FSBC also led to electrostatic expulsion between phosphate anion and FSBC. In general, FSBC exhibited stronger adsorption capacity for phosphate in acidic conditions. Similar results were reported for the adsorption of CIP, NOR and OFL onto Fe/Zn+H3PO4 modified SBC[26], tetracycline adsorption onto Fe/Zn modified sawdust biochar[36], and phosphate adsorption onto Mn/Al double oxygen sludge-derived biochar[1].
2.4 Effect of Coexisting Ions on Adsorption Capacity of FSBCFrom a practical point of view, real wastewater usually contains a mixture of various ions such as SO42-, NO3-, Cl-, CO32-, Cr (Ⅵ) and Cd2+. Specifically, the anions compete adsorption sites with phosphate ions. The effect of coexisting ions on the adsorption capacity of FSBC for phosphate was investigated at an FSBC dose of 1 g/L, pH of 5, initial phosphate of 10 mg/L, with the concentration of other ions being kept at 0.1 mol/L. It was evident from Fig. 5 that the interference of NH4+ could be negligible, while SO42-, NO3-, Cl- and CO32- ions could inhibit the adsorption of phosphate onto the FSBC to some extent. Among them, SO42-, NO3- and Cl- had little competition with phosphate for adsorption sites (adsorption capacity decreased by 14.1% (SO42-), 6.4% (NO3-) and 20.2% (Cl-) for FSBC), but CO32- exhibited strong competition with phosphate for adsorption sites (adsorption capacity decreased by 53.4% for FSBC). This may be because CO32- had two electrons, the electrostatic attraction between CO32- and adsorbents were higher than that of other ions such as NO3- and Cl- which only had one electron (phosphate existing as H2PO4- at pH 5). Therefore, CO32- could occupy the active adsorption sites on the adsorbents preferentially, and caused the decrease in removal efficiency of phosphate. The SO42- also belonged to divalent anions, but the inhibition of SO42- on phosphate adsorption was weaker than that of CO32-. This was because the hydration energy of CO32- was much lower than that of SO42-, and the lower hydration energy was conducive to the adsorption[37]. In comparison with SBC, the adsorption of phosphate onto FSBC was shown to be less influenced by these anions. This might be because the β-FeOOH loaded on the SBC had higher phosphate selectivity[25]. The Cr(Ⅵ) and Cd2+ ions could improve the removal rate of phosphate. It could be attributed to the fact that the heavy metal ions Cr(Ⅵ) and Cd2+ could react with phosphate ions to form insoluble precipitation. The presence of starch and bovine blood protein (BBP) slightly inhibited the adsorption of phosphate, possibly because macromolecular organic matter would be formed after starch or BBP dissolves in water, which could block some of the pores of the adsorbent, and then reduce the adsorption performance.

|
Fig.5 Effect of coexisting species on the adsorption capacity of FSBC and SBC for phosphate |
2.5 Adsorption Isotherms of FSBC
Langmuir and Freundlich models were applied to describe the adsorption isotherms to investigate the adsorption mechanism[38] and the results are shown in Fig. 6 and Table 3. As can be seen, Langmuir and Freundlich models were both fitted well with the experimental data at 15 ℃, 25 ℃ and 35 ℃. However, the R2 value of Langmuir model was higher than that of Freundlich model at the same reaction temperature (Table 3). It indicated that the adsorption of FSBC for phosphate may follow a monolayer adsorption process. The result was similar to the previous studies. For example, Fe-300 biochar adsorption for phosphorus[2]and Fe-coated cotton stalk biochar adsorption for Cr(Ⅵ)[39] were both fitted well with Langmuir model.

|
Fig.6 Adsorption isotherms for phosphate adsorption onto FSBC at different temperatures |
| Table 3 Linear correlation analysis and parameter values of adsorption isothermal models |
The theoretical maximum adsorption capacity of FSBC for phosphate at 25 ℃ calculated by Langmuir model was 27.17 mg/g, while it was 21.73 mg/g for SBC. The phosphate adsorption was influenced by the reaction temperature. It can be seen in Table 3 that the theoretical adsorption capacities of FSBC for phosphate were 22.49 (15 ℃), 27.17 (25 ℃) and 32.03 mg/g(35 ℃), respectively. It indicated that the phosphate removal rate can be improved when the reaction temperature is increased. Moreover, the K1 in Langmuir model were 0.0108 (15 ℃), 0.0111 (25 ℃) and 0.0116 (35 ℃), respectively, which suggests that higher temperature could enhance the affinity of phosphate to FSBC[40-41]. The result also indicated that the adsorption reaction was endothermic[40]. Furthermore, the RL in Langmuir model was between 0 and 1 at the three temperatures (0.1561-0.9022 (15 ℃), 0.1522-0.8998 (25 ℃), and 0.1471-0.8961 (35 ℃)), which also proved that the adsorption of phosphate on FSBC was favorable[40, 42-43].
2.6 Adsorption Kinetics for FSBCThe kinetic models were analyzed under 3 g/L FSBC, 10 mg/L phosphate and T=25 ℃. The parameters were presented in Table 4 and the fitted curves were given in Fig. 7. As can be seen, both pseudo-first-order kinetic model and pseudo-second-order kinetic model simulated the adsorption process well, but R2 of pseudo-second-order kinetic model (R2=0.9946) was higher than that of pseudo-first-order kinetic model (R2=0.9554). Therefore, the phosphate adsorption onto FSBC could be well fitted by the pseudo-second-order kinetic model, and the adsorption rate was mainly controlled by chemical adsorption[44].
| Table 4 Kinetic adsorption parameters of phosphate onto FSBC |
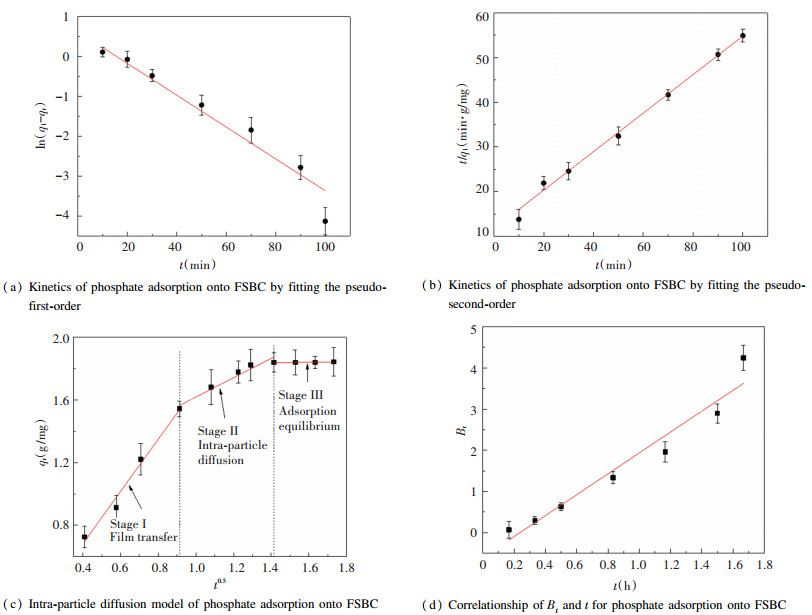
|
Fig.7 Kinetic models of phosphate adsorption onto FSBC |
In order to further reveal the mechanism of phosphate diffusion to FSBC, the intra-particle diffusion model was employed to fit the experimental data. Fig. 7(c) shows that the adsorption diffusion of phosphate onto FSBC exhibited multi-linearity through fitting the plots of qt versus t1/2. It includes three stages: liquid film diffusion, intra-particle diffusion and the adsorptive attachment-equilibrium phase[26, 45-46]. The result indicated that liquid film diffusion or intra-particle diffusion was not the unique rate-limiting step[26, 47-48]. Furthermore, the actual rate-controlling step involved in the phosphate adsorption process had been further analyzed using Eqs.(8)-(9)[49]:
| $ F=1-\frac{6}{\pi^{2}} \exp \left(-B_{t}\right) $ | (8) |
| $ F=q_{t} / q_{\mathrm{e}} $ | (9) |
where F is the fraction of adsorbate adsorbed at different times t; Bt is a mathematical function of F, which can be expressed as Eq.(9), and qt and qe represent the amount adsorbed (mg/g) at any time t and at infinite time.
The Bt value was calculated according to Eq.(10)-(11)[50]:
| $ F(t)>0.85: B_{t}=-0.4977-\ln (1-F(t)) $ | (10) |
| $ F(t) <0.85: B_{t}=\left(\sqrt{\pi}-\sqrt{\pi-\frac{\pi^{2} F(t)}{3}}\right)^{2} $ | (11) |
The Bt values were fitted with t as shown in Fig. 7(d). It can be seen from Fig. 7(d) that the plot Bt versus time is linear but it did not pass through the origin, which indicated that the adsorption rate was mainly controlled by liquid film diffusion[49]. It was also reported that liquid film diffusion was also the main rate-limiting step for the adsorption of tetracycline onto Fe/Zn modified sawdust biochar[36] and Cd (Ⅱ) onto Fennel biomass[50].
3 ConclusionsFSBC was prepared using dewatered waste activated sludge for the removal of phosphate. The specific adsorption capacity of FSBC was 57.8%, which is higher than that of CAC. FSBC worked well in acidic conditions (pH=3-5.35), but its adsorption ability for phosphate could be inhibited when anions coexist. The adsorption of phosphate onto FSBC was dominated by chemical adsorption, and the adsorption rate-limiting step was liquid film diffusion. Electrostatic interaction, metal phosphate precipitation and hydrogen bonding were all involved in the process. The adsorption capacity of FSBC for phosphate decreased by 36.4% after four cycles, but it still had good phosphate removal ability.
| [1] |
Peng G F, Jiang S Q, Wang Y X, et al. Synthesis of Mn/Al double oxygen biochar from dewatered sludge for enhancing phosphate removal. Journal of Cleaner Production, 2020, 251: 119725. DOI:10.1016/j.jclepro.2019.119725 (  0) 0) |
| [2] |
Wang H, Xiao K K, Yang J K, et al. Phosphorus recovery from the liquid phase of anaerobic digestate using biochar derived from iron-rich sludge: a potential phosphorus fertilizer. Water Research, 2020, 174: 115629. DOI:10.1016/j.watres.2020.115629 (  0) 0) |
| [3] |
Drenkova-Tuhtan A, Schneider M, Franzreb M, et al. Pilot-scale removal and recovery of dissolved phosphate from secondary wastewater effluents with reusable ZnFeZr adsorbent @ Fe3O4/SiO2 particles with magnetic harvesting. Water Research, 2017, 109: 77-87. DOI:10.1016/j.watres.2016.11.039 (  0) 0) |
| [4] |
Li B, Udugama I A, Mansouri S S, et al. An exploration of barriers for commercializing phosphorus recovery technologies. Journal of Cleaner Production, 2019, 229: 1342-1354. DOI:10.1016/j.jclepro.2019.05.042 (  0) 0) |
| [5] |
Li S J, Lei T, Jiang F, et al. Tuning the morphology and adsorption capacity of Al-MIL-101 analogues with Fe(3+) for phosphorus removal from water. Journal of Colloid And Interface Science, 2020, 560: 321-329. DOI:10.1016/j.jcis.2019.10.077 (  0) 0) |
| [6] |
Park T, Ampunan V, Maeng S, et al. Application of steel slag coated with sodium hydroxide to enhance precipitation-coagulation for phosphorus removal. Chemosphere, 2017, 167: 91-97. DOI:10.1016/j.chemosphere.2016.09.150 (  0) 0) |
| [7] |
Shepherd J G, Sohi S P, Heal K V. Optimising the recovery and re-use of phosphorus from wastewater effluent for sustainable fertiliser development. Water Research, 2016, 94: 155-165. DOI:10.1016/j.watres.2016.02.038 (  0) 0) |
| [8] |
Andalib M, Nakhla G, Zhu J. High rate biological nutrient removal from high strength wastewater using anaerobic-circulating fluidized bed bioreactor (A-CFBBR). Bioresource Technology, 2012, 118: 526-535. DOI:10.1016/j.biortech.2012.05.068 (  0) 0) |
| [9] |
Luo D C, Yuan L J, Liu L, et al. The mechanism of biological phosphorus removal under anoxic-aerobic alternation condition with starch as sole carbon source and its biochemical pathway. Biochemical Engineering Journal, 2018, 132: 90-99. DOI:10.1016/j.bej.2018.01.007 (  0) 0) |
| [10] |
Caravelli A H, de Gregorio C, Zaritzky N E. Effect of operating conditions on the chemical phosphorus removal using ferric chloride by evaluating orthophosphate precipitation and sedimentation of formed precipitates in batch and continuous systems. Chemical Engineering Journal, 2012, 209: 469-477. DOI:10.1016/j.cej.2012.08.039 (  0) 0) |
| [11] |
Xia W J, Guo L X, Yu L Q, et al. Phosphorus removal from diluted wastewaters using a La/C nanocomposite-doped membrane with adsorption-filtration dual functions. Chemical Engineering Journal, 2021, 405: 126924. DOI:10.1016/j.cej.2020.126924 (  0) 0) |
| [12] |
Lei Y, Saakes M, van der Weijden R D, et al. Electrochemically mediated calcium phosphate precipitation from phosphonates: implications on phosphorus recovery from non-orthophosphate. Water Research, 2020, 169: 115206. DOI:10.1016/j.watres.2019.115206 (  0) 0) |
| [13] |
Chang H, Yoo D, Lim H, et al. Granular crystallization using CFBC ash for phosphorus removal via hydroxyapatite. Environmental Technology & Innovation, 2020, 21: 101173. DOI:10.1016/j.eti.2020.101173 (  0) 0) |
| [14] |
Oginni O, Yakaboylu G A, Singh K, et al. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources. Journal of Environmental Chemical Engineering, 2020, 8(2): 103723. DOI:10.1016/j.jece.2020.103723 (  0) 0) |
| [15] |
Liu X L, Pang H W, Liu X W, et al. Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. The Innovation, 2021, 2(1): 100076. DOI:10.1016/j.xinn.2021.100076 (  0) 0) |
| [16] |
Zhang X N, Lin X Y, He Y, et al. Adsorption of phosphorus from slaughterhouse wastewater by carboxymethyl konjac glucomannan loaded with lanthanum. International Journal of Biological Macromolecules, 2018, 119: 105-115. DOI:10.1016/j.ijbiomac.2018.07.140 (  0) 0) |
| [17] |
Makita Y, Sonoda A, Sugiura Y, et al. Phosphorus removal from model wastewater using lanthanum hydroxide microcapsules with poly(vinyl chloride) shells. Separation and Purification Technology, 2020, 241: 116707. DOI:10.1016/j.seppur.2020.116707 (  0) 0) |
| [18] |
Shi W M, Fu Y W, Jiang W, et al. Enhanced phosphate removal by zeolite loaded with Mg-Al-La ternary (hydr)oxides from aqueous solutions: performance and mechanism. Chemical Engineering Journal, 2019, 357: 33-44. DOI:10.1016/j.cej.2018.08.003 (  0) 0) |
| [19] |
Mahardika D, Park H S, Choo K H. Ferrihydrite-impregnated granular activated carbon (FH@GAC) for efficient phosphorus removal from wastewater secondary effluent. Chemosphere, 2018, 207: 527-533. DOI:10.1016/j.chemosphere.2018.05.124 (  0) 0) |
| [20] |
Han C, Wang Z, Yang W J, et al. Effects of pH on phosphorus removal capacities of basic oxygen furnace slag. Ecological Engineering, 2016, 89: 1-6. DOI:10.1016/j.ecoleng.2016.01.004 (  0) 0) |
| [21] |
Kong L J, Han M, Shih K, et al. Nano-rod Ca-decorated sludge derived carbon for removal of phosphorus. Environmental Pollution, 2018, 233: 698-705. DOI:10.1016/j.envpol.2017.10.099 (  0) 0) |
| [22] |
Yang Q, Wang X L, Luo W, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresource Technology, 2018, 247: 537-544. DOI:10.1016/j.biortech.2017.09.136 (  0) 0) |
| [23] |
Michalekova-Richveisova B, Fristak V, Pipiska M, et al. Iron-impregnated biochars as effective phosphate sorption materials. Environmental Science and Pollution Research International, 2017, 24: 463-475. DOI:10.1007/s11356-016-7820-9 (  0) 0) |
| [24] |
Harijan D K L, Chandra V. Akaganeite nanorods decorated graphene oxide sheets for removal and recovery of aqueous phosphate. Journal of Water Process Engineering, 2017, 19: 120-125. DOI:10.1016/j.jwpe.2017.07.019 (  0) 0) |
| [25] |
Chitrakar R, Tezuka S, Sonoda A, et al. Phosphate adsorption on synthetic goethite and akaganeite. Journal of Colloid and Interface Science, 2006, 298: 602-608. DOI:10.1016/j.jcis.2005.12.054 (  0) 0) |
| [26] |
Ma Y F, Li P, Yang L, et al. Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal. Ecotoxicology and Environmental Safety, 2020, 196: 110550. DOI:10.1016/j.ecoenv.2020.110550 (  0) 0) |
| [27] |
Editorial Board of Water and Waste Water Monitoring and Analysis Method, National Environmental Protection Bureau. Water and Waste Water Monitoring and Analysis Method (Fourth Edition). Beijing: China Environmental Science Press, 2002: 243-249.
(  0) 0) |
| [28] |
Wu C X, Li L F, Zhou H, et al. Effects of chemical modification on physicochemical properties and adsorption behavior of sludge-based activated carbon. Journal of Environmental Sciences, 2021, 100: 340-352. DOI:10.1016/j.jes.2020.08.005 (  0) 0) |
| [29] |
Streit A F M, Côrtes L N, Druzian S P, et al. Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Science of the Total Environment, 2019, 660: 277-287. DOI:10.1016/j.scitotenv.2019.01.027 (  0) 0) |
| [30] |
Bing N C, Zhu X R, Tian Z, et al. Controllable imprinted polymer layer coated silica-gel for S-1-(1-Naphthyl) ethylamine recognition by ATRP. Advanced Materials Research, 2012, 508: 1770. DOI:10.4028/www.scientific.net/AMR.508.237 (  0) 0) |
| [31] |
Qiu M Q, Wang M, Zhao Q Z, et al. XANES and EXAFS investigation of uranium incorporation on nZVI in the presence of phosphate. Chemosphere, 2018, 201: 764-771. DOI:10.1016/j.chemosphere.2018.03.057 (  0) 0) |
| [32] |
Liu H C, Huang J, Zhang P P, et al. Preparation of magnetic fluorescent core-shell-structured nanoparticles and their application in immobilization of glucose oxidase. Proceedings of the 2015 2nd International Conference on Material Engineering and Application (ICMEA 2015).. Piscataway: IEEE, 2015, 560-565. DOI:10.12783/dtmse/icmea2015/7320 (  0) 0) |
| [33] |
Wu W Q. Study on Adsorption Characteristics of Metal Salt Modified Straw for Low Concentration Phosphorus in Water. Guangzhou: South China University of Technology, 2013. 50-54. (in Chinese)
(  0) 0) |
| [34] |
Harijan D K L, Chandra V. Akaganeite nanorods decorated graphene oxide sheets for removal and recovery of aqueous phosphate. Journal of Water Process Engineering, 2017, 19: 120-125. DOI:10.1016/j.jwpe.2017.07.019 (  0) 0) |
| [35] |
Chowdhury S R, Yanful E K. Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. Journal of Environmental Management, 2010, 91(11): 2238-2247. DOI:10.1016/j.jenvman.2010.06.003 (  0) 0) |
| [36] |
Zhou Y Y, Liu X C, Xiang Y J, et al. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: adsorption mechanism and modelling. Bioresource Technology, 2017, 245(A): 266-273. DOI:10.1016/j.biortech.2017.08.178 (  0) 0) |
| [37] |
Chen R, Yang Q, Zhong Y, et al. Sorption of trace levels of bromate by macroporous strong base anion exchange resin: influencing factors, equilibrium isotherms and thermodynamic studies. Desalination, 2014, 344: 306-312. DOI:10.1016/j.desal.2014.04.001 (  0) 0) |
| [38] |
Tang L, Yu J F, Pang Y, et al. Sustainable efficient adsorbent: alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chemical Engineering Journal, 2018, 336: 160-169. DOI:10.1016/j.cej.2017.11.048 (  0) 0) |
| [39] |
Duan S B, Ma W, Pan Y Z, et al. Synthesis of magnetic biochar from iron sludge for the enhancement of Cr (Ⅵ) removal from solution. Journal of the Taiwan Institute of Chemical Engineers, 2017, 80: 835-841. DOI:10.1016/j.jtice.2017.07.002 (  0) 0) |
| [40] |
Wei D N, Li B Y, Luo L, et al. Simultaneous adsorption and oxidation of antimonite onto nano zero-valent iron sludge-based biochar: indispensable role of reactive oxygen species and redox-active moieties. Journal of Hazardous Materials, 2020, 391: 122057. DOI:10.1016/j.jhazmat.2020.122057 (  0) 0) |
| [41] |
Zhao X Q, Dou X M, Mohan D, et al. Antimonate and antimonite adsorption by a polyvinyl alcohol-stabilized granular adsorbent containing nanoscale zero-valent iron. Chemical Engineering Journal, 2014, 247: 250-257. DOI:10.1016/j.cej.2014.02.096 (  0) 0) |
| [42] |
Bedin K C, Martins A C, Cazetta A L, et al. KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chemical Engineering Journal, 2016, 286: 476-484. DOI:10.1016/j.cej.2015.10.099 (  0) 0) |
| [43] |
Hu J, Chen G, Lo I M C. Removal and recovery of Cr(Ⅵ) from wastewater by maghemite nanoparticles. Water Research, 2005, 39(18): 4528-4536. DOI:10.1016/j.watres.2005.05.051 (  0) 0) |
| [44] |
Nuhoglu Y, Malkoc E. Thermodynamic and kinetic studies for environmentaly friendly Ni(Ⅱ) biosorption using waste pomace of olive oil factory. Bioresource Technology, 2009, 100(8): 2375-2380. DOI:10.1016/j.biortech.2008.11.016 (  0) 0) |
| [45] |
Li J, Yu G W, Pan L J, et al. Study of ciprofloxacin removal by biochar obtained from used tea leaves. Journal of Environmental Sciences, 2018, 73: 20-30. DOI:10.1016/j.jes.2017.12.024 (  0) 0) |
| [46] |
Li Y, Taggart M A, McKenzie C, et al. Utilizing low-cost natural waste for the removal of pharmaceuticals from water: mechanisms, isotherms and kinetics at low concentrations. Journal of Cleaner Production, 2019, 227: 88-97. DOI:10.1016/j.jclepro.2019.04.081 (  0) 0) |
| [47] |
Sun Y Y, Li H, Li G C, et al. Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresource Technology, 2016, 217: 239-244. DOI:10.1016/j.biortech.2016.03.047 (  0) 0) |
| [48] |
Wu F C, Tseng R L, Juang R S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chemical Engineering Journal, 2009, 153(1-3): 1-8. DOI:10.1016/j.cej.2009.04.042 (  0) 0) |
| [49] |
Maghsoodloo S, Noroozi B, Haghi A K, et al. Consequence of chitosan treating on the adsorption of humic acid by granular activated carbon. Journal of Hazardous Materials, 2011, 191(1-3): 380-387. DOI:10.1016/j.jhazmat.2011.04.096 (  0) 0) |
| [50] |
Rao R A K, Khan M A, Rehman F. Utilization of fennel biomass (Foeniculum vulgari) a medicinal herb for the biosorption of Cd(Ⅱ) from aqueous phase. Chemical Engineering Journal, 2010, 156: 106-113. DOI:10.1016/j.cej.2009.10.005 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


