With the development of scientific technology and clinical research, the structure modification and function optimization of biomedical materials have attracted much attention[1-2]. Therefore, biomedical materials get broader applications and have greater commercial value[3]. Alginate exhibits various outstanding characteristics, including biocompatibility, biodegradablity, hygroscopicity, oxygen permeability, and nontoxicity due to its diverse array of β-D-mannuronic acid residue (M) and α-L-guluronic acid residue (G) in its out-of-order molecular chain, which have been used in biomedicine, especially as medical dressings[4-5]. On the other hand, medical alginate fibers obviously lack antibacterial activity, which is unable to obstruct and kill bacteria effectively[6-7]. In previous studies about medical dressings, nano silver, nano-TiO2 and nano-ZnO have been utilized as antibacterial agents. However, nanosilver is mildly toxic as a result of multiple-transformations reactions[8-9], the antibacterial activity of nano-TiO2 is influenced by photocatalysis[10], and the antibacterial mechanism of nano-ZnO is still ambiguous[11]. Therefore, it is imperative to develop new antibacterial dressings, which have higher antibacterial activity and are safer.
The binding ability of various metal ions to alginate varies substantially because of the significantly different stereochemical structures and are in the order of Pb2+ > Cu2+ > Cd2+ > Ba2+ > Sr2+ > Ca2+ > Co2+ = Ni2+ = Zn2+ > Mn2+ according to the work of Qin[12] and Haug et al.[13] The ion-exchange capacity of the same metal ions on the alginate with different arrangements of M and G diversifies markedly[14]. The binding ability between Ca2+ and alginate is moderate[15], which makes it safer and easily form fibers gel by ion exchange. One promising strategy is to load chlorhexidine gluconate (CHG), a broad-spectrum antibacterial drug, in the manufacture of medical calcium alginate fibers. CHG is a commonly used disinfectant in the medical field at home and abroad. Selecting CHG as an antimicrobial agent can avoid antibiotic resistance and do no harm to the body cells. CHG loaded on calcium alginate fibers can be released through medium slowly and continuously to extend the medicinal time and inhibit wound infection.
Molecular docking is a theoretical simulation method to study the interactions between molecules ligands and receptors and to predict their binding patterns and affinity using Discovery Studio (DS), a professional molecular simulation software[16]. Controlled drug release is essential for drug delivery. Loaded drugs work only if they are released and reach the acting site through a medium, such as gel or solution[17-18]. As one of the best choices for drug-loaded dressings matrix, alginate fibers has been applied extensively to the high-tech wound dressing because alginate dressings can absorb wound exudate and form a stable hydrogel gel, which makes controlled drug release realizable[19-22].
To have a deep understanding of the fibrou structure and related properties, DS was implemented to simulate the internal morphology of CHG-loaded alginate fibers, and combined with specific experimental data to understand the combination mode of drug and fibers matrix, deeply analyze the release form of drug, and understand the structural change of CHG-loaded alginate fibers in the solvent state. The lowest energy of drug-binding state, the aqueous solvation modelling, standard dynamics cascade and feature mapping of CHG-loaded alginate fibers were predicted by DS. Furthermore, based on the previous performance characterization of alginate fibers loaded with CHG within our study, the cytotoxicity and drug release behavior of alginate fibers loaded with CHG were investigated to evaluate the further potential application in wound dressings.
1 Materials and Methods 1.1 MaterialsL-929 mouse fibroblast cells were purchased from Stem Cell Bank, Chinese Academy of Sciences; CHG solution (20 wt%) was purchased from Dasheng Pharmaceutical Technology Co., Ltd., Xi'an, China; all chemicals were purchased from Sinopharm Group Chemical Reagent Co., Ltd. in China.
All CHG-loaded fibers were fabricated by industrial wet spinning device. The concentration of spinning dope was 3.3% (w/v), and the mass ratios of chlorhexidine gluconat to CaCl2 coagulation were 0%, 0.3% and 0.9% in the manufacture to command the loading capacity of CHG into alginate fibers. A constant rate through spinneret (100 holes) at room temperature was set to extrude the spinning solutions into CaCl2 coagulation solution. The actual concentration of CHG-loaded alginate fibers were 0, 22.97 and 79.00 mg/g, and named as 1#, 2# and 3# fibers in this paper, which were detemined by High Performance Liquid Chromatography (HPLC).
1.2 Standard Dynamics CascadeThe energy changes of alginate molecules with diverse array of M and G were analyzed during the whole simulation process of molecular dynamics using standard dynamics cascade. Standard dynamics cascade can perform a set of minimization and equalization steps and then implement molecular dynamics using CHARMM. The parameters include automatic minmization, minmization 2, heating, equilbration, production, implict soluvial model, nonbond list radius and electrostaics. Among them, the initial and aimed temperatures are 50 ℃and 30 ℃, the storage interval is 2 ps, and the whole simulation time is 24 ps.
1.3 Drug Release and SolvationSample fibers (0.01 g) and media solution (PBS or A solution, 10 mL) were added in 15 mL centrifuge tube before shaking at (37.0 ± 0.5) ℃ for 120 min. 3 mL media solution was taken from the centrifuge tube and measured at 258 nm UV light at 5 fixed time points continuously. The concentration was calculated and calibrated using the standard curve. 3 mL fresh media solution was supplemented immediately. All centrifuge tubes remain shaking during the test, and all samples were determined in triplicate. The formula for calculating the cumulative release amount (CRM) of drug-loaded alginate fibers is as follows:
| $ {\mathop{\rm CRM}\nolimits} (\mu {\rm{g}}) = 10{C_n} + \sum\limits_{i = 1}^{n = 1} 3 {C_i} $ | (1) |
where Cn is the concentration of CHG measured for the n-th fixed time, Ci is the concentration of CHG measured before the i-th fixed time, 10 and 3 are the volume of the dielectric liquid respectively.
Preparation of solution A: 2.09 g sodium chloride and 0.06926 g calcium chloride was dissolved in water together, then fixed to 250 mL.
In addition, to simulate the state change of CHG-loaded alginate fibers in aqueous solvent, solvation is used CHARMm to add a system to the water molecule to simulate and observe the state change of target substance in aqueous solvent. There is an explicit periodic boundary with orthorhombic crystal in the related parameter settings. The minimum distance from boundary was 7.0, and the radius of sphere was 20.0. The added counterions were Na+ and Cl- with concentration of 0.145. The algorithm of minimization adopted basis NR.
1.4 Degradation Behavior of CHG-Loaded Alginate FibersThe degradation behavior of 1#, 2# and 3# fibers was observed by sealing sample fibers (0.02 g) in A solution or PBS (5 mL) at (25.0±1.0) ℃ for one week. Morphological changes of soaked fibers were recorded.
1.5 Cytotoxicity on L-929 Cells by MTT MethodThe cell growth curve was plotted ahead of time to find out the optimal concentration for cell suspensions. The extracting concentration of three fibers was prepared at 1 g/L using DMEM as the medium, then diluted gradiently in DMEM at 10%, 8%, 6%, 4% and 2% v/v ratio. The cytotoxicity of CHG-loaded alginate fiber was determined by MTT method. DMEM containing 10% FBS, 5% DMSO and pure CHG solution was set as control group.
The relative growth rate of cells was calculated via the following equation:
| $ \begin{array}{l} {\rm{Relative}}\;{\rm{growth}}\;{\rm{rate}} = \\ \frac{{{\rm{O}}{{\rm{D}}_{450}}{\rm{ \;of \;experimental\; group }}}}{{{\rm{O}}{{\rm{D}}_{450}}{\rm{\; of \;black \;group }}}} \times 100\% \end{array} $ | (2) |
Cell toxicity grading was rated by relative growth rate according to GB/T16886.5. Level 0 and 1 are considered to satisfy the criteria. Specifically, the relative growth rate and corresponding cell toxicity level are as follows: ≥100%, level 0; 75%-99%, level 1; 50%-74%, level 2; 25%-49%, level 3; 1%-24%, level 4; when 0%, level 5.
1.6 Statistical AnalysisSPSS 17.0 was used for statistical analysis. ANOVA was used to evaluate the significance of the differences between experimental groups. A statistically significant difference was set at p < 0.05, and the data were expressed as X ±S.
2 Results and Discussion 2.1 Standard Dynamics CascadeAs shown in Fig. 1, alginate molecular bond regions were simplified into -G-G-G-(polyG), -G-G-M-(polyGM) and -M-M-M-(polyM) according to the irregular linking of G and M residues and bonding difference between drug molecules and G and M residues during the simulation. The bonding gross between drug molecules and polyG was the tightest because of the "egg-box" structure formed by G residue and metal ions. Due to the bonding between metal ions and multi-G residue, the effect of drug molecules on the spatial conformation of polyG was much weaker than that of the other two regions. In addition to the pattern of residue arrangement of alginate itself, metal ions can enhance or weaken a property or function performed by the material.
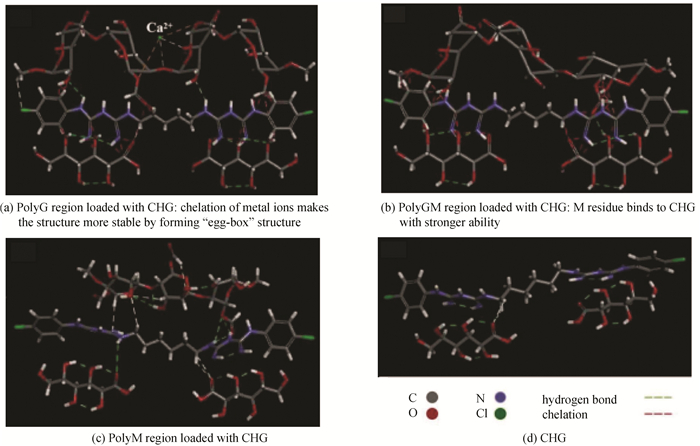
|
Fig.1 Conformation optimization by DS |
According to Fig. 2, the temperature changing range of the polyG is the largest, followed by polyGM and polyM. It indicated that alginate matrix with polyG is more suitable for research and development of thermos-sensitive tissue engineering products. As abrupt temperature increases, the polyG has the lowest torsion energy, and polyG releases the most energy during the cascade process, suggesting that the structure stability of multi-G region is the strongest. In the alginate molecular structure analysis, multi-G region has higher G/M value (the ratio of the number of G to M in alginate molecular chain) with the rising rigidity and the elasticity declining of alginate material[23]. Furthermore, polyG is chelated with Ca2+ to form "egg-box" structure, which enhances the intermolecular force dramatically[24]. Therefore, the dense multi-G regions enhance the binding forces and synergistic effect among different alginate molecular chains.

|
Fig.2 Temperature and energy changed by processing standard dynamics cascade |
2.2 Drug Release and Solvation
The solvent effect is helpful to investigate the dissolution and reaction process of compounds. In Fig. 3, 740, 697, and 739 water molecules were respectively added to polyG, polyGM and polyM as solvent molecules. It clearly shows that the molecular structures of all regions are substantially divergent in different solutions. Analysis indicates CHARMm energy, potential energy and van der Waals energy of all regions slumped upon solvation. For the neutral solute molecule, the heterolysis of the covalent bond can lead to the separation of charge to increase the polarity of the solvent, which will reduce the energy of the transition state and the activation energy of the reaction[25]. Therefore, it will be easier for the water molecules in the solvent to form hydrogen bonds with the hydroxyl, carboxyl or oxygen atoms in alginate molecules and destruct alginate daughter structures.

|
Fig.3 Effect of solvation on drug release |
Na+ and K+ in PBS or A solution swap out Ca2+ of "egg-box structure" by breaking fibrous structure to enhance the water absorption ability[26], then the bound hydroxyl group would be released again. The trend of CHG-loaded alginate fibers releasing drugs was roughly consistent, but was more rapid in PBS solution at a certain degree of instantaneous release. In vitro drug released curves indicated that the fibrous structure was more easily destroyed in PBS solution, and the degree damaged was more complete as shown in Fig. 4. The drug release increased significantly with the increase of the amount of loaded drug. The drug release rate of 2# fibers in PBS was 28.5%, which was much lower than 92% in A solution. The drug release rate of 3# fibers in PBS was 53%, slightly higher than 47.6% in A solution. These results revealed that degradation and release behaviors of sample fibers in PBS are distinct from those in A solution. Apparently, it is imperative to manage wound complications legitimately and apply different types of dressings correctly.
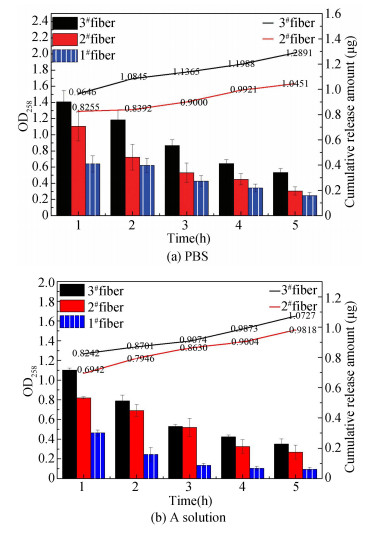
|
Fig.4 Drug release behavior of CHG-loaded fibers |
2.3 Degradation Behavior of CHG-Loaded Fibers
Controlled released drug is an essential function of excellent drug delivery system. Loaded drug works only if they are released from the system and reaches the acting site through a medium, such as gel or solution. The drug-loaded alginate fibers showed different degradation behaviors in the degradation experiment of CHG-loaded alginate fibers in Fig. 5 because of the various ion concentrations of PBS and A solution. Alginate fibers absorbed water and swelled, and was affected by metal ions, which increased the molecular gap of alginate and weakened the intermolecular entanglement. CHG-loaded alginate fibers released drugs easily in PBS solution with higher ion concentration.

|
Fig.5 Degradation behavior of CHG-loaded fibers |
This result can be analyzed and compared with the results of drug release. CHG-loaded fibers was swelled and broken into a large number of fine fibrous segments in PBS, but drug molecules were trapped in fibrous structure due to unformed hydrogels. The hydrogel formed after 2# fibers was swelled in A solution for a while, and fibrous structure was almost opened completely with a large number of drug molecules released successfully. However, fibrous structure becomes more compact with the increasing amount of CHG loaded, which makes it more difficult to form hydrogels. 3# fibers still had relatively kept fibrous morphology after being sealed for 148 h, which indicated that the appropriate concentration of loaded CHG can alleviate the swelling and fracture of alginate fibers in PBS and A solution because of its glucose molecule, especially in A solution.
2.4 Cytotoxicity on L-929 Cells by MTTCytotoxicity experiment is recognized as one of the basic index to evaluate biocompatibility in medical biology evaluation system[27-28], whose results reflect the effect of materials on the basic structure or physiological process of cells[29].
Based on the widespread applications in numerous medical aspects of CHG, such as hand sanitizer, medical disinfectant, and plaster, the security and feasibility of practical applications support its loading into alginate fibers as a drug model. MTT (Cell Counting Kit-8) method was adopted in this study and the results were demonstrated in Table 1. Compared with control groups, the experimental groups exhibited excellent biocompatibility to promote cell proliferation with 100% greater relative growth rates. Mostly, the relative growth rates of CHG-loaded fibers were lower than that of pure alginate fibers. When the concentration of the 2# fibers group was less than 4%, the relative growth rates were higher than that of the 1# fibers group. This finding displays that CHG at low concentration is conducive to cell proliferation but inhibits cell growth at high concentration. Notably, these results are consistent with that of TOPKAT simulation via DS, which, however, is not described in detail in this paper due to the straightforward results of TOPKAT.
| Table 1 Absorbance values, relative proliferation rate and cell toxicity grading of 24 h cultivated L-929 cells in leaching solution of 1# fibers, 2# fibers and 3# fibers at different concentrations (p < 0.05) |
3 Conclusions
Based on the fact that the composition of natural polymers varies from batch to batch, investigating the energy state and solvation at the molecular level can help to study how the two residues react to the macroscopic properties of alginate at the molecular level, such as staffness, brittleness, hygroscopicity, and solubility, and the appropriate batch of natural alginate materials can be selected according to the varied application. In this paper, the degradation, drug release behavior and cytotoxicity of CHG-loaded alginate fibers were further studied, and DS software was run to simulate the solvation effect of the fibers and the energy change of drug molecule loaded to alginate matrix.
According to the previous results of this experiment[30], CHG existed stably on the alginate fibers through hydrogen bond or other interaction, which was proved by relative characterization analysis. Then, CHG-loaded alginate fibers exhibited more excellent hygroscopicity than pure alginate fibers, inhibited the bioactivity of bacteria, especially gram-positive bacteria like S. aureus. It illustrates uprightly that CHG-loaded alginate fibers can be used as clinical dressings. With research and development, technology combining medical materials matrix with drug molecular, protein, growth factor and other characteristic substances will play a greater role in the field of biomedicine.
| [1] |
Ghalei S, Nourmohammadi J, Solouk A, et al. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressings application. Colloids and Surfaces B: Biointerfaces, 2018, 172: 82-89. DOI:10.1016/j.colsurfb.2018.08.028 (  0) 0) |
| [2] |
Hu W W, Ting J C. Gene immobilization on alginate/polycaprolactone fibers through electrophoretic deposition to promote in situ transfection efficiency and biocompatibility. International Journal of Biological Macromolecules, 2019, 121: 1337-1345. DOI:10.1016/j.ijbiomac.2018.09.043 (  0) 0) |
| [3] |
Hench L L, Polak J M. Third-generation biomedical materials. Science, 2002, 295(5557): 1014-1017. DOI:10.1126/science.1067404 (  0) 0) |
| [4] |
Martinsen A, Skjåk-Bræk G, Smidsrød O. Alginate as immobilization material: Ⅰ. Correlation between chemical and physical properties of alginate gel beads. Biotechnology and Bioengineering, 1989, 33: 79-89. DOI:10.1002/bit.260330111 (  0) 0) |
| [5] |
Sood A, Granick M S, Tomaselli N L. Wound dressings and comparative effectiveness data. Advances in Wound Care, 2014, 3(8): 511-529. DOI:10.1089/wound.2012.0401 (  0) 0) |
| [6] |
Li Y Q, Zhu B W, Li Y G, et al. A synergistic capture strategy for enhanced detection and elimination of bacteria. Angewandte Chemie International Edition, 2014, 53(23): 5837-5841. DOI:10.1002/anie.201310135 (  0) 0) |
| [7] |
Zhou J, Yao D Y, Qian Z Y, et al. Bacteria-responsive intelligent wound dressing: simultaneous in situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials, 2018, 161: 11-23. DOI:10.1016/j.biomaterials.2018.01.024 (  0) 0) |
| [8] |
de Souza T A J, Rodrigues L R R, Franchi L P, et al. Silver nanoparticles: an integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicology and Environmental Safety, 2019, 171: 691-700. DOI:10.1016/j.ecoenv.2018.12.095 (  0) 0) |
| [9] |
Scherer M D, Sposito J C V, Falco W F, et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: a close analysis of particle size dependence. Science of the Total Environment, 2019, 660: 459-467. DOI:10.1016/j.scitotenv.2018.12.444 (  0) 0) |
| [10] |
Montazer M, Behzadnia A, Pakdel E, et al. Photo induced silver on nano titanium dioxide as an enhanced antimicrobial agent for wool. Journal of Photochemistry and Photobiology B Biology, 2011, 103: 207-214. DOI:10.1016/j.jphotobiol.2011.03.009 (  0) 0) |
| [11] |
Jin G W, Kim J Y, Min B G. Superhydrophobic and antibacterial properties of cotton fabrics treated with pvdf and nano-zno through phase inversion process. Fibers and Polymers, 2018, 19: 1835-1842. DOI:10.1007/s12221-018-8313-x (  0) 0) |
| [12] |
Qin Y. Gel swelling properties of alginate fibers. Journal of Applied Polymer Science, 2004, 91: 1641-1645. DOI:10.1002/app.13317 (  0) 0) |
| [13] |
Haug A, Smidsrd O, Larsen B, et al. The Effect of divalent metals on the properties of alginate solutions. Ⅱ. Comparison of different metal ions. Acta Chemica Scandinavica, 1995, 19: 341-351. (  0) 0) |
| [14] |
Kołodyńska1 D, Gęca1 M, Skwarek E, et al. Titania-coated silica alone and modified by sodium alginate as sorbents for heavy metal ions. Nanoscale Research Letters, 2018, 13(1): 1-12. DOI:10.1186/s11671-018-2512-7 (  0) 0) |
| [15] |
Donati I, Holtan S, Mrch Y A, et al. New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules, 2005, 6(2): 1031-1040. DOI:10.1021/bm049306e (  0) 0) |
| [16] |
Huang Y, Chen C, Zhang Z Y, et al. Comparison of autodock vina and discovery studio in virtual screening for antibiotic resistance protein inhibitors. China Journal of Bioinformatics, 2012, 10(4): 248-253. DOI:10.3969/j.issn.1672-5565.2012.04.05 (  0) 0) |
| [17] |
Qiao L Y, Liu C D, Liu C, et al. Self-healing alginate hydrogel based on dynamic acylhydrazone and multiple hydrogen bonds. Journal of Materials Science, 2019, 54(10): 8814-8828. DOI:10.1007/s10853-019-03483-y (  0) 0) |
| [18] |
Zhang H X, Lu L, Li L H, et al. Alginate-based self-healing and pH-responsive hydrogels formed by dynamic covalent bonding. Acta Polymerica Sinica, 2016, 3: 368-374. DOI:10.11777/j.issn1000-3304.2016.15211 (  0) 0) |
| [19] |
Thomas S. Alginate dressings in surgery and wound management: Part 2. Journal of Wound Care, 2000, 9: 115-119. (  0) 0) |
| [20] |
Kheirabadi N R, Tabrizi N S, Sangpour P. Removal of nitrate from water by alginate-derived carbon aerogel modified by protonated cross-linked chitosan. Journal of Polymers and the Environment, 2019, 27: 1642-1652. DOI:10.1007/s10924-019-01458-3 (  0) 0) |
| [21] |
Selmi T A S, Verdonk P, Chambat P, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel. Journal of Bone and Joint Surgery-British Volume, 2008, 90B(5): 597-604. DOI:10.1302/0301-620X.90B5.20360 (  0) 0) |
| [22] |
Karadaǧ E, Öztürk Z D K, Vzüm Ö B, et al. Swelling performance studies of acrylamide/potassium 3-sulfopropyl methacrylate/sodium alginate/bentonite biohybrid sorbent hydrogels in binary mixtures of water-solvent. Journal of Encapsulation and Adsorption Sciences, 2019, 9(1): 35-61. DOI:10.4236/jeas.2019.91003 (  0) 0) |
| [23] |
Lee K Y, Mooney D J. Alginate: properties and biomedical applications. Progress in Polymer Science, 2012, 37: 106-126. DOI:10.1016/j.progpolymsci.2011.06.003 (  0) 0) |
| [24] |
Dechojarassri D, Omote S, Nishida K, et al. Preparation of alginate fibers coagulated by calcium chloride or sulfuric acid: application to the adsorption of Sr2+. Journal of Hazardous Materials, 2018, 355: 154-161. DOI:10.1016/j.jhazmat.2018.05.027 (  0) 0) |
| [25] |
Eisenberg D, McLachlan A D. Solvation energy in protein folding and binding. Nature, 1986, 319(6050): 199-203. DOI:10.1038/319199a0 (  0) 0) |
| [26] |
Li D H, Lv C X, Liu L, et al. Egg-Box structure in cobalt alginate: a new approach to multifunctional hierarchical mesoporous N-Doped carbon nanofibers for efficient catalysis and energy storage. ACS Central Science, 2015, 1(5): 261-269. DOI:10.1021/acscentsci.5b00191 (  0) 0) |
| [27] |
Gomes M E, Reis R L, Cunha A M, et al. Cytocompatibility and response of osteblastic-like cells to starch-based polymers: effect of several additives and processing conditions. Biomaterials, 2001, 22: 1911-1917. DOI:10.1016/S0142-9612(00)00377-X (  0) 0) |
| [28] |
International Organization for Standardization (ISO). Biological evaluation of medical devices-Part 5: Test for in vitro cytotoxicity. Geneva, Switzerland: ISO, 2009.
(  0) 0) |
| [29] |
Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved loads: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplantation, 2013, 49(4): 469-476. DOI:10.1038/bmt.2013.152 (  0) 0) |
| [30] |
Shi S S, Lei S J, Fu C X. Studies of the properties of CHG-Loaded alginate fibers for medical application. Polymer Testing, 2019, 83: 106141. DOI:10.1016/j.polymertesting.2019.106141 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


