Nowadays, water pollution is a global issue. According to the latest United Nations world water development report, there are more than 2 billion people who have limited access to safe drinking water[1]. Studies revealed that the river and air at Kuantan, Malaysia were polluted by bauxite, which consists of high amount of aluminium oxide and ferric oxide, due to mining activities[2]. Study reported by Hafiza et al.[3] showed that heavy metal concentration level in groundwater at Rosob village, Ampar Tenang and Machang exceeded the permissible limit set by Malaysian drinking water quality standard. Well water at Rosob village contains 409.05 ug/L of Mn2+. This is higher than 100 ug/L, which is the permissible limit set by Malaysian Ministry of Health. Recent study by Yunus et al.[4] showed that the coastal sediment in Peninsular Malaysia is contaminated by low, moderate and chronic level of heavy metals comprised of Pb, Hg, Cd, Cu, and Zn.
Among all the pollutants in water, heavy metal such as uranium exhibits high toxicity even at very low concentration level[5]. Heavy metal is non-degradable and persistent in the environment. Due to their high solubility in water, they can easily penetrate living organisms, and eventually accumulate in human body after entering the food chain. Trivial amount of heavy metals consumption such as copper is essential to know in human and animal bodies[6]. However, at higher dose of ingestion, they become threats, thus causing serious health disorders such as stunted growth, cancer, organ damage, damage of nervous system, and fatality[7].
Heavy metal polluted water is mainly generated from industrial activities such as mining, electroplating, wood processing, and painting. With the availability of advance technologies, heavy metals can be removed by chemical precipitation and membrane filtration[6]. However, chemical precipitation consumes large amount of chemical such as lime and oxidants which require physicochemical monitoring of effluent especially pH[7]. Additionally, toxic sludge is also produced[7-8]. Meanwhile, high pressure is required for both reverse osmosis and nanofiltration filtration[9-10]. Bisht et al.[11]reviewed several methods for heavy metals removal and concluded that adsorption is the most popular method due to its low cost, availability, and eco-friendliness.
The use of agricultural wastes for heavy metals gained a lot of attention from researchers lately due to its biodegradable properties, availability in large quantity, and high mechanical strength[12]. Numerous efforts have been done to process the waste to achieve high heavy metals adsorption, as shown in Table 1. Generally, the samples were firstly washed and dried in the oven before treatment and dried again before being pulverized for adsorption use. The findings showed that both acids and alkali treated samples have positive impacts to heavy metal removal[13-20]. However, limited research works have been done to provide alternative treatment process for agricultural waste. Additionally, the potential of other agricultural waste such as banana and dragon fruit peels for Cu2+ removal has not been discovered in a comprehensive manner.
| Table 1 The use of agricultural waste for heavy metal removal |
Thus, the objective of this study is to propose a new approach to treat banana peel by using ZnCl2 prior to alkali and acid treatment for Cu2+ removal and explore the potential of dragon fruit peels as an adsorbent for Cu2+ removal. Seven adsorption isotherms including Linear, Freundlich, Redlich-Peterson, Sips, Toth, Langmuir, and Volmer were studied to describe the adsorption mechanism.
1 Materials and Methods 1.1 MaterialsThe skins of banana and dragon fruit were firstly peeled, cleaned, and cut into small pieces before being dried in the oven for 24 h at 105 ℃. After the drying process, the samples were stored at room temperature. All the samples were pulverized before being used. The images of the dried samples are shown in Fig. 1. Chemicals such as CuCl2, NaOH, and HCl were used without further purification.

|
Fig.1 Images of dried and pulverized banana and dragon fruit peels |
1.2 Chemical Activation of Sample Adsorbents
The dried sample was chemically activated by using ZnCl2[21]. 20 g of dried sample was soaked in 10 g/mL of Zn solution for approximately 2 h. The sample was filtrated and dried in oven for 24 h at 80 ℃. After the drying process, the sample was treated with 0.1 M HCl and 0.1 M NaOH subsequently to remove the residual organic compounds. Then, the sample was washed with hot water until pH 7 was achieved. Lastly, the sample was dried in oven at 80 ℃ before being used for the Cu2+ adsorption experiment. Functional groups of the dried fruit peels were characterized by using Fourier Transform Infrared Spectroscopy (FTIR).
1.3 Batch Adsorption StudiesThe adsorption experiment was conducted at room temperature by dispersing 0.5 g to 1.5 g of dried and pulverized banana (B), dragon fruit (D), chemically activated banana (Act.-B), and dragon fruit (Act.-D) to 100 mL of 10×10-6 Cu2+ solutions. The pH of the solution was initially adjusted to pH 6. The experiment was conducted for 2 h. The sample was filtered using syringe filter (Thermo fisher, 0.22 micron). The concentration of Cu2+ before (Ci) and after (Cf) was measured using Atomic Absorption Spectrometer (Agilent Technologies, Model: 240 AA). The percentage of removed Cu2+ was calculated by using the following equation:
| $ {\rm{ Percentage\;of\;removal }} = \frac{{\left( {{C_{\rm{i}}} - {C_{\rm{f}}}} \right)}}{{{C_{\rm{i}}}}} \times 100\% $ | (1) |
Isotherm study was conducted by repeating the adsorption experiment using 1 g of adsorbent in 10×10-6 - 40×10-6 of Cu2+ solution. In this study, only Act.-B was selected due to the best adsorptive performance. The equilibrium relations for the best adsorbents were identified by fitting the data to isotherms, as shown in Table 2.
| Table 2 List of isotherms adopted in this study |
The equilibrium adsorption was calculated by using Eq. (2) below, where ce is the concentration of Cu2+ at equilibrium point, V is the volume of the sample, and m is the mass of the adsorbent used.
| $ {q_{\rm{e}}} = \frac{{\left( {{c_{\rm{i}}} - {c_{\rm{e}}}} \right)V}}{m} $ | (2) |
The following statistical parameters (Eqs. (3)-(6)) were calculated to verify the best isotherm to present the adsorption study.
| $ {R^2} = \frac{{\sum {{{\left( {{q_{{\rm{mean }}}} - {q_{{\rm{cal}}}}} \right)}^2}} }}{{\sum {{{\left( {{q_{{\rm{cal }}}} - {q_{{\rm{mean }}}}} \right)}^2}} + \sum {{{\left( {{q_{{\rm{cal }}}} - {q_{{\rm{exp }}}}} \right)}^2}} }} $ | (3) |
| $ {\chi ^2} = \frac{{\sum {{{\left( {{q_{\exp }} - {q_{{\rm{cal}}}}} \right)}^2}} }}{{q_{{\rm{cal}}}^2}} $ | (4) |
| $ {\rm{SSE}} = \sum {{{\left( {{q_{\exp }} - {q_{{\rm{cal}}}}} \right)}^2}} $ | (5) |
| $ {\rm{RMSE}} = \sqrt {\frac{1}{{{N_{\exp }}}}\sum {{{\left( {{q_{\exp }} - {q_{{\rm{cal}}}}} \right)}^2}} } $ | (6) |
qexp is the experimental adsorption capacity, qcal and qmean are the calculated and average value of experimental adsorption capacity respectively, Nexp is the number of data points.
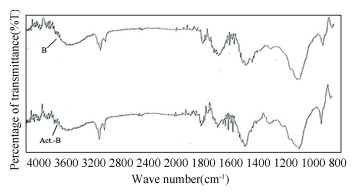
2 Results and Discussion 2.1 FTIRFigs. 2(a) and (b) show the FTIR spectra for both B and Act.-B. The broad band within the range of 3000 cm-1 to 3600 cm-1 and the peak at 3855 cm-1 indicate the existence of hydroxyl group (-OH), which is available in cellulose of fruit peels[22]. The peak around 2920 cm-1 is due to the stretching of alkane group (C-H)[23]. Peak at 1730 cm-1 in Fig. 2(a) is due to the presence of carboxylic acid or ester group (C=O)[23]. Meanwhile, the bands between 1450 cm-1and1700 cm-1 region are usually assigned to the alkene double bond (C=C). Lastly, the peak in the range of 1050 cm-1 to 1450 cm-1 is caused by the presence of C-O bond in carboxylic acid or alcohol group[22]. All the characteristic bands observed in this study is consistent with the FTIR spectra reported by other researchers[23-24]. It is notable that ZnCl2 activation treatment contributed different impact on different fruit peel. ZnCl2 caused the formation of new C=C bonds at 1506.83 cm-1 in Act.-B, while C-H and C=O bonding disappear in Act.-D as shown in Table 3. This finding is similar with the finding reported by Xie et al.[25] where the intensity for C-H and C=O bands was reduced after the activation treatment on the orange peel. This showed the crystallinity of cellulose was affected and destructed in the activation treatment.
|
Fig.2 FTIR spectra for B and Act.-B |
| Table 3 Characteristic adsorptive bands for B, Act.-B, D, and Act.-D |
Generally, intensities of the peaks would increase and slightly shift after the heavy metal adsorption process, especially the O-H, C-O bonding due to the sharing of electron valence[26]. Similar finding was also reported by Lahieb Faisal[27] where the dried banana peels used to adsorb copper showed significant shifts in the bonding between 1300 cm-1 and 1716 cm-1. Thus, it is reasonable to deduce that the copper adsorption would contribute similar impact to the adsorbents in this study.
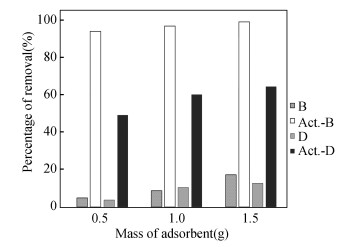
2.2 Adsorption PerformanceFig. 3 shows the adsorptive performance of 4 types of adsorbents on Cu2+ removal. Generally, when the mass of adsorbent increased, the Cu2+ removal rate increased. This is due to higher number of available active site for binding purpose when the mass of adsorbent increased[28]. This can be clearly seen from the adsorption trend of B. The Cu2+ removal increased from 4.92 % to 10.53 % and 12.67 % when the mass of B increased from 0.5 g to 1.0 g and 1.5 g. Similar trend is observed when D and Act.-D were used as the absorbent. However, it is notable that the degree of removal rate was reduced when the mass of D and Act.-D was increased from 1.0 g to 1.5 g. Additional 2% and 4% of Cu2+ removal was achieved when the mass of D and Act.-D was increased by 50%. It is reasonable to deduce that the active sites of the adsorbent were aggregated when the adsorbent was provided in excess[16]. This reduces the number of active binding sites for adsorption purpose.
|
Fig.3 Adsorption performance of B, Act.-B, D, and Act.-D for Cu2+ removal |
This effect is very significant when Act.-B was adopted as the adsorbent. Approximately 3% increment in Cu2+ removal rate was found when the mass was increased from 0.5 g to 1.0 g. This suggested that the optimum dosage to remove 10×10-6 of Cu2+ is 0.5 g of Act.-B, where the final concentration is 0.636×10-6. This meets the discharge limit of Standard B stated in The Environment Quality (Industrial Effluents) Regulation 2009, which is 1 mg/L[29]. However, in order to meet the discharge limit of Standard A (0.2 mg/L), 1.5 g of Act.-B can be used to treat the Cu2+ to 0.128 mg/L.
Fig. 3 also shows that Act.-B and Act.-D performed better in Cu2+ removal compared with untreated B and D. When dried B and D were immersed in ZnCl2 solution, the solution intercalated into the carbon matrix in both B and D. The reaction between ZnCl2 and carbon atoms occurred between the extended interlayers of carbon. Pores were generated within the sample when it was heated in oven[30]. The pores increase the surface area for Cu2+ adsorption, thus explaining the higher Cu2+ removal rate in Act.-B and Act.-D compared with B and D. This is further supported by the finding from Wirasnita et al.[31] where the surface of ZnCl2 activated empty fruit bunch (EFB) for adsorption purpose was 86.62 m2/g, while 9.09 m2/g was reported for untreated EFB. Similar finding was reported by Spagnoli et al.[32] where equilibrium adsorption capacity was 350 mg methylene blue/g for ZnCl2 treated cashew nut shell while approximately 70 mg methylene blue/g was reported for untreated cashew nut shell.
It is notable that Act.-B performed at least 30% better than Act.-D in terms of Cu2+ removal. This is due to the presence of hydroxyl and carboxyl groups in Act.-B, as shown in Fig. 2 and Table 3. Furthermore, only hydroxyl groups are present in Act.-D. The positive impact of oxygen containing functional groups for heavy metals removal is revealed by Yang et al.[33], and it has been proved by Li et al.[34] in Pb2+ removal, Tian et al.[35] in Cr6+, and Tan et al.[36] in Cu2+ and Pb2+.
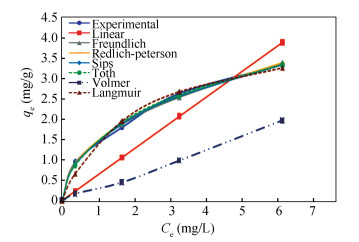
2.3 Adsorption IsothermAdsorption isotherm study was conducted on the best performed adsorbent Act.-B with a maximum qe value of 1872.8 mg/g. The adsorptive capacity of the adsorbents in this study is comparable with the data reported by Hossain et al.[16], Prabu et al.[17] and Feizi and Jalai[19], as shown in Table 1. Fig. 4 shows the predicted and experimental Cu2+ adsorption using Act.-B. Freundlich, Redlich-Peterson, Sips, and Toth models fitted the experimental data well with R2 more than 0.99, as shown in Table 4. Among these models, Freundlich model showed the lowest SSE, χ2, and RMSE values. This indicates heterogeneous adsorption due to the diversity of adsorption sites[23] and multilayer adsorption[37]. Redlich-Peterson and Sips models are the hybrid Freundlich and Langmuir models[38]. These models could be reduced to either Langmuir or Freundlich under specific condition. The condition includes low initial absorbate concentration of feed solution, which reduces Sips model to Freundlich model. This explains the high R2 obtained from Redlich-Peterson and Sips models in this case.
|
Fig.4 Predicted and experimental Cu2+adsorption data |
According to Wang and Hwang[39], layer thickness, layer coverage, effective coverage, deactivated fraction, isosteric heat and equilibrium constants of adsorption are the essential properties of a multilayer adsorption process. These properties are interrelated and it is strongly governed by the surface characteristic of adsorbents, especially the pore size distribution[40]. Attempts such as steam pyrolysis and surface modification could be done to improve the properties of Act.-B in the future work.
| Table 4 Adsorption isotherm constants and model verification |
3 Conclusions
A new approach to treat banana and dragon fruit peels by using ZnCl2 prior to alkali and acid treatment was conducted in this study. Maximum adsorption capacity at equilibrium was reported as 3.4 mg Cu2+/g Act-B and 3.0 mg Cu2+/g Act-D, respectively. This result is slightly better compared with the data reported in the literature. It is notable that Act-B performed at least 30% better than Act-D in terms of Cu2+ removal. This is due to the presence of hydroxyl and carboxyl groups in Act-B. Adsorption isotherm study showed that the experimental data for Act-B fitted well with Freundlich model, compared with the remaining 6 isotherms. This suggested multilayer adsorption, steam pyrolysis, and surface modification of adsorbent could further enhance the adsorption rate. Lastly, the finding of this study also showed that Act-B can be used to treat Cu2+ polluted water up to Malaysia water quality standard.
| [1] |
United Nations. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water, https://www.unwater.org/publications/world-water-development-report-2018/, 2021-03-01.
(  0) 0) |
| [2] |
Abdullah N H, Mohamed N, Sulaiman L, et al. Potential health impacts of Bauxite mining in Kuantan. The Malaysian Journal of Medical Sciences, 2016, 23(3): 1-8. (  0) 0) |
| [3] |
Razak N H A, Praveena S M, Aris A Z, et al. Drinking water studies: a review on heavy metal, application of biomarker and health risk assessment (a special focus in Malaysia). Journal of Epidemiology and Global Health, 2015, 5(4): 297-310. DOI:10.1016/j.jegh.2015.04.003 (  0) 0) |
| [4] |
Yunus K, Zuraidah M A, John A. A review on the accumulation of heavy metals in coastal sediment of Peninsular Malaysia. Ecofeminism and Climate Change, 2020, 1(1): 21-35. DOI:10.1108/EFCC-03-2020-0003 (  0) 0) |
| [5] |
Liu X, Wu J, Zhang S W, et al. Amidoxine-functionalized hollow carbon spheres for efficient removal of uranium from wastewater. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10800-10807. DOI:10.1021/acssuschemeng.9b01616 (  0) 0) |
| [6] |
Sharma S K. Heavy Metals in Water: Presence, Removal and Safety. Cambridge: The Royal Society of Chemistry, 2015, 1-24. (  0) 0) |
| [7] |
Barakat M A. New trends in removing heavy metals from industrial wastewater. Arabian Journal of Chemistry, 2011, 4(4): 361-377. DOI:10.1016/j.arabjc.2010.07.019 (  0) 0) |
| [8] |
Crini G, Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters, 2019, 17: 145-155. DOI:10.1007/s10311-018-0785-9 (  0) 0) |
| [9] |
Mikulasek P, Cuhorka J. Removal of heavy metal ions from aqueous solutions by nanofiltration. Chemical Engineering Transaction, 2016, 47: 379-384. DOI:10.3303/CET1647064 (  0) 0) |
| [10] |
Basaran G, Kavak D, Dizge N, et al. Comparative study for the removal of nickel (Ⅱ) and chromium (VI) heavy metals from metal plating wastewater by two nanofiltration membranes. Desalination and Water Treatment, 2016, 57: 21870-21880. DOI:10.1080/19443994.2015.1127778 (  0) 0) |
| [11] |
Bisht R, Agarwal M, Singh K. Methodologies for removal of heavy metal ions from wastewater: an overview. Interdisciplinary Environmental Review, 2017, 18(2): 124-142. DOI:10.1504/IER.2017.10008828 (  0) 0) |
| [12] |
Rajinipriya M, Nagalakshmaiah M, Rober M, et al. Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 2807-2828. DOI:10.1021/acssuschemeng.7b03437 (  0) 0) |
| [13] |
Tatah V S, Ibrahim K L C, Ezeonu C S, et al. Biosorption kinetics of heavy metals from fertilizer industrial waste water using groundnut husk powder as an adsorbent. Journal of Applied Biotechnology and Bioengineering, 2017, 2(6): 221-228. (  0) 0) |
| [14] |
Ahirwar B, Sharma A K, Sahrma S. Removal of copper from aqueous solution using low cost biosorbent (Potato Peel). International Journal of Applied Research, 2017, 3(9): 459-462. (  0) 0) |
| [15] |
Singha B, Kumar Das S. Removal of Pb (Ⅱ) ions from aqueous solution and industrial effluent using natural biosorbents. Environmental Science and Pollution Research, 2012, 19: 2212-2226. DOI:10.1007/s11356-011-0725-8 (  0) 0) |
| [16] |
Hossain M A, Ngo H H, Guo W S, et al. Removal of copper from water by adsorption onto banana peel as bioadsorbent. International Journal of GEOMATE, 2012, 2: 227-234. DOI:10.21660/2012.4.3c (  0) 0) |
| [17] |
Prabu D, Parthiban R, Senthil Kumar P, et al. Adsorption of copper ions onto nano-scale zero-valent iron impregnated cashew nut shell. Desalination and Water Treatment, 2016, 57: 6487-6502. DOI:10.1080/19443994.2015.1007488 (  0) 0) |
| [18] |
Sharma G, Naushad M. Adsorptive removal of noxious cadmium ions from aqueous medium using actovated carbon/zirconium oxide composite. Isotherm and Kinetic Modelling, 2020, 310: 113025. DOI:10.1016/j.molliq.2020.113025 (  0) 0) |
| [19] |
Feizi M, Jalali M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. Journal of the Taiwan Institute of Chemical Engineers, 2015, 54: 125-136. DOI:10.1016/j.jtice.2015.03.027 (  0) 0) |
| [20] |
Azmi S N H, Al-Balushi M, Al-Siyabi F, et al. Adsorptive removal of Pb (Ⅱ) ions from groundwater samples in Oman using carbonised Phoenix dactylifera seed (Date stone). Journal of King Saud University-Science, 2020, 32: 2931-2938. DOI:10.1016/j.jksus.2020.07.015 (  0) 0) |
| [21] |
Mohanty K, Jha M, Meikap B C, et al. Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed form Terminalia arjuna nuts activated with zinc chloride. Chemical Engineering Science, 2015, 60(11): 3049-3059. DOI:10.1016/j.ces.2004.12.049 (  0) 0) |
| [22] |
Romero-Cano L A, García-Rosero H, Gonzalez-Gutierrez L V, et al. Functionalized adsorbents prepared from fruit peels: equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. Journal of Cleaner Production, 2017, 162(20): 195-204. DOI:10.1016/j.jclepro.2017.06.032 (  0) 0) |
| [23] |
Kamel N A, El-messieh S L A, Saleh N M. Chitosan/banana peel powder nanocomposites for wound dressing application: preparation and characterization. Materials Science and Engineering C, 2017, 72: 543-550. DOI:10.1016/j.msec.2016.11.104 (  0) 0) |
| [24] |
Massimi L, Giuliona A, Astolfi M L, et al. Efficiency evaluation of food waste materials for the removal of metals and metalloids from complex multi-element solutions. Materials, 2018, 11(3): 334. DOI:10.3390/ma11030334 (  0) 0) |
| [25] |
Xie Z G, Guan W, Ji F Y, et al. Production of biological activated carbon from orange peel and landfill leachate subsequent treatment technology. Journal of Chemistry, 2014, 2014: 491912. DOI:10.1155/2014/491912 (  0) 0) |
| [26] |
Kamari A, Shamsudin R, Eljiedi A A A, et al. Treatment of electronics industry effluent using low-cost and commercial adsorbent: a comparative study. Journal of Physics: Conference Series, 2019, 1397: 012022. DOI:10.1088/1742-6596/1397/1/012022 (  0) 0) |
| [27] |
Lahieb Faisal M. Batch sorption of copper (Ⅱ) ions from simulated aqueous solution by banana peel. Al-Khwarizmi Engineering Journal, 2016, 4: 117-125. (  0) 0) |
| [28] |
Pathak P D, Mandavgane S A, Kulkarni B D. Fruit peel waste as a novel low-cost bio adsorbent. Reviews in Chemical Engineering, 2015, 31(4): 361-381. DOI:10.1515/revce-2014-0041 (  0) 0) |
| [29] |
Environmental Quality Act 1974-Environmental Quality (Industrial Effluent) Regulation 2009. http://www.doe.gov.my/portalv1/wp-content/uploads/2015/01/Environmental_Quality_Industrial_Effluent_Regulations_2009_-_P.U.A_434-2009.pdf, 2020-03-20.
(  0) 0) |
| [30] |
Hock P E, Zaini M A A. Activated carbons by zinc chloride activation for dye removal-a commentary. Acta Chimica Slovaca, 2018, 11: 99-106. DOI:10.2478/acs-2018-0015 (  0) 0) |
| [31] |
Wirasnita R, Hadibarata T, Yusoff A R M, et al. Preparation and characterization of activated carbon from oil palm empty fruit bunch wastes using zinc chloride. Jurnal Teknologi, 2015, 74(11): 77-81. DOI:10.11113/jt.v74.4876 (  0) 0) |
| [32] |
Spagnoli A A, Giannakoudakis D A, Bashkova S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: the role of surface and structural parameters. Journal of Molecular Liquids, 2017, 229: 465-471. DOI:10.1016/j.molliq.2016.12.106 (  0) 0) |
| [33] |
Yang X D, Wan Y S, Zheng Y L, et al. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chemical Engineering Journal, 2019, 366: 608-621. DOI:10.1016/j.cej.2019.02.119 (  0) 0) |
| [34] |
Li F F, Wang X L, Yuan T Q, et al. A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb (Ⅱ) removal. Journal of Materials Chemistry A, 2016, 4: 11888-11896. DOI:10.1039/C6TA03779H (  0) 0) |
| [35] |
Tian Z, Yang B, Cui G J, et al. Synthesis of poly (m-phenylenediamine)/iron oxide/acid oxidized multi-wall carbon nanotubes for removal of hexavalent chromium. RSC Advances, 2015, 5: 2266-2275. DOI:10.1039/C4RA10282G (  0) 0) |
| [36] |
Tan M T, Liu X, Li W, et al. Enhancing sorption capacities for copper (Ⅱ) and lead (Ⅱ) under weakly acidic conditions by L-tryptophan-functionalized graphene oxide. Journal of Chemical & Engineering Data, 2015, 60: 1469-1475. DOI:10.1021/acs.jced.5b00015 (  0) 0) |
| [37] |
Bulut Y, Aidin H. A kinetic and thermodynamics study of methylene blue adsorption on wheat shells. Deasalination, 2006, 194(1-3): 259-267. DOI:10.1016/j.desal.2005.10.032 (  0) 0) |
| [38] |
Wang J L, Guo X. Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere, 2020, 258: 127279. DOI:10.1016/j.chemosphere.2020.127279 (  0) 0) |
| [39] |
Wang C H, Hwang B J. A general adsorption isotherm considering multi-layer adsorption and heterogeneity of adsorbent. Chemical Engineering Science, 2000, 55: 4311-4321. DOI:10.1016/S0009-2509(00)00053-1 (  0) 0) |
| [40] |
Giraldo L, Rodriguez-Estupiñán P, Moreno-Piraján J C, et al. Isosteric heat: comparative study between Clausius-Clapeyron, CSK and Adsorption Calorimetry Methods. Processes, 2019, 7: 203. DOI:10.3390/pr7040203 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


