2. State Key Laboratory of Fluorinated Functional Membrane Materials, Zibo 256401, Shandong, China
With human consumption of the traditional fossil fuel and an increased sense of environment protection, research applied to regenerated energy conversion and storage is being promoted. During the last few years, fuel cells have been extensively investigated[1-4], which are considered to be the key technology with regard to energy saving, zero pollution emissions, and flexible use. In addition to practical processing and used material, there are two critical techniques prior to fuel cell use[5-7]. One is the slow rate of reaction in this system, especially the reduction of oxygen, which leads to low electricity and power levels. The other is that the used fuel like hydrogen cannot be obtained easily. To solve these issues, various fuel cell types would be developed. There are important differences between the various fuel cells, such as the working temperature, the fuel as well as the electrolyte. Typically, the fuel cells would be sorted into the following 5 types according to the used electrolyte[8]: alkaline fuel cells (AFCs), PEMFCs, phosphoric acid fuel cells (PAFCs), molten carbonate fuel cells (MCFCs), and solid oxide fuel cells (SOFCs). For AFCs, PAFCs, and MCFCs, their competitiveness and commercial viability are greatly reduced due to the highly corrosive electrolyte. Comparatively, PEMFCs and SOFCs are considered to be more reliable and feasible energy transformation technologies in the future[9].
PEMFCs use polymer membranes that normally contain negatively charged groups like -SO32-, -COO-, and -PO32- as electrolyte. Considering the different operating conditions, PEMFC is divided into LT-PEMFC (below 100 ℃), which works well at room temperature with an optimal operating temperature of approximately 80 ℃ and HT-PEMFC (up to 200 ℃). This fuel cell type has capabilities of high-power output and fast start-up/shut down cycles[9]. It is therefore particularly suitable for the clean power generation of transport vehicles. Since the LT-PEMFC runs at a relatively low operating temperature, the chemical reaction rate in system, especially the oxygen reduction at the cathode becomes the main concern in the research and development of PEMFCs. It requires the presence of an effective catalyst such as platinum. Compared with LT-PEMFC, the reaction rate is substantially improved with increasing working temperature in HT-PEMFC. Thus much less precious metal catalysts are consumed. However, HT-PEMFC is not superior to LT-PEMFC. It suffers from a few problems like insufficient durability in acidic high temperature environment. In fact, both technologies have the advantages and drawbacks in relation to different application scenarios. Previously, a few papers have reviewed the HT-PEMs[10-15], parts of which give special attention to PBI-based membranes[10, 13-15], while this paper aims to introduce recent research progress of PBI and some other membranes fabricated without the use of perfluorosulfonic acid (PFSA).
1 Perfluorosulfonic Acid Membrane for Proton Exchange Membrane Fuel CellsPEMs are crucial materials in the PEMFC system. The standard membranes in this field refer to sulfonated perfluorinated polymers including NafionⓇ (Chemours) and the following products with pendant side chains of different lengths, such as Gore-selectⓇ (Gore), AquivionⓇ (Solvay), AciplexTM(Asahi Kasei), FlemionⓇ(AGC), BAMⓇ(Ballard), and DF260 (Dongyue Group). As a well-developed polymer membrane for PEMFC, NafionⓇ (Scheme 1) is commercially available as Nafion 11x or Nafion 21x, with the membrane thickness to be x multiplied by 25.4 μm (1 mil), respectively.
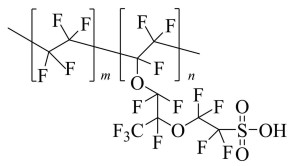
|
Scheme 1 Chemical structure of NafionⓇ |
Despite the superior chemical resistance, thermal stability, durability, and mechanical property under harsh conditions, shortcomings remain in NafionⓇ and become the stumbling stock for further extension. It is known that the production process for PFSA-based proton exchange membrane is complicated and expensive. Take NafionⓇas an example. The derivative is firstly prepared by the copolymerization of tetrafluoroethylene and perfluoro(alkyl vinyl ether) derivatives with sulphonylurea. Then, the resulting product is extruded into film, hydrolysed with a hot basic solution, and finally protonated by acid treatment[16-17]. Production costs of the membrane account for 10%-15% of the total cost in fuel cell system. PFSA membranes conduct protons by the co-work of sulfonic acid groups and water, meaning that behaviors of NafionⓇ rely heavily on its moisture content. When operating above 80 ℃[18-19] or under low humidity, NafionⓇ will lose water, causing a rapid reduction of the conductivity. So the membrane should be continuously humidified, which increases the complexity of equipment. In addition, the relatively low operating temperature of NafionⓇ has curbed further improvement of fuel cell performance and full utilization of residual heat. One method to this problem is the development of self-humidifying PEMs. Hence, a series of composite membranes have been developed. In this method, hydrophilic materials are normally added to NafionⓇ, which are generally organic[20] or inorganic fillers (i.e., SiO2[21-22], TiO2[23-24]) and heteropolyacids[25-28]. The high water retention property of these compounds allows a stronger interaction between polymer membranes and water molecules, avoiding membrane dehydration at elevated temperatures. With this approach, NafionⓇ-based membranes should be able to realize effective operation at an extended temperature range up to 120 ℃. Nevertheless, water performs a vital role in proton transfer for this type of membranes. Even if the humidifier exists, it restricts further improvement on the using temperature.
An alternative is the use of non-aqueous, low volatility acids[29-30] instead of water as the proton solvent. Thus acid doped basic composite membranes are developed, which can be applied well in the surrounding of higher temperature from 120 to 200 ℃[31] and bring a range of advantages to the H2/O2 fuel cell: accelerated electrode kinetics, no water management necessary, and utilization of exhaust heat from the chemical reactions. It is to be observed that the tolerance of the system to CO, which poisons platinum catalysts through strong adsorption[32], raises from 0.002 mol% below 100 ℃ to 3.000 mol% above 160 ℃[33]. That means with the increase of working temperature, high purity H2 as the fuel is no longer essential in PEMFC. Instead, a direct use of H2 produced by liquid alcohol fuels reforming (e.g., methanol) for PEMFC without CO removal is possible[34]. This concept enables a simpler and more cost-effective fuel cell system. Containing structural units like quaternary amine groups[35] or N-heterocyclic compounds, the typical structures for HT-PEMFC such as imidazole[36-37], pyridine[38], and tetrazole[39] possess the acid binding ability. Among those the polymer membranes made from non-PFSA materials could be a potential candidate.
2 PBI-Based Polymer Electrolyte MembranesDiscovered in the late 1960s by DuPont[40], heterocyclic polybenzimidazole (PBI) is a thermoplastic polymer with outstanding mechanical properties and a glass transition temperature of over 425 ℃[41]. Later, PBI products with different forms have been employed in various fields like the flame retardant material, the high temperature resistance adhesive, the high-performance fiber, and the sealing component in high temperature corrosion[42]. Until early 1991, when the researchers from Case Western Reserve University tried to produce a PEM that operates at about 200 ℃ to enhance the activity and stability of catalyst for direct methanol fuel cells, Hoel and Grunwald's work[43] reporting proton conductivity of PBI films was noticed. After that several PBI variants including poly(2, 2'-m-(phenylene)-5, 5'-bibenzimidazole) (mPBI), poly(2, 2'-p-(phenylene)-5, 5'-bibenzimidazole) (pPBI), poly(2, 5-benzimidazole) (ABPBI), and poly(2, 2'-(2, 5-dihydroxy-1, 4-phenylene) 5, 5'-bibenzimidazole)) (2OH-PBI) have been produced to study their impact on the membrane performances. Among the most common types of PBI family, ABPBI has the simplest configuration with the easily obtained raw material and relatively simple synthesizing procedure. However, mPBI has attracted considerable attention due to the good thermal stability and processability. It is also the only commercial PBI that was developed by Hoechst Celanese firstly. In comparison, pPBI shows a closer polymer chain packing with a relative high degree of crystallinity[44] and ABPBI encounters the same problem[45], which leads to a poor solvent-solubility of both structures and accompanying difficulties in membrane preparation. The fabrication methods of PBI were reviewed by Cong et al.[46] in detail. Based on the polycondensation of carboxylic acid and diamines (or their derivatives), there are normally two ways to fabricate PBI membranes. In the first methodology, a PBI containing solution (e.g., in DMAc) is employed to prepare the membrane using solvent evaporation, which is followed by the subsequent immersion in phosphoric acid (PA) to get access to a good conductivity. In the second case, the polycondensation of carboxylic acid with diamines is carried out in polyphosphoric acid (PPA) and the obtained mixture could be used directly for subsequent membrane casting. Doping with PA is not required in this procedure because the PPA can hydrolyze into PA. Accompanied by a sol-gel reaction, the PA content in manufactured PBI membrane can be as high as 95 wt%[47]. Scheme 2 illustrates the traditional synthesis methods for three common PBIs.
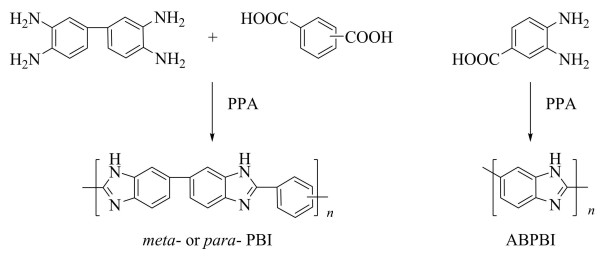
|
Scheme 2 Synthesis of m -or p-PBI[48] (Reprinted with permission from Ref. [48], Copyright 2005, American Chemical Society) and ABPBI in PPA |
It is known that PBI has chemical and thermal stability with a long-term working temperature of up to 300 ℃[49]. In the past decades, PBI was widely investigated and recognized as the most promising material for HT-PEMFC because of acceptable electrochemical performances and stability at high temperature[50]. The commercial PBI membranes under the trademark CeltecⓇ (BASF) have been available onto the market since 2005. The corresponding membrane electrode assemble that allows an operating temperature from 120 to 180 ℃ is especially suited for HT-PEMFC.
2.1 Phosphoric Acid Doped PBI MembranesPure PBI films suffer from low proton conductivity (about 10-9 mS/cm[51]) resulting from the insufficient conduction media. To increase the proton conductivity, high-boiling and low-volatile proton conductor should be imported in the structure. Since PBI appears alkaline (pKa = 5.5[52]), in practical application, the bonds -NH- and -N= in the N- heterocycles of PBIs not only serve as proton donor and acceptor, but also provide acid-base binding sites for the acids to further enhance the proton conducting capacity. As dopants a series of acids have been investigated, including H3PO4[53], H2SO4[54], HClO4[55], HNO3[55] and HCl[56]. The result indicates the mPBI conductivity with an acid doping concentration of more than 11 mol/L reduces in sequences of H2SO4 > H3PO4 > HClO4 > HNO3 > HCl[55]. Bouchet and Siebert[57] studied the interaction between PBI and the acids by infrared spectroscopy. It is evident that H2SO4 or H3PO4 in doped membranes would autoionize in varying degree, and the formed anions connect the protonated PBI via strong hydrogen bonds. For PBI/xH2SO4 system (x represents acid mole per repeating unit of PBI), the anion mainly exists in the form of SO42- at a relative low doping level of sulfuric acid (x < 0.6), while HSO4- is dominant with 0.6 < x < 1.5. Notably, the doped membranes' conductivity is rather low in the first instance and shows a significant trend for an increase with the appearance of HSO4-. For H3PO4 (PA) doped PBI, H2PO4- is the main anion in the whole range of acid doping concentration. The resulting conductivity is positively correlated with the number of acid and increases steadily. Thus, it can be concluded that the presence of anions, which can serve as both proton acceptor and proton donor, offers a key to ensure the high conductivity for acid doped membranes. According to the results, both acids can be used as dopants for PBI, yet the high vapor pressure of H2SO4[58] seems to be a drawback that hinders the further application more or less. By comparison, PA doped PBI[59-61] is always preferred as electrolyte for HT-PEMFC due to its low vapor pressure, outstanding thermal property, and excellent conductivity in low-humidity circumstance[62].Owning to the immobilization of PA in PBI matrix, humidity has a minimal impact on this membrane. The relevant mechanism of proton transfer through intermolecular hydrogen bonds is shown in Scheme 3.
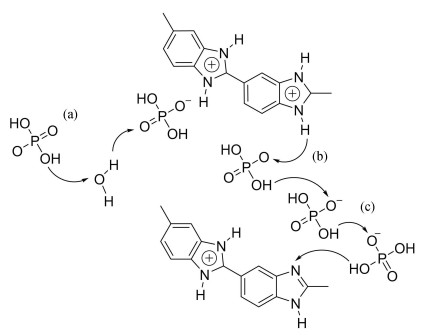
|
Scheme 3 Proton conduction mechanism in PA doped PBI membranes (a) Proton transfer between water and PA; (b) Proton transfer between PAs; (c) Proton transfer between PA and PBI[63] (Reprinted with permission from Ref. [63], Copyright 2010, Royal Society of Chemistry) |
To achieve good proton conductivity, a high PA doping level is required for synthesized HT-PEMs. In this case, van der Waals force between the polymer main chains will decrease substantially when large amounts of free PA gather around macromolecular chains, causing mechanical stability of PEMs to degrade. The loss of PA in long-time running is another critical problem that affects the application of HT-PEMs. Plenty of free PA molecules in the membrane will migrate to one side of the membrane under the action of electronic filed due to their weak interaction with the polymer chains. Furthermore, different chemical potentials may be formed inside and outside the membrane by water produced at the cathode in running fuel cells, which leads to the outward diffusion of free PA. The combination of above factors has an enormous influence on the characteristics of polymer membranes. To extend the service life of PBI-based membranes in fuel cell system, effective strategies to guarantee their stability at high doping level and PA retention are desirable.
2.2 Cross-Linked PBI MembranesCross-linking is an import technological process for polymer modification, which is often adapted in rubber reinforcement and equally applicable for PEM. With enhanced cross-linking, hydrolysis of the polymer membrane is accordingly weakened, thus effectively reducing the polymer decomposition under actual working conditions. Simultaneously, the mechanical strength, chemical resistance, and dimensional stability of the membrane increase. This method is normally divided into covalent and ionic cross-linking. In 1977, Celanese[64] patented the method to form covalently cross-linked PBIs with halides containing two or more functional groups, which provided a basis for the following relevant studies. Accordingly, these researches refer to an amide-type linkage generated between the imidazole moiety in PBI and low-molecular compounds or halomethylated arylene polymers[65]. As low-molecular cross-linkers, 1, 3-bis(2, 3-epoxypropoxy)-2, 2-dimethylpropan[66], divinylsulfone[67], multifunctional triglycidylisocyanurate[68], 1, 3, 5-tris(bromomethyl)-benzene, and 1, 3, 5-tris(bromomethyl)-2, 4, 6-triethylbenzene[69] have been applied for this use, while the covalent cross-linking could be also generated via alkylation of N-H group in PBI with macromolecular compounds such as chloromethylated polysulfone[70] and bromomethylated polyetherketone[71-72]. Ionical cross-linking of PBIs refers to flexible acid-base blend membranes obtained by mixing PBI with an acid polymer, which commonly belongs to polymeric sulfonic[73-74] or phosphoric acids[75-76].
Compared with the pristine PBI membrane, cross-linked PBI membranes display remarkable improvements on relevant functions. Wang et al.[71] produced a series of macromolecular cross-linked PBIs with enhanced mechanical and thermal stability via using bromomethylated poly(aryl ether ketone) (BrPAEK) as cross-linker. Özdemir et al.[77] prepared modified PBI by using various cross-linkers such as bisphenol A diglycidyl ether, ethylene glycol diglycidyl ether, α-α'-dibromo-p-xylene, and terephthalaldehyde. It was confirmed that the cross-linking of PBI contributed to the membrane acid retention properties. A conductivity of up to 151 mS/cm was observed among these membranes. Further, the sample peaked at 123 mW/cm2 without humidification. Outside of the above-mentioned benefits, there are some points that should be noticed in adopting this technology. By using of ionical cross-linking, polymer precipitates easily due to the simultaneous dissolution of the acidic and the basic polymers in a common solvent, resulting in difficult processing for the target membranes.
In addition, excessive cross-linking degree means a decline of adsorption sites for PA in PBI, which depresses the proton conductivity eventually. Actually, the structure and properties of the adopted cross-linker have always influenced over the characteristics of cross-linked membranes. It is found that cross-linkers with long soft chains are more beneficial to the membranes' mechanical stability than short ones[78]. Experiments proved that long cross-linking chains are formed for modified PBI membranes by using benzoxazines as cross-linker[79]. These membranes exhibit enhanced acid doping level, better mechanical and chemical stability compared with PBI membranes. However, the decomposition temperature is reduced. Another example is the imidazole containing cross-linkers, which are demonstrated to be beneficial for the acid uptake of PBI due to the affinity for PA molecules[80-81]. Since each of the available cross-linkers has its own advantages, the suitable one should be chosen carefully based on actual need. In fact, thermal curing is another valid cross-linking method for PBI without the participation of nitrogen atoms[82], although the mechanism has not been completely understood. For instance, chemically highly stable aromatic sulfones can be obtained by a short heat treatment, which is related to the thermally activated Friedel-Crafts reaction of the sulfonic groups and the phenyl groups[83]. Yang[78] and Krishnan et al.[84] demonstrated that thermal curing offers possibility for the transformation from an ionically crosslinked blend of PBI and a sulfonated polymer into a covalently crosslinked system. This step was carried out in accordance with the route described in Scheme 4.
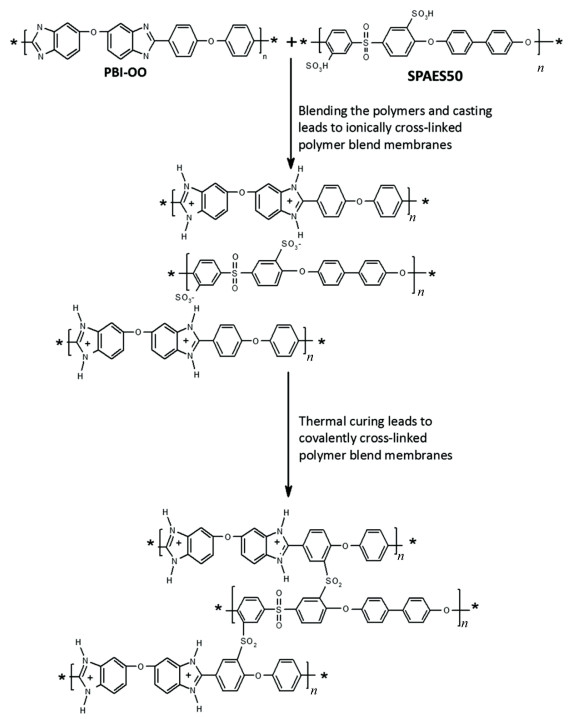
|
Scheme 4 Formation of covalently cross-linked PBI/sulfonated polysulfone[83] (Reprinted with permission from Ref. [83], Copyright 2016 Royal Society of Chemistry) |
By using poly(2, 2'-(p-phenylene-2-sulfonic acid)-5, 5'-bibenzimidazole) (MS-p-PBI, Scheme 5), the above showed procedure was simplified. It is thought that the protons in sulfonic acid can reduce unnecessary consumption of phosphoric acid molecules which have strong attraction with PBI. A 44% higher conductivity (214 mS/cm under 5% relative humidity at 160 ℃) with 63% lower voltage decay rate in fuel cell than pure m-PBI[47] was observed for cross-linked MS-p-PBI.
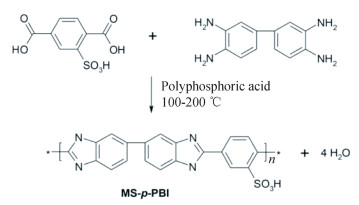
|
Scheme 5 Formation of MS-p-PBI[47] (Reprinted with permission from Ref. [47], Copyright 2019 Elsevier) |
Rational dimensional change of polymer membranes can be ensured by cross-linking. However, there are several problems for cross-linked membranes, including the decrease of proton conductivity and difficulty in accurate control of the cross-linking degree. To reduce the loss of proton conductivity caused by cross-linking, it should be avoided that PA absorption sites work as the junction points in future research of cross-linked membrane, and how to fabricate the membranes with controllable cross-linking is a valuable research issue.
2.3 PBI-Based Composite MembranesThe application of composite membranes is an effective way to retain the original advantages of relevant raw materials. As one of the main strategies to improve the functional stability of PBIs, this approach designs composite structure by combining PBI with organic or inorganic additives to raise the holding acid capacity and steadiness of PBI-based membrane under elevated temperature. Experiments indicated that the performance of PA doped PBI could be further enhanced by incorporation of individual dispersed fillers such as SiO2[85-86], TiO2[87-88], or Fe2TiO5[89], which could prevent the acid leaching due to their strong hydrogen-bond donor capability to acids, leading to improved durability of the membranes in PEMFCs. Further, for composite membranes, the compatibility between PBI and fillers is required, and the additives should be well distributed in the composite material to the most extent. The preparation procedure is related to a homogeneously dispersing of fillers within PBI substrate, which would be usually achieved through either direct addition in a PBI contained solution[80] or in-situ sol-gel process[90]. Chemically functionalized inorganic substances as fillers have been studied as well. Angioni et al.[91] reported composite membranes with the addition of mesoporous silica-based hybrids or functionalized hybrids, modified by different units, such as -SO3H, -NH2, or amphoteric SO3H-NH2 to PBI. In comparison with neat PBI, composite membranes display higher PA doping level and conductivity (90 mS/cm) under the condition of 30% relative humidity above 100 ℃. This value was not upgraded further for the samples with functionalized fillers, which could be induced by the interaction between these fillers and PA. Improved performance for PBI composite membranes was demonstrated by Lee et al.[92] using proton conductive sulfophenylated titanium oxide (s-TiO2) particles as fillers. A highest proton conductivity (96 mS/cm) was observed for the PA doped PBI/s-TiO2 at 150 ℃, which is more significant than PBI and PBI/TiO2 membranes[93]. The fuel cell test revealed that the peak power density of PBI/s-TiO2 membranes arrived 621 mW/cm2, whereas pure PBI reached 471 mW/cm2 under the same test condition.
As a kind of molten salt at room temperature, weak-volatilized ionic liquid (IL) can be used as proton transmission medium. Nevertheless, IL is easily leached in IL doped PBI and this membrane does not show a good-enough conductivity, which is traced back to the high resistance of PBI substrate. Strikingly, IL can be conductive to PA adsorption capacity of PBI and promote the associated proton conductivity. Skorikova et al.[94] immobilized bis(trifluoromethanesulfonyl)-based protic IL in PA doped PBI, which exhibited a highest output (320 mW/cm2) at 200 ℃. On the basis of OH group containing PBI and through a sol-gel process germinated ionic-liquid-functionalized silica particles, a series of composite materials were fabricated by Liu et al.[95] Their PA uptake and corresponding conductivity value get greatly improved through introducing ILs into PBI system, indicating a peak of 106 mS/cm at 170 ℃.
Moreover, inert materials with high mechanical strength used as supporting matrix to develop reinforced pore-filling substance has been widely applied to manufacture composite membranes[96-98]. In term of this technology, the pores of the substrate would be packed with the polymer electrolyte, which allow for exceptionally thin polymer membranes (less than 15 μm[97, 99]) while maintaining good mechanical stability. However, the reinforced membranes may show lower proton conductivity than the original one owning to their low swelling ability under lower moisture content. One proposed solution is a highly packed structure, which delivers high conducting without excessive swelling[100]. The superior durable Core-SelectⓇ is one of the most representative reinforced membranes generated by embedding PFSA solution into expanded polytetrafluoroethylene (PTFE) porous sheet. Park et al.[101] applied this approach to PA doped PBI membranes with PTFE film as the matrix. To increase the interfacial adhesion between PTFE and PBI, a pretreatment was employed to create a hydrophilic surface on porous PTFE, by which a coupling agent can be avoided. It was also confirmed that various commercial hydrophobic polymers (e.g., PTFE, polyvinylidene fluoride (PVDF)[102], poly(VDF-co-hexafluoro propylene)[103] or polyethylene (PE)[96]) can be used to manufacture the reinforced membranes and through this approach obtained materials are suitable for HT-PEMFC.
2.4 PBI Derivatives-Based MembranesProton conductivity reflects the final electrochemical property of PBI-based PEMs in the fuel cell system. Namely, higher acid-doping level is a necessary precondition for HT-PEMs to achieve a higher performance. Particularly, the amount of doped PA correlates with the alkaline of the base polymer, in which N atoms serve not only as proton acceptor but also as the complexation sites to form acid-base ion pairs. To this aim, various PBI derivatives were prepared to modulate the properties of pristine material, including the increase of basicity in the polymer backbone using different monomers[104] and introduction of additional basic sites by substitution reaction[105-106], which can assure a stable conductivity in obtained membranes because of the reduced acid leaching[107].
Bipyridine containing PBI (Bipy-PBI) membranes with different molecular weights (MWs) were fabricated by Berber and Nakashima[108]. The obtained conductivity of Bipy-PBI is 36% higher than PBI with similar MW on account of the additional nitrogen atoms provided by the polymer main chain. The influence of controlling the polymer MWs to gain a sufficient mechanical strength is also demonstrated. Chen et al.[109] found similar results for grafted PBI membranes with pendant quaternary ammonium end groups, which were designed to promote a stronger acid-base interaction.
In recent researches, the acid doping level of PBI-based PEMs can be affected by the free volume in the structure. According to the research of Wang et al.[110], a type of amino-modified PBI membranes, grafted by ethyl phosphoric acid (EPA) was produced. The presence of EPA groups on the side chains allows an increase in the free volume. Higher doping level and stability of PA were measured compared with pristine PBI membrane. As depicted in Scheme 6, Ni[111], Hu[112] and Wang et al.[113-114] proposed a novel approach using highly branched PBIs as HT-PEMs. It is thought that the formed unique three-dimensional structures provide a large free volume with an extensive number of open and accessible cavities, which contributes to the acid uptake of the branched PEMs, while also avoids suffering from acid leaching due to their ability to encapsulate the absorbed acid. Moreover, Wang et al.[115] successfully develop the segmented block copolymer based on OPBI and p-PBI oligomers with obvious nanophase-separated structures. This polymer membrane indicates high conductivity value of 100 mS/cm, coupled with the output of 360 mW/cm2 in fuel cell at 160 ℃ without the need of additional humidification. By combination of branched PBIs and block PBIs, a PBI derivative membrane with a stellate branched block structure was first synthesized, which exhibits superior performance by integrating advantages of both strategies. A maximum power density that is more than 700 mW/cm2 was recorded in a single cell[116]. Yet it is worth noting that sufficient stability of PBI derivatives-based membranes is essential as the acid doping level has been improved significantly.
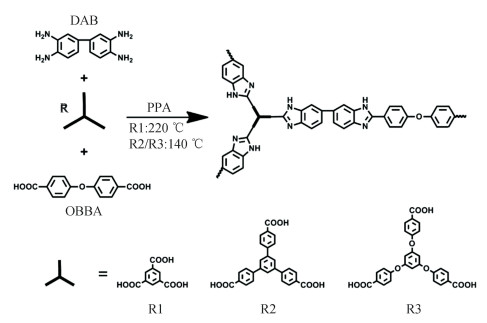
|
Scheme 6 Synthesis and structures of different branched OPBIs[113](Reprinted with permission from Ref. [113], Copyright 2018 Elsevier) |
3 Other Hydrocarbon-Based Polymer Electrolyte Membranes
In addition to the application of PBI and PBI derivative membranes, researchers also focus on the development of new compounds as HT-PEMs due to the harsh synthetic and processing condition of PBI. Since the performance of PEMs depends on the selected macromolecular main chain, the basicity, the quantity, and the position of introduced functional groups, numerous structural designs have been attempted, for example, polyetheretherketone (PEEK), polysulfone (PSU), polyarylene ether (PAE), and polyvinylpyrrolidone (PVP). This approach aims to synthesize polymer with tailored property, which has the capacity for absorbing acid and offers the possibility for proton transfer. Basically, it can be realized by three ways: application of alkaline polymer with N-heterocycles, phosphonation of the material, and introduction of the basic group via post-polymerization substitution[117].
Just as the sulfonation process to introduce sulfonic acid groups in LT-PEMs, phosphonated membranes are considered as potential HT-PEMs, which have been explored with several hydrogen carbon polymers like PSU[118], PAE[119], and polypenylene oxide[120]. Despite the properties of generated membranes conform to the demand as electrolyte in HT-PEMFC, i.e., working at elevated temperature without humidification, there are two problems in gaining access to wide application of this idea. Firstly, the feasible synthesis routes of phosphonated polymer is finite, which might be carried out under stringent reaction conditions; besides, the proton conductivity of these membranes is another issue that merits attention due to the low acidity of PA. Based on this, Bano et al.[121] introduced phosphonic acid functional groups onto PEEK as protogenic units via chloromethylation and subsequent phosphonation (Michaelis-Arbuzov reaction).What PA groups lack in acidity is compensated by the high substitution degree while 47 mS/cm was recorded for the proton conductivity at 120 ℃.
In the case of polymer-PA composite membranes, basic units are introduced in the structure. Generally, the adsorption capacity of these membranes to PA rises proportionately with the basicity of the functional groups and the flexibility of polymer backbone. High PA doping level is a premise for an outstanding proton conductivity in HT-PEMs, yet excessive branching chains doping with PA could significantly degrade the membranes' mechanical properties, so a trade-off between the two needs to be found. The researchers have attempted to strike this balance by quantitative improvements of the functional groups at the same grafting site. Bu et al.[122] used polyarylene ether ketone (PEAK) as HT-PEM backbone, which is produced by bromomethyated with subsequent nucleophilic substitution between the groups Ar-CH2Br and -SH to form 1, 2, 4-triazole side chains. The relevant conductivity (up to 51 mS/cm) was observed at 190 ℃. Meanwhile, the modifications of PSU by using 2, 4, 6-tri(dimethylaminomethyl)-phenol (TDAP) were studied by Zhang et al[123]. The preparation of the substance containing several tertiary amine groups is shown in Scheme 7. It was verified that the resulted polymer membranes exhibit higher proton conductivity, power density, and lower dimensional change rate compared with dimethylamine (DMA, single amine group in the structure) grafted PSU membranes with the same grafting density (Fig. 1).
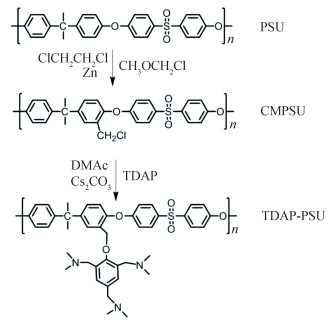
|
Scheme 7 Preparation of TDAP-g-PSU membranes[122] (Reprinted with permission from Ref. [122], Copyright 2019 Elsevier) |
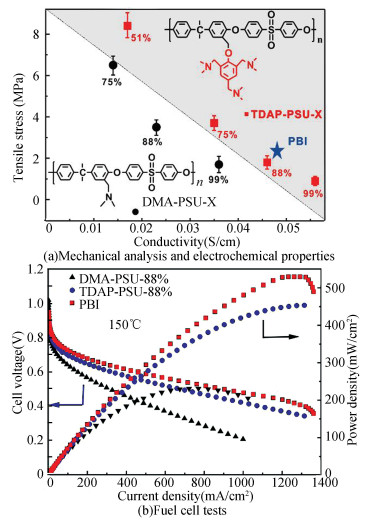
|
Fig.1 Performance comparison of TDAP-g-PSU, DMA-g-PSU, and PBI membranes (X stands for the grafting degree of CMPSU[122]) (Reprinted with permission from Ref. [122], Copyright 2019 Elsevier) |
Furthermore, the mechanical property of the PA-doped PEMs ties in nicely with their swelling behavior. Under the condition of guaranteeing high performance in terms of proton conductivity, a new strategy by separating functional units that absorb PA from the polymer backbone might effectively control the membranes dimensional stability.
On this basis, poly(1-vinylimidazole) (PVIm) grafted PSU (Scheme 8) via atom transfer radical polymerization (ATRP) was reported by Bai et al[124]. Based on the formed micro-phase separated structure (Fig. 2), excellent conductivity was recorded for this membrane type, which amounted to 125 mS/cm at 160 ℃. As expected, these membranes maintain high mechanical property of 7.94 MPa in tensile strength after doping with PA due to the reduced accompanying plasticizing effect.
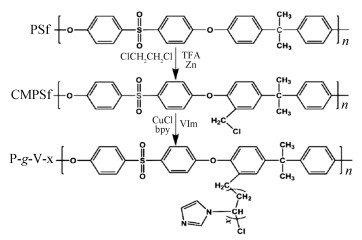
|
Scheme 8 Synthesis of PSU-g-VIm membranes via ATRP process[124] (Reprinted with permission from Ref. [124], Copyright 2019 Elsevier) |
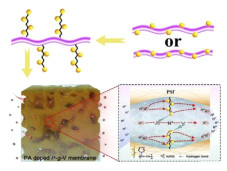
|
Fig.2 Illustration of ionic clusters and proton transfer pathway in HT-PEM with side chains[124] (Reprinted with permission from Ref. [124], Copyright 2019 Elsevier) |
Polymer PVP containing N-heterocycle is widely applied in industry and is a potential alternative material of PBI as "PA absorber"[125]. But in practical use, PVP is brittle and shows limited film forming ability. As a solution, it is considered to blend PVP with components, which exhibit excellent mechanical strength and chemical inertness like PVDF[126] or polyethersulfone (PES) with a higher glass transition temperature of 230 ℃[125]. Excellent electrochemical performance could be observed for these membranes, of which a highest proton conductivity of 210 mS/cm was recorded[125], but the mechanical property of the PVP-based membranes needs to be improved further. It was confirmed that the modification methods such as reinforced membrane technology[127-129] and use of organic or inorganic fillers applied for PBI are available to PVP-based membranes as well. Among the different types of filler, heteropoly acids possess a proton conductivity up to 180 mS/cm[130]. However, phosphotungstic acid (PWA) added PBI shows a low conductivity of 2.2 mS/cm, which is much less than the desired value in an HT-PEMFC, while 144 mS/cm was achievable by PA doped PWA/PBI composite membrane with considerable mechanical stability[131].
In addition, hydrocarbon matrix like polyamide-imde (PAI)[132], polyether sulfone benzotriazole (PESB)[133], and AB type polyphenylquinoxaline (ABPPQ)[134] has also been approved for the application in HT-PEMFC, yet the durable performance in fuel cells needs further verification.
4 Fluorinated Polymer Electrolyte Membranes Produced via Radiation Induced PolymerizationRadiation-induced polymerization was started in the 1950s, which is one of the important methods for modification of polymers. To understand this process, a lot of study has been done in recent years referring to the effects of reaction conditions, the type and thickness of the polymer matrix, the type and concentration of monomers on graft reaction kinetics, and the structural behavior of polymers. Due to the simple operation and good control over the process, radiation induced polymerization is an attractive method for the production of graft polymers. This approach to produce PEMs involves the radical polymerization of functional monomers on the backbone material surface or in its interior. Here, electromagnetic UV, X-ray, γ-ray as well as β-rays[135] are often applied for creating radicals, namely active centers in the substrate. The units gray (Gy, equivalent to J/kg) or kilogray (kGy) are commonly used in discussing the intensity of irradiation dose.
The radiation grafting methods include co-irradiation[136-137] and pre-irradiation [138-140] grafting. Generally, the difference between the two depends primarily on the applied step of the substrate. As for the former, the backbone material and the vinyl monomers are placed in the same system and irradiated simultaneously. That is to say, the graft polymerization occurs during the irradiation and this process can be expected to possess a high radical utilization rate up to 100%. The existing problem, however, is the additional formation of homopolymer from the monomer by the radiation, which tends to lower the grafting yield; for the latter, pre-irradiation and grafting are two separate courses. The base material is irradiated with or without oxygen, which forms stable peroxides or free radicals in the polymer. Then, the polymerization of monomers is carried out onto the activated substrate under inert gas protection. Although the rate of radical usage is lower in pre-irradiation method, this approach is easy to be controlled and therefore more and more laboratories and enterprises have actively made use of it.
To synthesize fluorinated PEMs, the radiation induced grafting employs partially or perfluorinated polymers, namely poly(ethylene-alt-tetrafluoroethylene) (ETFE) [141-143], poly(tetrafluoroethylene-co-hexafluoropropylene) (FEP)[144-145], PVDF[146-148], PTFE[149-150], poly(tetrafluoroethylene-co-perfluoro-(alkyl vinyl ether)) (PFA)[151-153], etc. as the matrix. In preparation of the polymer membranes, these commercially available base films are firstly irradiated by high-energy electrons, i.e., the produced radicals initiate the subsequent graft polymerization of functional side-chain polymers. The grafted membranes were fabricated according to the method shown in Fig. 3.
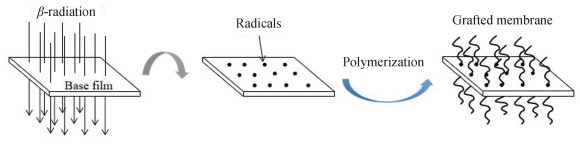
|
Fig.3 General synthetic route for grafted membrane via e-beam treatment |
As far as is known, for getting an elevated working temperature of the obtained PEMs, the three-proton containing PA instead of water is considered as an ideal proton carrier and conductor. One approach to this issue is the synthesis of grafted membranes containing pendant R-PO(OH)2. Schmidt-Naake et al.[154] synthesized this membrane type via irradiation induced polymeization of vinylbenzyl chloride (VBC) on ETFE or FEP membranes with subsequent Arbuzov phosphonation. The phosphonation degree, water swelling behaviors, stability, and the electrical chemical feature of the synthesized membranes were evaluated. Another proposed solution is the application of acid doped basic polymer membranes[155-156]. Functional groups in side chains are considered to directly affect the affinity between doped phosphoric acid and the generated membranes. Hence, the grafting of basic monomers like 1-vinylimidazole (1-VIm)[157], 1-vinyl-2-pyrrolidone (1-V-2-P)[152], and 4-vinylpyridine (4VP)[141] onto the backbone material is considered to be responsible for the synthesis of the acid doped HT-PEMs. In addition, suitable reactive monomers such as N-vinylformamid (NVF)[141] and glycidyl methacrylate (GMA), in which basic functional groups could be introduced by further modification, are viable for this application as well. For homo-grafted membranes, the incorporation of comonomers like acrylonitrile (AN) and butyl acrylate (BA), or various cross-linkers[158] could be considered to enhance the properties of these PEMs.
Nevertheless, the researches have shown that the use of neutral hydrophilic monomers grafted polymer membranes can serve as an alternate concept for the application in HT-PEMFC as well. Li et al.[159] studied the pre-irradiation induced graft copolymerization of 2-hydroxyethylmethacrylate (HEMA) and 2-hydroxyethyl acrylate (HEA) with 50 μm commercial ETFE films as substrate. Functional groups of both monomers provide access to connect PA in two ways. On the one hand, strong hydrogen bonds with steric network structure were generated between PA and the carboxyl groups; on the other, PA could be linked by covalent bonds, which stem from phosphoric acid esters generated by condensation reaction of PA and the hydroxyl groups in HEMA and HEA[160]. Using the acrylate monomer HEA benefits, the polymerization rate and ensures a better contact of the PEM in the membrane electrode assembly owing to the decreased glass temperature of the resulted copolymer. With this approach, stable ETFE-g-(HEMA-co-HEA) membranes with high PA doping level up to 300% were prepared. Then another monomer acrylate acid (AA) was investigated as copolymer for HEMA[161]. Obviously, the influence of steric effect on the grafting of small AA molecules is relatively weak compared with HEA. Experimental results confirmed that the grafting degree of obtained PEMs raised considerably in HEMA/AA system, along with 113 mW/cm2 output power under dry condition (120 ℃). The aforementioned synthetic routes were summarized in Fig. 4.
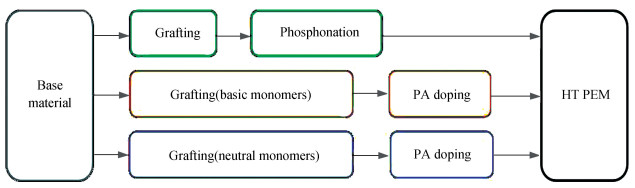
|
Fig.4 Synthetic routes of HT PEM via irradiation induced grafting |
The properties of these PEMs are essentially determined by the graft polymerization. The grafting degree and the ratio of the main chain to branches depend on the e-beam dose, the mole composition in monomer feed, and the fraction of solvent in the reaction mixture. To find the optimum reaction conditions, the pertinent parameters could be varied by linear models or the experiment designs of the multi-response linear models[156]. Another influence on the irradiated graft involves the employed base material. For instance, there are three different bonds C-C, C-H, and C-F in ETFE, while FEP possesses C-C and C-F. Owing to the significantly lower binding energy of 345 kJ/mol[162], the bond breaking process occurs mainly at C-C in both materials. However, the mobility of the broken C-C is rather limited, which tends to cause a quick recombination of these bonds. Thus, in ETFE, most radicals should be supplied from the broken C-H bonds (416 kJ/mol[162]). By contrast, the generation of radicals in FEP bases on the break of C-F bonds, which is more difficult than the former due to the highest bond energy of 489 kJ/mol[162]. It is obvious that higher radical density can be achieved in partially fluorinated polymer ETFE rather than perfluorinated FEP under the same e-beam dose. In addition, operating temperature of these membrane is limited by the relatively low glass transition temperature of fluorinated backbone material. For example, use temperature of PVDF is less than 160 ℃, which means a PVDF-based HT-PEM might decay very fast at a higher temperature.
Despite pre-irradiation induced graft polymerization is a significant way to prepare HT-PEMs, this approach has its limitations. First, the mechanical strength of the base material suffers damage during the radiation and grafting process. Therefore, excessive radiation dose or exorbitant grafting degree should be avoided. Second, there is more or less a restricted accessibility of the radicals for the monomer molecules as a result of the grafting onto activated base materials. In case of a copolymerization with this approach, the polymer composition may differ from the radical polymerization using free movable initiator, especially for the monomers, which are relatively large or electrically charged[140]. Additionally, it should be noted that the chain transfer occurs frequently during the polymerization, which causes a transport of radicals from base materials to the monomers. This phenomenon may be accompanied by the polymer formation in the solvent, or the reaction mixture gradually becomes gel with increasing viscosity, which is a process known as gelling. To prevent such a situation, the reaction conditions need to be optimized. For example, the reduction in monomer fraction may be useful for the reaction when the set temperature is very high. Apart from gelling, there is another drawback in radiation grafting process. Actually, this method belongs to a type of free radical polymerization. Thus, the obtained branches with varying lengths in the structure may upset the uniformity of the PEMs. For this, researchers have tried to combine this procedure with controlled radical polymerization (CRP), during which pre-irradiation modified fluorinated films containing C-Cl or C-Br[163] could be applied as macroinitiator for the consequent ATRP[164-165] to get access to well-defined graft copolymers.
5 Long-Term Durability of High Temperature Polymer Electrolyte Membrane Fuel CellsAs the central component in PEMFC, membrane electrode assembly (MEA) has been increasingly concerned. Typical units in MEA include a PEM, two catalyst layers, and two gas diffusion layers. However, finite service life of MEA is always a bottleneck restriction of commercial application for HT-PEMFC technology. Since high temperature brings a great challenges to materials in fuel cell system, the safe and stable operation of MEA has great significance and remains a challenging task with a target (over 80000 h)[166] stated by US Department of Energy. For this purpose, the durability of MEA should be evaluated under various conditions, which may provide a comprehensive feedback. Representative HT-PEM product CeltecⓇ1000 shows an average degradation rate of approximately 5μV/h in a continuous durability test for more than 6000 h, while running in a start/stop cycling mode the corresponding value was tripled to 11 μV/h[167]. Quite a few long-term tests were conducted under stable operational conditions of 200 mA/cm2, while an accelerated attenuation may be observed at higher current density. Generally, the voltage decay is mainly attributable by the degradation of PEM, catalyst, and the acid leakage[168].
Various HT-PEM systems were investigated in order to mitigate aging. Ossiander et al.[169] compared the property and degradation of PBI-based PEMs before and after 1300 h fuel cell operation by using two different molecular weight distributions (MWDs) of PBI and three different reinforce strategies, namely cross-linking, organic-inorganic composite, and substrate-strengthening. As a result, longer lasting PBIs favor a narrow MWD and an increased interaction between polymer chains. A remarkable long-term test of HT-PEMFC for 13000 h at 200 mA/cm2 was recorded by Søndergaard et al.[170] The cross-linked m-PBI membrane formed via thermal treatment exhibits a performance degradation of less than 1.5 μV/h compared with 4.6 μV/h of linear m-PBI in average. Results of the cross-sectional SEM (Fig. 5) revealed the changes of the membrane thickness during these tests, which offer yet another confirmation of the membrane degradation.
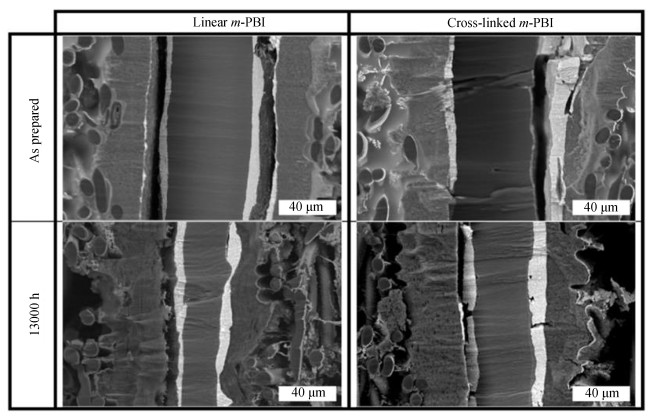
|
Fig.5 Cross-sectional backscatter electron images of linear and cross-linked m-PBI in MEAs before and after 13000 h operation[170] (Reprinted with permission from Ref. [170], Copyright 2017 Elsevier) |
Besides the composite and cross-linked structures mentioned above, molecular weight enrichment has been proven as a practical approach to reduce performance attenuation of HT-PEMFC. In general, properties of PBI materials like mechanical and chemical stability could be greatly influenced by corresponding molecular weight. Commercial available PBI (CelazoleⓇ), however, is inapplicable to fuel cell, since the linear molecular weight is relative low, less than 40 kDa[171]. Balance between mechanical strength and proton conductivity is essential for a PA doped PBI membrane, so PBI polymer with higher molecular weight was seen as a feasible way to ensure its mechanical stability at high PA doping level. He at al.[172] reported the tensile stress at break of PBI film (acid doping level: 5.5 to 6.7) rises from 3.5 to 12.0 MPa with increasing molecular weight from 25.0 to 36.8 kDa. Though the formed membranes were physicochemically analyzed, the data on the durability of membranes was limited. PBI films with varied molecular weight up to 94 kDa were investigated by Yang et al.[173], especially the fuel cell durability. The sample PBI-78kDa/10.8PA shows a tensile strength of 30.3 MPa with a high doping level. The relevant fuel cell test was carried out under stable operational condition of 300 mA/cm2 at 60 ℃. The result indicated a lifetime exceeding 1400 h with 1.5 μV/h decline. Several durability tests were carried out for alternative hydrocarbon-based membranes as well, for instance, reinforced PVP/PES membrane shows a stability for 1000 h at 0.7 V (160 ℃)[129] and PA doped polyarylene piperidine membrane was measured in HT-PEMFC for 1600 h (120 mA/cm2, 150 ℃)[174]. All the results prove excellent performance of these synthesized HT-PEMs in fuel cell, but in act some of them were tested with different measurement condition, so a unified testing standard is very necessary for a better evaluation between different membranes.
Moreover, Li et al.[175] has proved that the degradation of membrane during the test is mainly resulted from the attack of H2O2, -OH, and -OOH generated in chemical processes. That means the use of migrate additive like CeO2 and ZrO2[176-178] as regenerative free radical scavengers would be an appropriate way to extend service life of MEA in PEMFC. Despite the tests of PBI and PBI-based membranes appeared to be more than the other membranes systems, each type of membrane has their own inferior position in HT-PEMFC application. The comparison of different membrane systems is summarized in Table 1, based on different synthesis methods.
| Table 1 Comparison of different membrane systems for HT-PEMFC |
6 Conclusions
HT-PEM that meets relevant requirements for practical application in fuel cell is the primary factor to a large-scale promotion of HT-PEMFC. Since the PA doped polymer membranes possess favorable stability and significant proton conductivity at low water activities in high temperature condition, they have emerged nowadays as the most promising HT-PEMs. Meanwhile, distinct swelling of membranes occurs at high PA doping degree, which brings about the decreased mechanical and dimensional stability. So, during actual operation of these membranes, a certain balance between electrochemical and mechanical properties is often necessary to be found. Besides, free phosphoric acid may be gradually lost in the operation process of fuel cell. Aiming at these issues, scientists have made broad research about approaches like cross-linked membranes, composite materials or design of new structures with increased basicity. Moreover, introduction of the basic functional groups onto the polymer main chain via long branches, which can weaken the influence of the plasticizer PA on polymer matrix or grafting of PA groups directly into polymer membranes, might be an appropriate way to overcome the current shortcomings of HT-PEMs. In summary, improving the durability of high temperature polymer membranes while maintaining sufficient performance should be the focus of future research.
| [1] |
Parhizkar T, Roshandel R. Long term performance degradation analysis and optimization of anode supported solid oxide fuel cell stacks. Energy Conversion and Management, 2016, 133: 20-30. DOI:10.1016/j.enconman.2016.11.045 (  0) 0) |
| [2] |
Cha D, Jeon S W, Yang W, et al. Comparative performance evaluation of self-humidifying PEMFCs with short-side-chain and long-side-chain membranes under various operating conditions. Energy, 2018, 150: 320-328. DOI:10.1016/j.energy.2018.02.133 (  0) 0) |
| [3] |
Lu C L, Chang C P, Guo Y H, et al. High-performance and low-leakage phosphoric acid fuel cell with synergic composite membrane stacking of micro glass microfiber and nano PTFE. Renewable Energy, 2018, 134: 982-988. DOI:10.1016/j.renene.2018.11.011 (  0) 0) |
| [4] |
Liu X D, Luo X H, Chen X H, et al. Perfluorinated membrane electrode assembly containing metal-free-catalyst cathode for anion exchange membrane fuel cells. Journal of Electroanalytical Chemistry, 2020, 871: 114283. DOI:10.1016/j.jelechem.2020.114283 (  0) 0) |
| [5] |
Mohideen M M, Liu Y, Ramakrishna S. Recent progress of carbon dots and carbon nanotubes applied in oxygen reduction reaction of fuel cell for transportation. Applied Energy, 2019, 257: 114027. DOI:10.1016/j.apenergy.2019.114027 (  0) 0) |
| [6] |
Kim J, Kim S I, Jo S G, et al. Enhanced activity and durability of Pt nanoparticles supported on reduced graphene oxide for oxygen reduction catalysts of proton exchange membrane fuel cells. Catalysis Today, 2019, 352: 10-17. DOI:10.1016/j.cattod.2019.11.016 (  0) 0) |
| [7] |
Lv C C, Guo S, Yang T T, et al. Improvement of oxygen reduction capacity by activated carbon doped with broccoli-like Co-Ni2P in microbial fuel cells. Chemical Engineering Journal, 2020, 399: 125601. DOI:10.1016/j.cej.2020.125601 (  0) 0) |
| [8] |
Pachauri R K, Chauhan Y K. A study, analysis and power management schemes for fuel cells. Renewable and Sustainable Energy Reviews, 2014, 43: 1301-1319. DOI:10.1016/j.rser.2014.11.098 (  0) 0) |
| [9] |
Wang S Y, Jiang S P. Prospects of fuel cell technologies. National Science Review, 2017, 4(2): 163-166. DOI:10.1093/nsr/nww099 (  0) 0) |
| [10] |
Asensio J A, Sánchez E M, Gómez-Romero P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chemical Society Reviews, 2010, 39: 3210-3239. DOI:10.1039/B922650H (  0) 0) |
| [11] |
Bose S, Kuila T, Nguyen T X H, et al. Polymer membrane for high temperature proton exchange membrane for fuel cell: recent advances and challenges. Progress in Polymer Science, 2011, 36(6): 813-843. DOI:10.1016/j.progpolymsci.2011.01.003 (  0) 0) |
| [12] |
Araya S S, Zhou F, Liso V., et al. A comprehensive review of PBI-based high temperature PEM fuel cells. International Journal of Hydrogen Energy, 2016, 41(46): 21310-21344. DOI:10.1016/j.ijhydene.2016.09.024 (  0) 0) |
| [13] |
Quartarone E, Angioni S, Mustarelli P. Polymer and composite membranes for proton-conducting, high-temperature fuel cells: a critical review. Materials, 2017, 10: 687. DOI:10.3390/ma10070687 (  0) 0) |
| [14] |
Kalathil A, Raghavan A, Kandasubramanian B. Polymer fuel cell based on polybenzimidazole membrane: a review. Polymer-Plastics Technology and Engineering, 2019, 58(5): 465-497. DOI:10.1080/03602559.2018.1482919 (  0) 0) |
| [15] |
Aili D, Henkensmeier D, Martin S, et al. Polybenzimidazole-based high-temperature polymer electrolyte membrane fuel cells: new insights and recent progress. Electrochemical Energy Reviews, 2020, 3: 793-845. DOI:10.1007/s41918-020-00080-5 (  0) 0) |
| [16] |
E. I. du Pont de Nemours and Company. Fluorocarbon Sulfonyl Fluorides. U.S.A. : US 3041317. 1962-06-26.
(  0) 0) |
| [17] |
E. I. du Pont de Nemours and Company. Fluorocarbon Vinyl Ether Polymers. U.S.A. : US 3282875, 1966-11-01.
(  0) 0) |
| [18] |
Li Z, He G W, Zhang B, et al. Enhanced proton conductivity of Nafion hybrid membrane under different humidities by incorporating metal-organic frameworks with high phytic acid loading. ACS Applied Materials & Interfaces, 2014, 6: 9799-9807. DOI:10.1021/am502236v (  0) 0) |
| [19] |
He G W, Li Z Y, Li Y F. Zwitterionic microcapsules as water reservoirs and proton carriers within a Nafion membrane to confer high proton conductivity under low humidity. ACS Applied Materials & Interfaces, 2014, 6: 5362-5366. DOI:10.1021/am500626f (  0) 0) |
| [20] |
Li Y, Wu H, Yin Y H, et al. Fabrication of Nafion/zwitterion-functionalized covalent organic framework composite membranes with improved proton conductivity. Journal of Membrane Science, 2018, 568: 1-9. DOI:10.1016/j.memsci.2018.09.050 (  0) 0) |
| [21] |
Wang L, Advani S G, Prasad A K. PBI/Nafion/SiO2 hybrid membrane for high-temperature low-humidity fuel cell applications. Electrochimica Acta, 2013, 105: 530-534. DOI:10.1016/j.electacta.2013.05.043 (  0) 0) |
| [22] |
Wang H, Li X J, Zhuang X P. Modification of Nafion membrane with biofunctional SiO2 nanofiber for proton exchange membrane fuel cells. Journal of Power Sources, 2017, 340: 201-209. DOI:10.1016/j.jpowsour.2016.11.072 (  0) 0) |
| [23] |
Yang H N, Lee W H, Choi B S, et al. Preparation of Nafion/Pt-containing TiO2/graphene oxide composite membranes for self-humidifying proton exchange membrane fuel cell. Journal of Membrane Science, 2016, 504: 20-28. DOI:10.1016/j.memsci.2015.12.021 (  0) 0) |
| [24] |
Yin C S, Li J J, Zhou Y W. Phase separation and development of proton transport pathways in metal oxide nanoparticle/Nafion composite membranes during water uptake. The Journal of Physical Chemistry C, 2018, 122: 9710. DOI:10.1021/acs.jpcc.8b02535 (  0) 0) |
| [25] |
Costamagna P, Yang C, Bocarsly A B, et al. NafionⓇ115/zirconium phosphate composite membranes for operation of PEMFCs above 100 ℃. Electrochimica Acta, 2002, 47: 1023-1033. DOI:10.1016/S0013-4686(01)00829-5 (  0) 0) |
| [26] |
Kim Y T, Song M K, Kim K H, et al. Nafion/ZrSPP composite membrane for high temperature operation of PEMFCs. Electrochimica Acta, 2004, 50(2-3): 645-648. DOI:10.1016/j.electacta.2003.12.079 (  0) 0) |
| [27] |
Amirinejad M, Madaeni S S, Rafiee E, et al. Cesium hydrogen salt of heteropolyacids/Nafion nanocomposite membranes for proton exchange membrane fuel cells. Journal of Membrane Science, 2011, 377(1-2): 89-98. DOI:10.1016/j.memsci.2011.04.014 (  0) 0) |
| [28] |
Chen L, Tang H, Pan M. Periodic Nafion-silica-heteropolyacids electrolyte for PEM fuel cell operated near 200 ℃. International Journal of Hydrogen Energy, 2012, 37(5): 4694-4698. DOI:10.1016/j.ijhydene.2011.04.116 (  0) 0) |
| [29] |
Yang C, Costamagna P, Srinivasan S., et al. Approaches and technical challenges to high temperature operation of proton exchange membrane fuel cells. Journal of Power Sources, 2001, 103(1): 1-9. DOI:10.1016/S0378-7753(01)00812-6 (  0) 0) |
| [30] |
Jang J, Kim D H, Min C M, et al. Azole structures influence fuel cell performance of phosphoric acid-doped poly(phenylene oxide) with azoles on side chains. Journal of Membrane Science, 2020, 605: 118096. DOI:10.1016/j.memsci.2020.118096 (  0) 0) |
| [31] |
Chang C P, Wu Y C, Chen W Y, et al. A hybrid phosphorus-acid fuel cell system incorporated with oxidative steam reforming of methanol (OSRM) reformer. Renewable Energy, 2020, 153: 530-538. DOI:10.1016/j.renene.2020.01.137 (  0) 0) |
| [32] |
Li Q F, He R H, Jensen J O, et al. Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 ℃. Chemistry of Materials, 2003, 15: 4896-4915. DOI:10.1021/cm0310519 (  0) 0) |
| [33] |
Li Q F, He R H, Jensen J O, et al. PBI-based polymer membranes for high temperature fuel cells-preparation, characterization and fuel cell demonstration. Fuel Cells, 2004, 4(3): 147-159. DOI:10.1002/fuce.200400020 (  0) 0) |
| [34] |
Mocoteguy P, Ludwig B, Scholta J, et al. Long-term testing in dynamic mode of HT-PEMFC H3PO4/PBI celtec-P based membrane electrode assemblies for micro-CHP applications. Fuel Cells, 2010, 10: 299-311. DOI:10.1002/fuce.200800134 (  0) 0) |
| [35] |
Lee K S, Spendelow J S, Choe Y K, et al. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs. Nature Energy, 2016, 1: 16120. DOI:10.1038/nenergy.2016.120 (  0) 0) |
| [36] |
Yang J S, Li Q F, Jensen J O, et al. Phosphoric acid doped imidazolium polysulfone membranes for high temperature proton exchange membrane fuel cells. Journal of Power Sources, 2012, 205: 114-121. DOI:10.1016/j.jpowsour.2012.01.038 (  0) 0) |
| [37] |
Li Q, Liu L, Liang S E, et al. A novel poly(2, 6-dimethyl-1, 4-phenylene oxide) with pendant imidazolium groups for high-temperature proton exchange membrane. Polymer Chemistry, 2014, 5: 2425-2432. DOI:10.1039/C3PY01435E (  0) 0) |
| [38] |
Hink S, Duong N M H, Henkensmeier D, et al. Radel-based membranes with pyridine and imidazole side groups for high temperature polymer electrolyte fuel cells. Solid State Ionics, 2015, 275: 80-85. DOI:10.1016/j.ssi.2015.03.026 (  0) 0) |
| [39] |
Henkensmeier D, Duong N M H, Brela M, et al. Tetrazole substituted polymers for high temperature polymer electrolyte fuel cells. Journal of Materials Chemistry A, 2015, 3: 14389-14400. DOI:10.1039/C5TA01936B (  0) 0) |
| [40] |
E. I. du Pont de Nemours and Company. Polybenzimidazoles. U.S.A. : US2895948, 1959-07-21.
(  0) 0) |
| [41] |
Aili D, Cleemann L N, Li Q, et al. Thermal curing of PBI membranes for high temperature PEM fuel cells. Journal of Materials Chemistry, 2012, 22: 5444-5453. DOI:10.1039/C2JM14774B (  0) 0) |
| [42] |
Hedberg F L, Marvel C S. A new single-step process for polybenzimidazole synthesis. Journal of Polymer Science: Polymer Chemistry Edition, 1974, 12(8): 1823-1828. DOI:10.1002/pol.1974.170120822 (  0) 0) |
| [43] |
Hoel D, Grunwald E. High protonic conduction of polybenzimidazole films. The Journal of Physical Chemistry, 1977, 81(22): 2135-2136. DOI:10.1021/j100537a021 (  0) 0) |
| [44] |
Vogel H, Marvel C S. Polybenzimidazoles, new thermally stable polymers. Journal of Polymer Science, 1961, 50(154): 511-539. DOI:10.1002/pol.1961.1205015419 (  0) 0) |
| [45] |
Chaudhari H. D, Illathvalappil R, Kurungot S, et al. Preparation and investigations of ABPBI membrane for HT-PEMFC by immersion precipitation method. Journal of Membrane Science, 2018, 564: 211-217. DOI:10.1016/j.memsci.2018.07.026 (  0) 0) |
| [46] |
Cong S Z, Wang J X, Wang Z, et al. Polybenzimidazole (PBI) and Benzimidazole-Linked Polymer (BILP) Membranes. https://www.sciencedirect.com/science/article/pii/S266695 282030042X, 2010-03-08.
(  0) 0) |
| [47] |
Krishnan N N, Konovalova A, Aili D, et al. Thermally crosslinked sulfonated polybenzimidazole membranes and their performance in high temperature polymer electrolyte fuel cells. Journal of Membrane Science, 2019, 588: 117218. DOI:10.1016/j.memsci.2019.117218 (  0) 0) |
| [48] |
Xiao L X, Zhang H F, Scanlon E, et al. High-temperature polybenzimidazole fuel cell membranes via a sol-gel process. Chemistry of Materials, 2005, 17(21): 5328-5333. DOI:10.1021/cm050831+ (  0) 0) |
| [49] |
DeMeuse M T. High Temperature Polymer Blends. Dordrecht: Woodhead Publishing, 2014: 174-175.
(  0) 0) |
| [50] |
Liu G, Zhang H M, Hu J W, et al. Studies of performance degradation of a high temperature PEMFC based on H3PO4-doped PBI. Journal of Power Sources, 2006, 162: 547-552. DOI:10.1016/j.jpowsour.2006.07.008 (  0) 0) |
| [51] |
Mader J, Xiao L X, Schmidt T J, et al. Polybenzimidazole/acid complexes as high-temperature membrane. Scherer G G. Fuel Cells Ⅱ. Advances in Polymer Science, Vol. 216. Berlin: Springer, 2008: 63-124.
(  0) 0) |
| [52] |
Wainright J S, Wang J T, Weng D, et al. Acid-doped polybenzimidazoles: a new polymer electrolyte. Journal of The Electrochemical Society, 1995, 142(7): L121-123. DOI:10.1149/1.2044337 (  0) 0) |
| [53] |
Li X B, Ma H W, Wang H L, et al. Novel PA-doped polybenzimidazole membranes with high doping level, high proton conductivity and high stability for HT-PEMFCs. RSC Advances, 2015, 5: 53870-53873. DOI:10.1039/C5RA05953D (  0) 0) |
| [54] |
Pu H T, Meyer W H, Wegner G. Proton transport in polybenzimidazole blended with H3PO4 or H2SO4. Journal of Polymer Science Part B: Polymer Physics, 2002, 40: 663-669. DOI:10.1002/polb.10132 (  0) 0) |
| [55] |
Xing B, Savadogo O. The effect of acid doping on the conductivity of polybenzimidazole (PBI). Journal of New Materials for Electrochemical Systems, 1999, 2(2): 95-101. (  0) 0) |
| [56] |
Kawahara M, Morita J, Rikukawa M, et al. Synthesis and proton conductivity of thermally stable polymer electrolyte: poly(benzimidazole) complexes with strong acid molecules. Electrochimica Acta, 2000, 45: 1395-1398. DOI:10.1016/s0013-4686(99)00349-7 (  0) 0) |
| [57] |
Bouchet R, Siebert E. Proton conduction in acid doped polybenzimidazole. Solid State Ionics, 1999, 118(3-4): 287-299. DOI:10.1016/S0167-2738(98)00466-4 (  0) 0) |
| [58] |
Li Q, Aili D, Hjuler H A, et al. High Temperature Polymer Electrolyte Membrane Fuel Cells: Approaches, Status, and Perspectives. Switzerland: Springer International Publishing, 2016: 198-201.
(  0) 0) |
| [59] |
Jang J, Kim D H, Ahn M K, et al. Phosphoric acid doped triazole-containing cross-linked polymer electrolytes with enhanced stability for high-temperature proton exchange membrane fuel cells. Journal of Membrane Science, 2019, 595: 117508. DOI:10.1016/j.memsci.2019.117508 (  0) 0) |
| [60] |
Wang L L, Liu Z R, Ni J P, et al. Preparation and investigation of block polybenzimidazole membranes with high battery performance and low phosphoric acid doping for use in high-temperature fuel cells. Journal of Membrane Science, 2019, 572: 350-357. DOI:10.1016/j.memsci.2018.10.083 (  0) 0) |
| [61] |
Wang K, Yang L, Wei W X, et al. Phosphoric acid-doped poly(ether sulfone benzotriazole) for high-temperature proton exchange membrane fuel cell applications. Journal of Membrane Science, 2018, 549: 23-27. DOI:10.1016/j.memsci.2017.11.067 (  0) 0) |
| [62] |
Li Q F, Jensen J O, Savinell R F, et al. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Progess in Polymer Science, 2009, 34: 449-477. DOI:10.1016/j.progpolymsci.2008.12.003 (  0) 0) |
| [63] |
Asensio J A, Sánchez E M, Gómez-Romero P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chemical Society Reviews, 2010, 39(8): 3210-3239. DOI:10.1039/b922650h (  0) 0) |
| [64] |
Celanese Corporation. Chemical Modification of Polybenzimidazole Semipermeable. U.S.A. : US4020142. 1977-04-26.
(  0) 0) |
| [65] |
Duan C X, Luo H C, Li J L, et al. A novel strategy to construct polybenzimidazole linked crosslinking networks for polymer electrolyte fuel cell applications. Polymer, 2020, 201: 122555. DOI:10.1016/j.polymer.2020.122555 (  0) 0) |
| [66] |
Wang S, Zhang G, Han M M, et al. Novel epoxy-based cross-linked polybenzimidazole for high temperature proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 2011, 36(14): 8412-8421. DOI:10.1016/j.ijhydene.2011.03.147 (  0) 0) |
| [67] |
Aili D, Li Q F, Christensen E, et al. Crosslinking of polybenzimidazole membranes by divinylsulfone post-treatment for high-temperature proton exchange membrane fuel cell applications. Polymer International, 2011, 60(8): 1201-1207. DOI:10.1002/pi.3063 (  0) 0) |
| [68] |
Sun P, Li Z F, Wang S W, et al. Performance enhancement of polybenzimidazole based high temperature proton exchange membranes with multifunctional crosslinker and highly sulfonated polyaniline. Journal of Membrane Science, 2018, 549: 660-669. DOI:10.1016/j.memsci.2017.10.053 (  0) 0) |
| [69] |
Yang J S, Jiang H X, Gao L P, et al. Fabrication of crosslinked polybenzimidazole membranes by trifunctional crosslinkers for high temperature proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 2018, 43(6): 3299-3307. DOI:10.1016/j.ijhydene.2017.12.141 (  0) 0) |
| [70] |
Yang J S, Li Q F, Cleemann L N, et al. Crosslinked hexafluoropropyliden polybenzimidazole membranes with chloromethyl polysulfone for fuel cell applications. Advanced Energy Materials, 2013, 3(5): 622-630. DOI:10.1002/aenm.201200710 (  0) 0) |
| [71] |
Wang S, Zhao C J, Ma W J, et al. Macromolecular cross-linked polybenzimidazole based on bromomethylated poly (aryl ether ketone) with enhanced stability for high temperature fuel cell applications. Journal of Power Sources, 2013, 243: 102-109. DOI:10.1016/j.jpowsour.2013.05.181 (  0) 0) |
| [72] |
Zhang N, Zhao C J, Ma W J, et al. Macromolecular covalently cross-linked quaternary ammonium poly(ether ether ketone) with polybenzimidazole for anhydrous high temperature proton exchange membranes. Polymer Chemistry, 2014, 5(17): 4939-4947. DOI:10.1039/c4py00234b (  0) 0) |
| [73] |
Song M F, Lu X W, Li Z F, et al. Compatible ionic crosslinking composite membranes based on SPEEK and PBI for high temperature proton exchange membranes. International Journal of Hydrogen Energy, 2016, 41(28): 12069-12081. DOI:10.1016/j.ijhydene.2016.05.227 (  0) 0) |
| [74] |
Tashvigh A A, Luo L, Chung T S, et al. Performance enhancement in organic solvent nanofiltration by double crosslinking technique using sulfonated polyphenylsulfone (sPPSU) and polybenzimidazole (PBI). Journal of Membrane Science, 2018, 551: 204-213. DOI:10.1016/j.memsci.2018.01.047 (  0) 0) |
| [75] |
Atanasov V, Gudat D, Ruffmann B, et al. Highly phosphonate polypentafluorostyrene: characterization and blends with polybenzimidazole. European Polymer Journal, 2013, 49(12): 3977-3985. DOI:10.1016/j.eurpolymj.2013.09.002 (  0) 0) |
| [76] |
Tang T H, Su P H, Liu Y C, et al. Polybenzimidazole and benzyl-methyl-phosphoric acid grafted polybenzimidazole blend crosslinked membrane for proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 2014, 39(21): 11145-11156. DOI:10.1016/j.ijhydene.2014.05.020 (  0) 0) |
| [77] |
Özdemir Y, Özkan N, Devrim Y. Fabrication and characterization of cross-linked polybenzimidazole based membranes for high temperature PEM fuel cells. Electrochimica Acta, 2017, 245: 1-13. DOI:10.1016/j.electacta.2017.05.111 (  0) 0) |
| [78] |
Yang J S, Xu Y X, Liu P P, et al. Epoxides cross-linked hexafluoropropylidene polybenzimidazole membranes for application as high temperature proton exchange membranes. Electrochimica Acta, 2015, 160: 281-287. DOI:10.1016/j.electacta.2015.01.094 (  0) 0) |
| [79] |
Liu C, Wang X, Li Y M., et al. Novel cross-linked membranes based on polybenzoxazine and polybenzimi-dazole containing 4-phenyl phthalazinone moiety for high-temperature proton exchange membrane. Journal of Polymer Research, 2017, 24: 23. DOI:10.1007/s10965-016-1173-4 (  0) 0) |
| [80] |
Cai Y B, Yue Z Y, Teng X. Phosphoric acid doped crosslinked polybenzimidazole/modifed graphene oxide composition membranes for high temperature proton exchange membrane applications. Journal of The Electrochemical Society, 2018, 165(11): F914-F920. DOI:10.1149/2.0051811jes (  0) 0) |
| [81] |
Li X B, Ma H W, Wang P., et al. Construction of high performance high-temperature proton exchange membranes through incorporating SiO2 nanoparticles into novel cross-linked polybenzimidazole networks. ACS Applied Materials & Interfaces, 2019, 11(34): 30735-30746. DOI:10.1021/acsami.9b06808 (  0) 0) |
| [82] |
Aili D, Cleemann L N, Li Q F, et al. Thermal curing of PBI membranes for high temperature PEM fuel cells. Journal of Materials Chemistry, 2012, 22(12): 5444-5453. DOI:10.1039/C2JM14774B (  0) 0) |
| [83] |
Joseph D, Nambi Krishnan N, Henkensmeier D, et al. Thermal crosslinking of PBI/sulfonated polysulfone based blend membranes. Journal of Materials Chemistry A, 2016, 5(1): 409-417. DOI:10.1039/C6TA07653J (  0) 0) |
| [84] |
Krishnan N N, Joseph D, Duong N M H, et al. Phosphoric acid doped crosslinked polybenzimidazole (PBI-OO) blend membranes for high temperature polymer electrolyte fuel cells. Journal of Membrane Science, 2017, 544: 416-424. DOI:10.1016/j.memsci.2017.09.049 (  0) 0) |
| [85] |
Devrim Y, Devrim H, Eroglu I. Polybenzimidazole/SiO2 hybrid membranes for high temperature proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 2016, 41(23): 10044-10052. DOI:10.1016/j.ijhydene.2016.02.043 (  0) 0) |
| [86] |
Cheng Y, Zhang J, Lu S, et al. High CO tolerance of new SiO2 doped phosphoric acid/polybenzimidazole polymer electrolyte membrane fuel cells at high temperatures of 200-250℃. International Journal of Hydrogen Energy, 2018, 43(49): 22487-22499. DOI:10.1016/j.ijhydene.2018.10.036 (  0) 0) |
| [87] |
Lobato J, Cañizares P, Rodrigo M A, et al. A novel titanium PBI-based composite membrane for high temperature PEMFCs. Journal of Membrane Science, 2011, 369(1-2): 105-111. DOI:10.1016/j.memsci.2010.11.051 (  0) 0) |
| [88] |
Pinar F J, Cañizares P, Rodrigo M. A, et al. Long-term testing of a high-temperature proton exchange membrane fuel cell short stack operated with improved polybenzimidazole-based composite membranes. Journal of Power Sources, 2015, 274: 177-185. DOI:10.1016/j.jpowsour.2014.08.136 (  0) 0) |
| [89] |
Moradi M, Moheb A, Javanbakht M, et al. Experimental study and modeling of proton conductivity of phosphoric acid doped PBI-Fe2TiO5 nanocomposite membranes for using in high temperature proton exchange membrane fuel cell (HT-PEMFC). International Journal of Hydrogen Energy, 2016, 41(4): 2896-2910. DOI:10.1016/j.ijhydene.2015.12.100 (  0) 0) |
| [90] |
Ossiander T, Heinzl C, Gleich S, et al. Influence of the size and shape of silica nanoparticles on the properties and degradation of a PBI-based high temperature polymer electrolyte membrane. Journal of Polymer Science, 2014, 454: 12-19. DOI:10.1016/j.memsci.2013.12.004 (  0) 0) |
| [91] |
Angioni. S, Villa D C, Cattaneo A S, et al. Influence of variously functionalized SBA-15 fillers on conductivity and electrochemical properties of PBI composite membranes for high temperature polymer fuel cells. Journal of Power Sources, 2015, 294: 347-353. DOI:10.1016/j.jpowsour.2015.06.096 (  0) 0) |
| [92] |
Lee S, Seo K, Ghorpade R V, et al. High temperature anhydrous proton exchange membranes based on chemically-functionalized titanium/polybenzimidazole composites for fuel cells. Materials Letters, 2020, 263: 127167. DOI:10.1016/j.matlet.2019.127167 (  0) 0) |
| [93] |
Pinar F J, Cañizares P, Rodrigo M A, et al. Titanium composite PBI-based membranes for high temperature polymer electrolyte membrane fuel cells. Effect on titanium dioxide amount. RSC Advances, 2012, 2(4): 1547-1556. DOI:10.1039/c1ra01084k (  0) 0) |
| [94] |
Skorikova G, Rauber D, Aili D, et al. Protic ionic liquids immobilized in phosphoric acid-doped polybenzimidazole matrix enable polymer electrolyte fuel cell operation at 200 ℃. Journal of Membrane Science, 2020, 608: 118188. DOI:10.1016/j.memsci.2020.118188 (  0) 0) |
| [95] |
Liu F X, Wang S, Li J S, et al. Polybenzimidazole/ionic-liquid-functional silica composite membranes with improved proton conductivity for high temperature proton exchange membrane fuel cells. Journal of Membrane Science, 2017, 541: 492-499. DOI:10.1016/j.memsci.2017.07.026 (  0) 0) |
| [96] |
Kim K, Kim S K, Park J O, et al. Highly reinforced pore-filling membranes based on sulfonated poly(arylene ether sulfone)s for high-temperature/low-humidity polymer electrolyte membrane fuel cells. Journal of Membrane Science, 2017, 537: 11-21. DOI:10.1016/j.memsci.2017.05.014 (  0) 0) |
| [97] |
Oshiba Y, Tomatsu J, Yamaguchi T. Thin pore-filling membrane with highly packed-acid structure for high temperature and low humidity operating polymer electrolyte fuel cells. Journal of Power Sources, 2018, 394: 67-73. DOI:10.1016/j.jpowsour.2018.05.013 (  0) 0) |
| [98] |
W. L. Gore & Associates, Inc.; W. L. Gore & Associates, Co., Ltd. Highly reinforced ionomer membranes for high selectivity and high strength. U.S.A. : US 20200243887A1, 2020-07-30.
(  0) 0) |
| [99] |
Kim K, Kim S K, Park J O, et al. Highly reinforced pore-filling membranes based on sulfonated poly(arylene ether sulfone)s for high-temperature/low-humidity polymer electrolyte membrane fuel cells. Journal of Membrane Science, 2017, 537: 11-21. DOI:10.1016/j.memsci.2017.05.014 (  0) 0) |
| [100] |
Ichimura S, Sota Y, Ishikawa J, et al. Poly(p-phenylene sulfonic acid-ran-2, 5-benzophenone) pore-filling membranes with highly packed acid structure and their polymer electrolyte fuel cell performances. International Journal of Hydrogen Energy, 2016, 41(46): 21461-21469. DOI:10.1016/j.ijhydene.2016.06.261 (  0) 0) |
| [101] |
Park J, Wang L, Advani S G, et al. Mechanical stability of H3PO4-dope PBI/hydrophilic-pretreated PTFE membranes for high temperature PEMFCs. Electrochimica Acta, 2014, 120: 30-38. DOI:10.1016/j.electacta.2013.12.030 (  0) 0) |
| [102] |
Singha S, Jana T. Effect of composition on the properties of PEM based on polybenzimidazole and poly(vinylidene fluoride) blends. Polymer, 2014, 55(2): 594-601. DOI:10.1016/j.polymer.2013.12.021 (  0) 0) |
| [103] |
Hazarika M, Jana T. Novel proton exchange membrane for fuel cell developed from blends of polybenzimiadzole with fluorinated polymer. European Polymer Journal, 2013, 49(6): 1564-1576. DOI:10.1016/j.eurpolymj.2013.01.028 (  0) 0) |
| [104] |
Carollo A, Quartarone E, Tomasi C, et al. Developments of new proton conducting membranes based on different polybenzimidazole structures for fuel cells applications. Journal of Power Sources, 2006, 160(1): 175-180. DOI:10.1016/j.jpowsour.2006.01.081 (  0) 0) |
| [105] |
Xu N, Guo X X, Fang J H. Synthesis of novel polybenzimidazoles with pendant amino groups and the formation of their crosslinked membranes for medium temperature fuel cell applications. Journal of Polymer Science Part A Polymer Chemistry, 2009, 47(24): 6992-7002. DOI:10.1002/pola.23738 (  0) 0) |
| [106] |
Bhadra S, Kim N H, Lee J H. A new self-cross-linked, net-structured, proton conducting polymer membrane for high temperature proton exchange membrane fuel cells. Journal of Membrane Science, 2010, 349(1-2): 304-311. DOI:10.1016/j.memsci.2009.11.061 (  0) 0) |
| [107] |
Mustarelli P, Quartarone E, Grandi S., et al. Increasing the permanent conductivity of PBI membranes for HT-PEMs. Solid State Ionics, 2012, 225: 228-231. DOI:10.1016/j.ssi.2012.04.007 (  0) 0) |
| [108] |
Berber M R, Nakashima N. Bipyridine-based polybenzimidazole membranes with outstanding hydrogen fuel cell performance at high temperature and non-humidifying conditions. Journal of Membrane Science, 2019, 591: 117354. DOI:10.1016/j.memsci.2019.117354 (  0) 0) |
| [109] |
Chen H, Wang S, Liu F X., et al. Base-acid doped polybenzimidazole with high phosphoric acid retention for HT-PEMFC applications. Journal of Membrane Science, 2020, 596: 117722. DOI:10.1016/j.memsci.2019.117722 (  0) 0) |
| [110] |
Wang D, Wang S, Tian X, et al. Ethyl phosphoric acid grafted amino-modified polybenzimidazole with improved long-term stability for high-temperature proton exchange membrane applications. International Journal of Hydrogen Energy, 2020, 45(4): 3176-3185. DOI:10.1016/j.ijhydene.2019.11.219 (  0) 0) |
| [111] |
Ni J P, Hu M S, Liu D, et al. Synthesis and properties of highly branched polybenzimidazoles as proton exchange membranes for high-temperature fuel cells. Journal of Materials Chemistry C, 2016, 4(2): 4814-4821. DOI:10.1039/C6TC00862C (  0) 0) |
| [112] |
Hu M S, Ni J P, Zhang B, et al. Crosslinked polybenzimidazoles containing branching structure as membrane materials with excellent cell performance and durability for fuel cell applications. Journal of Power Sources, 2018, 389: 222-229. DOI:10.1016/j.jpowsour.2018.04.025 (  0) 0) |
| [113] |
Wang L, Ni J, Liu D, et al. Effects of branching structures on the properties of phosphoric acid-doped polybenzimidazole as a membrane material for high-temperature proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 2018, 43(34): 16694-16703. DOI:10.1016/j.ijhydene.2018.06.181 (  0) 0) |
| [114] |
Wang L, Liu Z R, Liu Y, et al. Crosslinked polybenzimidazole containing branching structure with no sacrifice of effective N-H sites: towards high-performance high-temperature proton exchange membranes for fuel cells. Journal of Membrane Science, 2019, 583: 110-117. DOI:10.1016/j.memsci.2019.04.030 (  0) 0) |
| [115] |
Wang L, Liu Z R, Ni J P, et al. Preparation and investigation of block polybenzimidazole membranes with high battery performance and low phosphoric acid doping for use in high-temperature fuel cells. Journal of Membranes Science, 2019, 572: 350-357. DOI:10.1016/j.memsci.2018.10.083 (  0) 0) |
| [116] |
Wang L, Wu Y N, Fang M L, et al. Synthesis and preparation of branched block polybenzimidazole membranes with high proton conductivity and single-cell performance for use in high temperature proton exchange membrane fuel cells. Journal of Membrane Science, 2020, 602: 117981. DOI:10.1016/j.memsci.2020.117981 (  0) 0) |
| [117] |
Bai H J, Peng H Q, Xiang Y., et al. Poly(arylene piperidine)s with phosphoric acid doping as high temperature polymer electrolyte membrane for durable, high-performance fuel cells. Journal of Power Sources, 2019, 443: 227219. DOI:10.1016/j.jpowsour.2019.227219 (  0) 0) |
| [118] |
Lafitte B, Jannasch P. Phosphonation of polysulfones via lithiation and reaction with chlorophosphonic acid esters. Journal of Polymer Science Part A Polymer Chemistry, 2004, 43(2): 273-286. DOI:10.1002/pola.20487 (  0) 0) |
| [119] |
Abouzari-Lotf E, Ghassemi H, Shockravi A, et al. Phosphonated poly(arylene ether)s as potential high temperature proton conducting materials. Polymer, 2011, 52(21): 4709-4717. DOI:10.1016/j.polymer.2011.08.020 (  0) 0) |
| [120] |
Ingratta M, Elomaa M, Jannasch P, et al. Grafting poly(phenylene oxide) with poly(vinylphosphoric acid) for fuel cell membranes. Polymer Chemistry, 2010, 1(5): 739-746. DOI:10.1039/b9py00390h (  0) 0) |
| [121] |
Bano S, Negi Y S, Ramya K. Studies on new highly phosphonated poly (ether ether ketone) based promising proton conducting membranes for high temperature fuel cell. International Journal of Hydrogen Energy, 2019, 44(54): 28968-28983. DOI:10.1016/j.ijhydene.2019.09.067 (  0) 0) |
| [122] |
Bu F Z, Zhang Y R, Hong L H, et al. 1, 2, 4-Triazole functionalized poly(arylene ether ketone) for high temperature proton exchange membrane with enhanced oxidative stability. Journal of Membrane Science, 2018, 545: 167-175. DOI:10.1016/j.memsci.2017.09.072 (  0) 0) |
| [123] |
Zhang J J, Zhang J, Bai H J, et al. A new high temperature polymer electrolyte membrane based on tri-functional group grafted polysulfone for fuel cell application. Journal of Membrane Science, 2019, 572: 496-503. DOI:10.1016/j.memsci.2018.11.035 (  0) 0) |
| [124] |
Bai H J, Wang H N, Zhang J, et al. High temperature polymer electrolyte membrane achieved by grafting poly (1-vinylimidazole) on polysulfone for fuel cells application. Journal of Membrane Science, 2019, 592: 117395. DOI:10.1016/j.memsci.2019.117395 (  0) 0) |
| [125] |
Xu X, Wang H N, Lu S F, et al. A novel phosphoric acid doped poly(ethersulphone)-poly(vinylpyrrolidone) blend membrane for high-temperature proton exchange membrane fuel cells. Journal of Power Sources, 2015, 286: 458-463. DOI:10.1016/j.powsour.2015.04.028 (  0) 0) |
| [126] |
Guo Z B, Xu X, Xiang Y, et al. New anhydrous proton exchange membranes for high-temperature fuel cells based on PVDF-PVP blended polymers. Journal of Materials Chemistry, 2015, 3: 148-155. DOI:10.1039/C4TA04952G (  0) 0) |
| [127] |
Lu S F, Xiu R J, Xu X, et al. Polytetrafluoroethylene (PTFE) reinforced poly(ethersulphone)-poly(vinyl pyrrolidone) composite membrane for high temperature proton exchange membrane fuel cells. Journal of Membrane Science, 2014, 464: 1-7. DOI:10.1016/j.memsci.2014.03.053 (  0) 0) |
| [128] |
Bai H J, Wang H N, Zhang J, et al. Simultaneously enhancing ionic conduction and mechanical strength of poly(ether sulfones)-poly(vinyl pyrrolidone) membrane by introducing graphitic carbon nitride nanosheets for high temperature proton exchange membrane fuel cell application. Journal of Membrane Science, 2018, 558: 26-33. DOI:10.1016/j.memsci.2018.04.039 (  0) 0) |
| [129] |
Niu B B, Yi W D, Liang J T, et al. Solid electrolyte Sm0.2Ce0.8O2-δ reinforced polymer composite membranes for high temperature proton exchange membrane fuel cells. Materials Letters, 2021, 286: 12941. DOI:10.1016/j.matlet.2020.129241 (  0) 0) |
| [130] |
Kourasi M, Wills R G A, Shah A A, et al. Heteropolyacids for fuel cell applications. Electrochimica Acta, 2014, 127: 454-466. DOI:10.1016/j.electacta.2014.02.006 (  0) 0) |
| [131] |
Zhang J, Chen S A, Bai H J, et al. Effects of phosphotungstic acid on performance of phosphoric acid doped polyethersulfone-polyvinylpyrrolidone membranes for high temperature fuel cells. International Journal of Hydrogen Energy, 2021, 46(19): 11104-11114. DOI:10.1016/j.electacta.2014.02.006 (  0) 0) |
| [132] |
Kowsari E, Ansari V, Moradi A, et al. Poly(amide-imide) bearing imidazole groups/sulfonated polyimide blends for low humidity and medium temperature proton exchange membranes. Journal of Polymer Research, 2015, 22: 77. DOI:10.1007/s10965-015-0729-z (  0) 0) |
| [133] |
Wang K L, Yang L, Wei W X, et al. Phosphoric acid-doped poly(ether sulfone benzotriazole) for high-temperature proton exchange membrane fuel cell applications. Journal of Membrane Science, 2018, 549: 23-27. DOI:10.1016/j.memsci.2017.11.067 (  0) 0) |
| [134] |
Hsu S L C, Liu C W, Tu C H, et al. Synthesis and properties of ABPPQ for high-temperature proton exchange membrane fuel cells. Polymer Bulletin, 2018, 75: 5321-5331. DOI:10.1007/s00289-018-2332-z (  0) 0) |
| [135] |
Güler E, Sadeghi S, Gürsel S A. Characterization and fuel cell performance of divinylbenzene crosslinked phosphoric acid doped membranes based on 4-vinylpyridine grafting onto poly(ethylene-co-tetrafluoroethylene)films. International Journal of Hydrogen Energy, 2018, 43(16): 8088-8099. DOI:10.1016/j.ijhydene.2018.03.087 (  0) 0) |
| [136] |
Li R, Wang H D, Wang W F, et al. Gamma-ray co-irradiation induced graft polymerization of NVP and SSS onto polypropylene non-woven fabric and its blood compatibility. Radiation Physics and Chemistry, 2013, 91: 132-137. DOI:10.1016/j.radphyschem.2013.05.034 (  0) 0) |
| [137] |
Li H, Shen X, Hua H M, et al. A novel single-ion conductor gel polymer electrolyte prepared by co-irradiation grafting and electrospinning process. Solid State Ionics, 2020, 347: 115246-115254. DOI:10.1016/j.ssi.2020.115246 (  0) 0) |
| [138] |
Guan Y J, Fang J, Fu T, et al. Preparation and characterization of mono-sheet bipolar membranes by pre-irradiation grafting method for fuel cell applications. Journal of Power Sources, 2016, 327: 265-272. DOI:10.1016/j.jpowsour.2016.07.060 (  0) 0) |
| [139] |
Wang L Q, Brink J J, Liu Y, et al. Non-fluorinated pre-irradiation-grafted (peroxidated) LDPE-based anion-exchange membranes with high performance and stability. Energy & Enviromental Science, 2017, 10(10): 2154-2167. DOI:10.1039/C7EE02053H (  0) 0) |
| [140] |
Chu H Y, Fu C, Wu X Y, et al. Enhancing released electrical energy density of poly(vinylidene fluoride-co-trifluoroethylene)-graft-poly(methyl methacrylate) via the pre-irradiation method. Applied Surface Science, 2018, 465: 643-655. DOI:10.1016/j.apsusc.2018.08.222 (  0) 0) |
| [141] |
Schmidt C, Schmidt-Naake G. Proton conducting membranes obtained by doping radiation-grafted basic membrane matrices with phosphoric acid. Macromolecular Materials and Engineering, 2007, 292(10-11): 1164-1175. DOI:10.1002/mame.200700188 (  0) 0) |
| [142] |
SanlıL I, Tas S, Yürüm Y, et al. Water free operated phosphoric acid doped radiation-grafted proton conducting membranes for high temperature polymer electrolyte membrane fuel cells. Fuel Cells, 2014, 14(6): 914-925. DOI:10.1002/fuce.201300110 (  0) 0) |
| [143] |
Sithambaranathana P, Nasef M M, Ahmad A, et al. Crosslinked composite membrane by radiation grafting of 4-vinylpyridine/trially-cyanurate mixtures onto poly(ethylene-co-tetrafluoroethylene) and phosphoric acid doping. International Journal of Hydrogen, 2017, 42(14): 9333-9341. DOI:10.1016/j.ijhydene.2016.03.140 (  0) 0) |
| [144] |
Li X, Zhao Y, Li W W, et al. Molecular dynamics simulation of radiation grafted FEP films as proton exchange membranes: Effects of the side chain length. International Journal of Hydrogen Energy, 2017, 42(50): 29977-29987. DOI:10.1016/j.ijhydene.2017.09.043 (  0) 0) |
| [145] |
Kim B N, Lee D H, Han D H. Characteristics of fuel cell membranes prepared by EB radiation grafting onto FEP with styrene derivatives, styrene and 2-methylstyrene. Journal of The Electrochemical Society, 2008, 155(7): B680-B685. DOI:10.1149/1.2909867 (  0) 0) |
| [146] |
Sadeghi S, Şanli L I, Guler E, et al. Enhancing proton conductivity via sub-micron structures in proton conducting membranes originating from sulfonated PVDF powder by radiation-induced grafting. Solid State Ionics, 2018, 314: 66-73. DOI:10.1016/j.ssi.2017.11.017 (  0) 0) |
| [147] |
Abdel-Hady E E, EI-Toony M M, Abdel-Hamed M O. Grafting of glycidyl methacrylate/styrene onto polyvinyldine fluoride membranes for proton exchange fuel cell. Electrochimica Acta, 2013, 103: 32-37. DOI:10.1016/j.electacta.2013.03.193 (  0) 0) |
| [148] |
Ke X, Zhang Y F, Gohs U, et al. Polymer electrolyte membranes prepared by graft copolymerization of 2-acrylamido-2-methylpropane sulfonic acid and acrylic acid on PVDF and ETFE activated by electron beam treatment. Polymers, 2019, 11(7): 1175-1187. DOI:10.3390/polym11071175 (  0) 0) |
| [149] |
Chen J, Asano M, Yamaki T, et al. Preparation of sulfonated crosslinked PTFE-graft-poly(alkyl vinyl ether) membranes for polymer electrolyte membrane fuel cells by radiation processing. Journal of Membrane Science, 2005, 256(1-2): 38-45. DOI:10.1016/j.memsci.2005.02.005 (  0) 0) |
| [150] |
Yoshida M, Kimura Y, Chen J H, et al. Preparation of PTFE-based fuel cell membranes by combining latent track formation technology with graft polymerization. Radiation Physics and Chemistry, 2009, 78(12): 1060-1066. DOI:10.1016/j.radphyschem.2009.06.021 (  0) 0) |
| [151] |
Park K R, Kang P H, Nho Y C. Preparation of PFA-g-polystyrene sulfonic acid membranes by the γ-radiation grafting of styrene onto PFA films. Reactive and Functional Polymers, 2005, 65(1-2): 47-56. DOI:10.1016/j.reactfunctpolym.2004.11.009 (  0) 0) |
| [152] |
Kang S, Jung D, Shin J, et al. Long-term durability of radiation-grafted PFA-g-PSSA membranes for direct methanol fuel cells. Journal of Membrane Science, 2013, 447: 36-42. DOI:10.1016/j.memsci.2013.07.005 (  0) 0) |
| [153] |
Ghobashy M M, Elbarbary A M, EI-Sawy N M, et al. Proton-conducting polymers derived from radiation grafting and sulphonation of poly(tetraflouroethylene-perflourovinyl ether) film with three rare-earth elements. Macromolecular Research, 2017, 25: 924-930. DOI:10.1007/s13233-017-5136-3 (  0) 0) |
| [154] |
Schmidt-Naake G, Böhme M, Cabrera A. Synthesis of proton exchange membranes with pendent phosphonic acid groups by irradiation grafting of VBC. Chemical Engineering & Technology, 2005, 28(6): 720-724. DOI:10.1002/ceat.200407143 (  0) 0) |
| [155] |
Schmidt-Naake G. Polymer-Säure-Komposite auf Basis strahlungsinduzierter Pfropfpolymerisation für Hochtemperatur-PEM-Brennstoffzellen. Chemie Ingenieur Technik, 2009, 81(5): 599-609. DOI:10.1002/cite.200900003 (  0) 0) |
| [156] |
Chandan A, Hattenberger M, El-kharouf A., et al. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC) - A review. Journal of Power Sources, 2013, 231: 264-278. DOI:10.1016/j.jpowsour.2012.11.126 (  0) 0) |
| [157] |
Saidi H, Uthman H. Phosphoric acid doped polymer electrolyte membrane based on radiation grafted poly(1- vinylimidazole-co-1-vinyl-2-pyrrolidone)-g-poly(ethylene/ tetrafluoroeth-ylene) copolymer and investigation of grafting kinetics. International Journal of Hydrogen Energy, 2017, 42(14): 9315-9332. DOI:10.1016/j.ijhydene.2016.06.187 (  0) 0) |
| [158] |
Sithambaranathan P, Nasef M M, Ahmad A. Crosslinked composite membrane by radiation grafting of 4-vinylpyridine/triallyl-cyanurate mixtures onto poly(ethylene-co-tetrafluoroethylene) and phosphoric acid doping. International Journal of Hydrogen Energy, 2017, 42(14): 9333-9341. DOI:10.1016/j.ijhydene.2016.03.140 (  0) 0) |
| [159] |
Li X, Drache M, Gohs U, et al. Novel concept of polymer electrolyte membranes for high-temperature fuel cells based on ETFE grafted with neutral acrylic monomers. Journal of Membrane Science, 2015, 495: 20-28. DOI:10.1016/j.memsci.2015.07.056 (  0) 0) |
| [160] |
De Klerk A. Key catalyst types for the efficient refining of Fischer-Tropsch syncrude: alumina and phosphoric acid. Catalysts, 2011, 23: 1-49. DOI:10.1039/9781849732772-00001 (  0) 0) |
| [161] |
Li X, Drache M, Ke X, et al. Fuel cell application of high temperature polymer electrolyte membranes obtained by graft copolymerization of acrylic acid and 2-hydroxyethylmethacrylate on ETFE backbone material. Macromolecular Materials and Engineering, 2016, 301: 56-64. DOI:10.1002/mame.201500207 (  0) 0) |
| [162] |
Benedix R. Bauchemie: Einführung in die Chemie für Bauingenieure und Architekten. Wiesbaden: Springer, 2020: 513.
(  0) 0) |
| [163] |
Zhang M F, Russell T P. Graft copolymers from poly(vinylidene fluoride-co-chlorotrifluoroethylene) via atom transfer radical polymerization. Macromolecules, 2016, 39(10): 3531-3539. DOI:10.1002/mame.201500207 (  0) 0) |
| [164] |
Holmberg S, Holmlund P, Wilén C-E, et al. Synthesis of proton-conducting membranes by the utilization of preirradiation grafting and atom transfer radical polymerization techniques. Journal of Polymer Science Part A Polymer Chemistry, 2002, 40(4): 591-600. DOI:10.1002/pola.10146 (  0) 0) |
| [165] |
Zhai M L, Chen J H, Hasegawa S, et al. Synthesis of fluorinated polymer electrolyte membranes by radiation grafting and atom transfer radical polymerization techniques. Polymer, 2009, 50(5): 1159-1165. DOI:10.1016/j.polymer.2009.01.014 (  0) 0) |
| [166] |
Eberhardt S H, Lochner T, Büchi F N. Correlating electrolyte inventory and lifetime of HT-PEFC by accelerated stress testing. Journal of the Electrochemical Society, 2015, 162(12): 1367-1372. DOI:10.1149/2.0591512jes (  0) 0) |
| [167] |
Schmidt T J, Baurmeister J. Properties of high-temperature PEFC CeltecⓇ-P 1000 MEAs in start/stop operation mode. Journal of Power Sources, 2008, 176(2): 428-434. DOI:10.1016/j.jpowsour.2007.08.055 (  0) 0) |
| [168] |
Kreuer K D. Fuel Cells. New York: Springer, 2013: 249-303.
(  0) 0) |
| [169] |
Ossiander T, Perchthaler M, Heinzl C, et al. Influence of membrane type and molecular weight distribution on the degradation of PBI-based HTPEM fuel cells. Journal of Membrane Science, 2016, 509: 27-35. DOI:10.1016/j.memsci.2016.02.037 (  0) 0) |
| [170] |
SØndergaard T, Cleemann L N, Becker H, et al. Long-term durability of HT-PEM fuel cells based on thermally cross-linked polybenzimidazole. Journal of Power Sources, 2017, 342: 570-578. DOI:10.1016/j.jpowsour.2016.12.075 (  0) 0) |
| [171] |
Choe E W, Choe D D. Polymeric Materials Encyclopedia. New York: CRC Press, 1996: 5619.
(  0) 0) |
| [172] |
He R H, Li Q F, Bach A, et al. Physicochemical properties of phosphoric acid doped polybenzimidazole membranes for fuel cells. Journal of Membrane Science, 2006, 277(1-2): 38-45. DOI:10.1016/j.memsci.2005.10.005 (  0) 0) |
| [173] |
Yang J S, Cleemann L N, Steenberg T, et al. High molecular weight polybenzimidazole membranes for high temperature PEMFC. Fuel Cells, 2014, 14: 7-15. DOI:10.1002/fuce.201300070 (  0) 0) |
| [174] |
Bai H J, Peng H Q, Yan X, et al. Poly(arylene piperidine)s with phosphoric acid doping as high temperature polymer electrolyte membrane for durable, high-performance fuel cells. Journal of Power Sources, 2019, 443: 227219. DOI:10.1016/j.jpowsour.2019.227219 (  0) 0) |
| [175] |
Li Q F, Jensen J O, Savinell R F, et al. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Progress in Polymer Science, 2009, 34(5): 449-477. DOI:10.1016/j.progpolymsci.2008.12.003 (  0) 0) |
| [176] |
Wang L, Advani S G, Prasad A K. Degradation reduction of polymer electrolyte membranes using CeO2 as a free-radical scavenger in catalyst layer. Electrochimica Acta, 2013, 109: 775-780. DOI:10.1016/j.electacta.2013.07.189 (  0) 0) |
| [177] |
Weissbach T, Peckham T J, Holdcroft S. CeO2, ZrO2 and YSZ as mitigating additives against degradation of proton exchange membranes by free radicals. Journal of Membrane Science, 2016, 498: 94-104. DOI:10.1016/j.memsci.2015.10.004 (  0) 0) |
| [178] |
Hao J K, Jiang Y Y, Gao X Q, et al. Degradation reduction of polybenzimidazole membrane blended with CeO2 as a regenerative free radical scavenger. Journal of Membrane Science, 2017, 522: 23-30. DOI:10.1016/j.memsci.2016.09.010 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


