The technique of thermal energy storage (TES) is defined as the ability of the material to collect and release energy in the form of heat, depending on the operating temperature. In a solar energy system, TES material absorbs heat during daytime, and the collected energy is used later when the source of solar irradiation is intermittent or during night time[1]. TES is divided into two categories, sensible heat storage (SHS) and latent heat storage (LHS). SHS is a technique, in which the energy is stored when an interior temperature of the material increases and a reduction in temperature is observed when the energy is released. The performance of SHS depends on the specific heat of material (Cp) and value of change in material's temperature (ΔT). Generally, high Cp and high ΔT are desired for a TES material as a large amount of heat can be collected. LHS is a technique in which the material stores and releases the heat when it is undergoing a change in a physical state under constant temperature condition, where latent heats are concerned[2]. Heat is collected when solid is changed to a liquid phase, and the latent heat associated in this process is named as the heat of fusion.
SHS approach is widely used in oil refining factories, formatting steel factories, chemical compound manufacturers, and energy plants. The water, rock bed, and oil are examples of SHS mediums, where the water is typically widely used as the coolant in a heat exchanger to remove heat[3]. LHS method is used for building application, where the heat is adsorbed by TES materials, such as paraffin wax and fatty acids, to reduce the temperature in the building during day time. During cold night, the heat is released to maintain the desired temperature in the building. Besides, LHS approach is also used in solar energy systems and heat recovery process, where TES materials were integrated with heat exchangers for heating and cooling purpose[4].
Phase change material (PCM), which is also known as LHS material attracted the attention of researchers as it exhibits high energy storage capacity without changing the temperature[5]. PCM is classified into inorganic and organic groups. Salt hydrates and metallic alloys are inorganic PCM that exhibit the high value of phase-change heats and good thermal conductance for thermal energy storage applications. However, phase separation, chemical instability, and supercooling of salt hydrates are the drawbacks for its wide utilization[6]. On the other hand, the metallic alloy has a low-heat capacity and low phase-change heat per unit mass, and high melting temperature[7-8], which limits its utilization as well. In the last decade, extensive research works have been done on organic PCMs that include paraffin wax, fatty acid, and fatty acid mixtures. It is because these PCMs are characterized by congruous melting temperature, no supercooling during the solidification process, and excellent chemical-thermal stability for long-duration utilization[9].
Among the PCMs, fatty acids and their binary and ternary mixtures are considered the most promising thermal storage mediums for various energy storage applications because their thermal properties can be readily manipulated to cater to the need of different heat storage systems[6]. Moreover, the resources of the fatty acids are natural and sustainable, which are derived from animal and vegetable oils, while the paraffin wax is extracted from oil shale rock fragments or petroleum derivatives[10]. However, the low thermal conductivity (λF) of organic PCMs is the main drawback that limits their wide utilization, where a considerable amount of time is required for the charging and discharging process[11]. Table 1 shows the range of λF for fatty acids and their binary and ternary mixtures is within 0.17 to 0.26 W/(m·K), which is lower than that of salt hydrates which is between 0.5 to 1.97 W/(m·K)[12].
| Table 1 Enhancement of the thermal conductivity of fatty acid PCMs by using EG additive |
Several endeavours have been performed to improve the thermal conductivity of fatty acid and fatty mixture based PCMs by using fin type heat exchanger to increase heat transfer rate, adding high heat conductivity additives into the fatty acids mixture, and integrating PCM mixture with porous structure materials which have high thermal conductivity and spacious area of surface. Various type of carbon-based materials with porous structures such as carbon nano-tube (CNT), graphene nano- platelets (GNPs), expanded graphite (EG), graphene aerogel, and 3D graphene sponge (GS) have been applied as a thermal reinforcer to improve the thermal performance of organic PCMs[13-14]. According to the study made by Song et al.[15], EG enhanced the thermal conductivity of fatty acid PCMs significantly compared with carbon nano-tube (CNT) and graphene nano-platelets (GNP) as shown in Table 1, in which λF represents thermal conductivity of fatty acid mixture, λC represents thermal conductivity of fatty acid composite, wt% represents mass ratio of additive. Properties of EG such as high thermal conductivity, excellent chemical stability, low density, and highly porous structure made it an ideal thermal enhancer. Furthermore, it is available at reasonable price.
Throughout last year, new composite PCMs have been formulated by merging EG with fatty acid and their eutectic mixture PCMs. The thermal-physical properties, chemical-thermal stability, the thermal conductivity and thermal reliability of fatty acid composite PCMs were analyzed. The results show a significant enhancement of the thermal conductivity of fatty acid composite PCMs as shown in Table 1. Furthermore, fatty acid composite PCMs exhibit preferable thermal properties and excellent thermal-chemical stability and reliability for long-term utilization in various thermal storage applications.
Palmitic acid (PA) and lauric acid (LA) eutectic mixture is an ideal PCM for low temperature heating applications[22]. This mixture has a melting temperature of almost 37 ℃ and latent heat of fusion of 176 J/g. However, its low thermal conductivity properties limit its utilization. Thus, the purpose of this work is to prepare a new form-stable with PA-LA mixture and EG as thermal conductivity reinforcer. The chemical-thermal stability of this mixture is also investigated to find out the reliability of this mixture for real life application.
1 Materials and Experiments 1.1 MaterialsLauric acid CH3(CH2)10COOH (LA) and Palmitic acid CH3(CH2)14COOH (PA) (98% purity for each, Merck) were used as received. Expandable graphite (treated graphite) with 80 meshes, expansion coefficient of 250 mL/g, and 99% carbon content was purchased from Qingdao Millikun Graphite products Factory, China.
1.2 Preparation of PA-LA Mixture and PA-LA/EG Composite PCMsThe composite PCMs of the PA-LA/EG with varied concentrations of EG (Table 2) were prepared. Fig. 1 shows the procedures of the experiment. Firstly the PA-LA mixture was melted in a thermostatic water bath at 70 ℃, followed by 45 min stirring at 80 ℃ to ensure homogeneous mixing. EG, which was prepared by heating treated graphite in a microwave oven at 750 W for 35 s, was added to the PA-LA mixture. The PA-LA/EG composites was exposed to heat in the oven for 24 h at 70 ℃. Lastly, the solution was cooled at room temperature.
| Table 2 Formulation of pure mixture and composite PCMs |
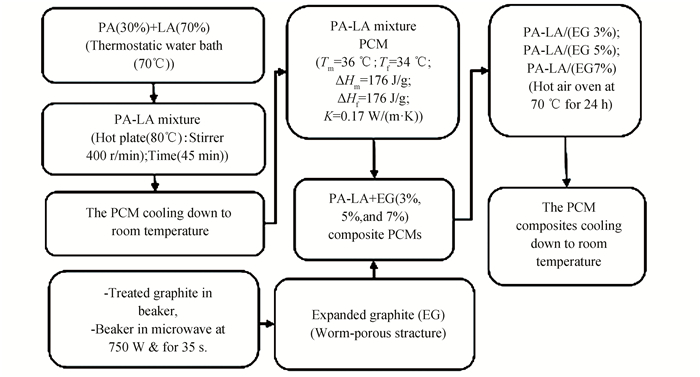
|
Fig.1 Schema of preparation steps of the form-staple composite PCMs |
1.3 Characterization
The latent heat of melting and freezing process along with phase-change temperatures of both pure mixture PA-LA and PA-LA/EG composite PCMs were determined by using differential scanning calorimetry (DSC) (PerkinElmer Jade DSC). All measurements were made at 5.00 ℃/min within the temperature range of 20-70 ℃ under a stable stream of argon gas at a flow rate of 50 mL/min at atmospheric pressure. Melting and freezing temperatures were gained by dropping a line from the peak point to the initial point of the slope phase-change curves, and the latent heats were computed by mathematical integration of the area below the slope of the DSC peak. Morphologies of PA-LA/EG PCM composite, expandable graphite, and EG were analyzed by using a field emission-scanning electron microscope (FE-SEM, Zeiss, MERLIN). Chemical bondings of PA-LA/EG mixture were identified by using Fourier transform infrared spectrometer (FT-IR, IRTracer-100, Shimadzu, Japan). The FT-IR spectra were recorded on a KBr pellet at the frequency range of 4000 cm-1 to 400 cm-1. TEMPOS thermal analyzer was used to measure the thermal conductivities of pure mixture PA-LA and PA-LA/EG composite PCMs at room temperature below the melting and freezing temperature of PCMs. Reliability and chemical-physical stability of composite for long-term applications were conducted by repeating the melting and freezing processes for 360 and 1080 thermal cycles. Thermostatic chamber method was used, where the samples were melted by thermostatic water bath and cooled down to 20 ℃.
2 Results and Discussion 2.1 Thermal Properties of PA and LA Fatty Acid PCMPhase-change temperatures and latent heats of PA and LA were analyzed by using differential scanning calorimetry equipment, where the results are tabulated in Table 3.
| Table 3 Thermal properties of PA and LA fatty acid PCM |
2.2 Predication of Melting Temperature and Latent Melting Heat of Fatty Acid Mixtures
Generally, the phase-change temperature of the binary and ternary fatty acid mixture PCMs used for low-temperature applications is lower than that of their components[23-24]. According to Zhang et al.[25], the melting temperature value (Tm) of the mixture PCMs of fatty acids can be predicted by using the following equation:
| $ T_{\mathrm{m}}=\left[\frac{1}{T_i}-\frac{R \cdot \ln x_i}{\Delta H_i}\right]^{-1} $ | (1) |
where Tm is melting temperature of the fatty acid mixture PCM (K); Ti and ΔHm are the melting temperature and latent heat of fusion, respectively; xi is the mass ratio of the component which has a higher mass fraction in the mixture.
The latent melting heat of the fatty acid mixture (ΔHm) can be computed by the following equation:
| $ \Delta {H_{\rm{m}}} = {T_{\rm{m}}}\sum\limits_{i = 1}^n {\left( {\frac{{\Delta {H_i} \cdot {x_i}}}{{{T_i}}}} \right)} $ | (2) |
The theoretical results show that the melting point and latent heat of the pure mixture of PA-LA fatty acid is 35.4 ℃ and 177.1 J/g, respectively. The diagram of the melting temperature and latent heat curve of the PA-LA mixture versus the mass ratio of the mixture components is shown in Fig. 2.
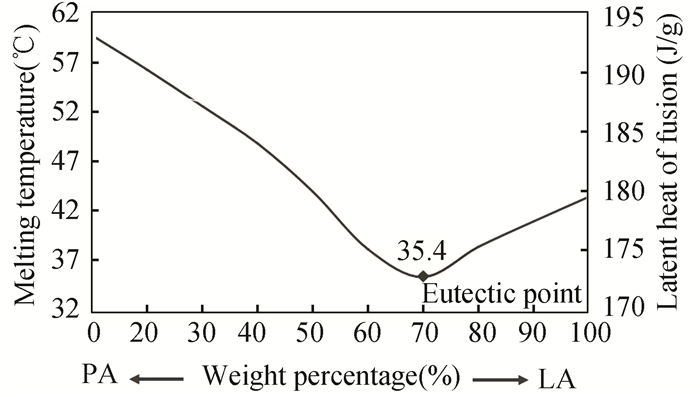
|
Fig.2 Theorical results of melting temperature of PA-LA mixture PCM |
2.3 Thermal Conductivity Enhancement of Composite PCMs
The thermal conductivity of the pure mixture was measured as shown in Fig. 3. Without the presence of EG, the thermal conductivity of the pure mixture was 0.17 W/(m·K). When the mass ratio of 3 wt %, 5 wt %, and 7 wt % of EG was added into the pure mixture, the thermal conductivity values increased to 0.42, 1.19, and 1.68 W/(m·K), respectively.

|
Fig.3 Thermal conductivity of PA-LA mixture and PA-LA/EG composites |
Generally, intermolecular forces and thermal motion of molecules of the fatty acid mixture are the main parameters that significantly influence the thermal effectiveness of the heat transfer between molecules of the mixture. Due to the ability of EG to absorb the mixture, the number of the molecules of the mixture are replaced by the EG molecules that have higher thermal conductivity. Therefore, intermolecular forces and thermal movement of composite molecules increased, which leads to accelerating the heat transfer process from one region to another in the composite[26-27]. This range of thermal conductivity values is higher compared with the commercial grade PCM, RT 35 where the heat conductivity was 0.2 W/(m·K)[28]. Additionally, according to the list of commercially available PCMs compiled by Whiffen and Rfiffa[29] and Cabeza et al[30]. in their review papers, the range of thermal conductivity was within 0.43 to 1.48 W/(m·K).
According to Fig. 3, PA-LA/ (7% EG) composite PCM shows highest thermal conductivity, but PA-LA/ (5% EG) composite PCM is considered to be the optimum dosage for practical application. This is because the sample of the PA-LA/(3% EG) composite showed a little agglomeration of EG additive. As for the PA-LA/(7% EG) composite, its thermal conductivity was highly increased, which results in an extreme decline in its latent melting heat. Thus, the rest of the study focuses only on the properties of the optimum PA-LA/(5% EG) composite PCM.
2.4 Morphology and Compatibility of Form-Stable Composite PCMFig. 4 shows the FE-SEM images of the treated graphite, EG, and form-stable of the composite PCM. Fig. 4(a) of treated graphite exhibits the form of graphite crystal structure which consists of numerous symmetrical layers of graphene.Figs. 4 (b-c) are the FE-SEM images of EG illustrating that the EG has porous structure which looks like a worm. This porous structure is characterized by a huge active surface area of graphite. The numerous pores of EG structure play a big role in enhancing the homogeneity between the pure mixture and EG. This sort of structure allows the melted PA-LA mixture to easily penetrate inside its pores, which helps to obtain a coherent composite PCM[30]. Fig. 4(d) of the composite PCM reveals that the pure mixture was uniformly distributed into the pore structure of EG.

|
Fig.4 FE-SEM images of treated graphite (a), expanded graphite (b-c) and PA-LA/EG composite (d) |
The structural analysis of composite PCM were conducted by FT-IR spectroscopy to study the chemical conformity between the pure mixture and EG. Fig. 5 shows the FT-IR spectra of the pure mixture and composite PCMs. The stretching and bending vibration of -OH group has been presented by the peak 3280 cm-1, while the asymmetric vibration of -CH3 and -CH2 groups have been presented by peaks 2916-2854 cm-1. The peaks within 1700 cm-1 - 1580 cm-1 are the distinctive adsorption bands for the stretching vibration of C=O. The - CH2 bending has been represented by the peak of 1442 cm-1, while C-H and C-C bending by the peak of 1271 cm-1. These are the characteristic bonds for an aliphatic chain of PA-LA mixture[17]. PA-LA/EG mixture shows similar adsorption bands as PA-LA mixture. The absence of new bonding or missing adsorption bands shows that the mixture is homogeneous and exhibits excellent inter-element chemical compatibility. This finding also explains the well-connected two-phase interfaces of PA-LA and EG, as shown in FE-SEM image Fig. 4(d). It may be a result of the high penetrating capacity of the pure mixture into the porous surface of EG[31].

|
Fig.5 FT-IR spectra of the pure mixture and composite PCM |
2.5 Thermal Properties and Reliability of Form-Stable Composite PCM
Curves of DSC results of the pure mixture and the composite PCM have been illustrated in Fig. 6. Also, the values of phase-change temperatures and heats of the pure mixture and composite PCM have been tabulated in Table 4. The temperatures of the pure mixture during the melting and solidification process are 36.76 ℃ and 34.29 ℃, respectively. Meanwhile, PA-LA/EG composite PCM melted at 35.53 ℃ and froze at 34.84 ℃. It is notable that the presence of EG reduced by approximately 1 ℃ of the melting points of the pure mixture. This result is consistent with the result reported by Zhang et al.[32] and Sari and Karaipekli[33], which put forward that slight changes in phase transition temperatures will occur when the appealing interaction between PCM and the internal surface of the porous supporting material is not strong enough.

|
Fig.6 DSC curves of pure mixture and composites PCM before and after thermalcycling test |
| Table 4 Thermal properties of pure mixture and form-stable composites PCM before and after thermal reliability analysis |
The latent melting heat of both pure mixture and composite PCM are fallen within the range from 176 to 174 J/g, respectively, as shown in Table 4. This data is comparable with the commercially available PCM, RT 35 where the reported latent melting heat is 157 J/g and Tm= 35 ℃[28, 34]. It is notable that the change of latent heats is minimum after 360 and 1080 thermal cycles. Additionally, slight changes in phase-change temperatures are observed after 360-1080 thermal cycles. Transition temperatures during melting and solidification process changed by - 0.37 ℃ and - 0.55 ℃ after 360 thermal cycles. Meanwhile, a small change of 0.20 ℃ and - 0.34 ℃ for melting and freezing temperatures were observed in 1080 thermal cycles. This result indicated that the composite PCM exhibited good thermal reliability and stability for long-term usage in low-temperature thermal storage application[35].
3 ConclusionsA new form-stable PA-LA composite with 5 wt % of EG was successfully prepared with thermal conductivity of 1.19 W/ (m·K), melting temperature of ~35 ℃, and latent melting heat of 175 J/g. The 360-1080 thermal cycle study proved that an excellent thermal stability and reliability of the mixture is achievable for long-term usage due to the negligible changes in the melting point, freezing point, and latent melting heat. Good chemical compatibility was observed from the FTIR spectra, where no formation of new bonding or absence of the existing adsorption bands was observed. This finding also explains the well-connected two-phase interfaces of PA-LA and EG in the SEM images. Therefore, PA-LA/EG is the potential alternative PCM for TES in solar heat space, overheat treatment systems, and other energy building systems.
| [1] |
Farid M M, Khudhair A M, Razack S A K, et al. A review on phase change energy storage: materials and applications. Energy Conversion and Management, 2004, 45(9-10): 1597-1615. DOI:10.1016/j.enconman.2003.09.015 (  0) 0) |
| [2] |
Akeiber H, Nejat P, Abd. Majid M Z, et al. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renewable and Sustainable Energy Reviews, 2016, 60: 1470-1497. DOI:10.1016/j.rser.2016.03.036 (  0) 0) |
| [3] |
Pugh S J, Hewitt G F, Müller-Steinhagen H. Fouling during the use of seawater as coolant-the development of a 'user guide'. Heat Transfer Engineering, 2005, 26(1): 35-43. DOI:10.1080/01457630590890148 (  0) 0) |
| [4] |
Hasnain S M. Review on sustainable thermal energy storage technologies, Part Ⅰ: heat storage materials and techniques. Energy Conversion and Management, 1998, 39(11): 1127-1138. DOI:10.1016/S0196-8904(98)00025-9 (  0) 0) |
| [5] |
Kalnæs S E, Jelle B P. Phase change materials for building applications: a state-of-the-art review and future research opportunities. Energy and Buildings, 2015, 94: 150-176. DOI:10.1016/j.enbuild.2015.02.023 (  0) 0) |
| [6] |
Kenisarin M, Mahkamov K. Solar energy storage using phase change materials. Renewable and Sustainable Energy Reviews, 2007, 11(9): 1913-1965. DOI:10.1016/j.rser.2006.05.005 (  0) 0) |
| [7] |
Sharma A, Tyagi V V, Chen C R, et al. Review on thermal energy storage with phase change materials and applications. Renewable and Sustainable Energy Reviews, 2009, 318-345. DOI:10.1016/j.rser.2007.10.005 (  0) 0) |
| [8] |
Pielichowska K, Pielichowski K. Phase change materials for thermal energy storage. Progress in Materials Science, 2014, 65: 67-123. DOI:10.1016/j.pmatsci.2014.03.005 (  0) 0) |
| [9] |
Sharma R K, Ganesan P, Tyagi V V, et al. Developments in organic solid-liquid phase change materials and their applications in thermal energy storage. Energy Conversion and Management, 2015, 95: 193-228. DOI:10.1016/j.enconman.2015.01.084 (  0) 0) |
| [10] |
Singh S, Gaikwad K K, Lee Y S. Phase change materials for advanced cooling packaging. Environmental Chemistry Letters, 2018, 16(3): 845-859. DOI:10.1007/s10311-018-0726-7 (  0) 0) |
| [11] |
Tao Y B, He Y L. A review of phase change material and performance enhancement method for latent heat storage system. Renewable and Sustainable Energy Reviews, 2018, 93: 245-259. DOI:10.1016/j.rser.2018.05.028 (  0) 0) |
| [12] |
Xie N, Huang Z W, Luo Z G, et al. Inorganic salt hydrate for thermal energy storage. Applied Sciences, 2017, 7(12): 1317. DOI:10.3390/app7121317 (  0) 0) |
| [13] |
Yang X J, Yuan Y P, Zhang N, et al. Preparation and properties of myristic-palmitic-stearic acid/expanded graphite composites as phase change materials for energy storage. Solar Energy, 2014, 99: 259-266. DOI:10.1016/j.solener.2013.11.021 (  0) 0) |
| [14] |
Chen T, Liu C, Mu P, et al. Fatty amines/graphene sponge form-stable phase change material composites with exceptionally high loading rates and energy density for thermal energy storage. Chemical Engineering Journal, 2020, 382: 122831. DOI:10.1016/j.cej.2019.122831 (  0) 0) |
| [15] |
Song Y L, Zhang N, Yuan Y P, et al. Prediction of the solid effective thermal conductivity of fatty acid/carbon material composite phase change materials based on fractal theory. Energy, 2018, 170: 752-762. DOI:10.1016/j.energy.2018.12.162 (  0) 0) |
| [16] |
Li C C, Zhang B, Xie B S, et al. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustainable Cities and Society, 2019, 44: 458-464. DOI:10.1016/j.scs.2018.10.041 (  0) 0) |
| [17] |
Zhang N, Yuan Y P, Du Y X, et al. Preparation and properties of palmitic-stearic acid eutectic mixture/expanded graphite composite as phase change material for energy storage. Energy, 2014, 78: 950-956. DOI:10.1016/j.energy.2014.10.092 (  0) 0) |
| [18] |
Zhu H, Zhang P, Meng Z N, et al. Thermal characterization of lauric-stearic acid/expanded graphite eutectic mixture as phase change materials. Journal of Nanoscience and Nanotechnology, 2015, 15(4): 3288-3294. DOI:10.1166/jnn.2015.9659 (  0) 0) |
| [19] |
Yuan J W, Liu Y C, Zhou Y H, et al. Palmitic acid-stearic acid/expanded graphite as form-stable composite phase-change material for latent heat thermal energy storage. Journal of Nanomaterials, 2020, 1648080. DOI:10.1155/2020/1648080 (  0) 0) |
| [20] |
Zhou D Y, Yuan J W, Zhou Y H, et al. Preparation and properties of capric-myristic acid/expanded graphite composite phase change materials for latent heat thermal energy storage. Energies, 2020, 13(10): 2462. DOI:10.3390/en13102462 (  0) 0) |
| [21] |
Zhang N, Yuan Y P, Wang X, et al. Preparation and characterization of lauric-myristic-palmitic acid ternary eutectic mixtures/expanded graphite composite phase change material for thermal energy. Chemical Engineering Journal, 2013, 231: 214-219. DOI:10.1016/j.cej.2013.07.008 (  0) 0) |
| [22] |
Tuncbileka K, Saria A, Tarhanb S, et al. Lauric and palmitic acids eutectic mixture as latent heat storage material for low temperature heating applications. Energy, 2005, 30(5): 677-692. DOI:10.1016/j.energy.2004.05.017 (  0) 0) |
| [23] |
Yuan Y P, Tao W Q, Cao X L, et al. Theoretic prediction of melting temperature and latent heat for a fatty acid eutectic mixture. Journal of Chemical & Engineering Data, 2011, 56(6): 2889-2891. DOI:10.1021/je200057j (  0) 0) |
| [24] |
Yuan J W, Liu Y C, Luo X Z, et al. Preparation and performance of capric-myristic acid binary eutectic mixtures for latent heat thermal energy storages. Journal of Nanomaterials, 2019, 1-9. DOI:10.1155/2019/2094767 (  0) 0) |
| [25] |
Zhang Y P, Su Y H, Ge X S. Prediction of the melting temperature and the fusion heat of (quasi-)eutectic PCM. Journal of China University of Science and Technology, 1995, 25(4): 474-478. (  0) 0) |
| [26] |
Raza G, Shi Y M, Deng Y. Expanded graphite as thermal conductivity enhancer for paraffin wax being used in thermal energy storage systems. Proceedings of 13th International Bhurban Conference on Applied Sciences and Technology (IBCAST). Piscataway: IEEE, 2016. DOI: 10.1109/IBCAST.2016.7429846.
(  0) 0) |
| [27] |
Zhao Y J, Min X, Huang Z H, et al. Honeycomb-like structured biological porous carbon encapsulating PEG: a shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy and Buildings, 2018, 158: 1049-1062. DOI:10.1016/j.enbuild.2017.10.078 (  0) 0) |
| [28] |
Rubitherm Technologies GmbH. Technisches Datenblatt RT35. https://www.rubitherm.eu/media/products/datasheets/Techdata_-RT35_DE_09102020.PDF, 2020-12-12.
(  0) 0) |
| [29] |
Whiffen T R, Riffat S B. A review of PCM technology for thermal energy storage in the built environment: Part I. International Journal of Low-Carbon Technologies, 2012, 8(3): 147-158. DOI:10.1093/ijlct/cts021 (  0) 0) |
| [30] |
Cabeza L F, Castell A, Barreneche C, et al. Materials used as PCM in thermal energy storage in buildings: a review. Renewable and Sustainable Energy Reviews, 2011, 15(3): 1675-1695. DOI:10.1016/j.rser.2010.11.018 (  0) 0) |
| [31] |
Wi S, Li T X, Yan T, et al. High-performance form-stable expanded graphite/stearic acid composite phase change material for modular thermal energy storage. International Journal of Heat and Mass Transfer, 2016, 102: 733-744. DOI:10.1016/j.ijheatmasstransfer.2016.06.066 (  0) 0) |
| [32] |
Zhang D, Tian S L, Xiao D Y. Experimental study on the phase change behaviour of phase change material confined in pores. Solar Energy, 2007, 81(5): 653-660. DOI:10.1016/j.solener.2006.08.010 (  0) 0) |
| [33] |
Sarı A, Karaipekli A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Solar Energy Materials & Solar Cells, 2009, 93(5): 571-576. DOI:10.1016/j.solmat.2008.11.057 (  0) 0) |
| [34] |
Sharma S D, Sagara K. Latent heat storage materials and systems: a review. International Journal of Green Energy, 2005, 2(1): 1-56. DOI:10.1081/GE-200051299 (  0) 0) |
| [35] |
Zhang X G, Huang Z H, Yin Z Y, et al. Form stable composite phase change materials from palmitic-lauric acid eutectic mixture and carbonized abandoned rice: preparation, characterization, and thermal. Energy and Buildings, 2017, 154: 46-54. DOI:10.1016/j.enbuild.2017.08.057 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


