2. Department of Chemistry and the Tsinghua Center for Frontier Polymer Research, Tsinghua University, Beijing 100084, China
Considering the ongoing advancements in science and technology, several problems are becoming increasingly prominent, such as the energy crises and environment pollution. The key to solving current problems is exploring and designing new friendly energy storage devices[1-3]. Therefore, it is very crucial to create a large number of eminent energy storage and conversion devices. Electrochemical capacitors, also known as supercapacitors (SCs), have become people's choice due to their excellent electrochemical characteristics such as excellent power density, stable safety and ultralong cycle lifetime[4-6].
MOF is considered a promising advanced material due to its high porosity and tunable crystal structure, which is usually used in catalysis, gas adsorption, supercapacitor and sensing[7-8]. In particular, its porous structure is always modified to increase the specific capacity and electrical conductivity to achieve the desired high energy density. Compared with monometallic systems, bimetallic MOF exhibits superior electrochemical performance[9-11]. Therefore, it has received more and more attention in supercapacitors. Particularly, LDH derived from MOFs is becoming prominent competitor. LDH is an application material which is suitable for supercapacitors. It is characterized by the rapid propagation of charged ions[12-13]. Some reports indicate that the hydrophilicity of LDH facilitates the dispersion of ions in aqueous electrolytes. Faraday redox charge transfer occurs on the surface of LDH[14-16]. Ran et al. revealed a carboxylated carbon nanotubes (C/CNTs) initiated the method of crystal growth to blend MOF polyhedrons associated by C-CNTs "bridge"[17]. The results demonstrate that hollow nanostructures of NiCo-LDH/C-CNTs derived from MOF exhibit excellent electrochemical properties and extraordinary rate properties when used as battery-type electrodes for supercapacitor applications. However, due to the large size of MOFs, in situ growth of their conductive substrates has been a challenge[18-19], and related studies are mostly performed on powders[20]. However, the powder electrode is easy to agglomerate, resulting in poor conductivity, which seriously hinders the application of powder in the area of energy storage.
Herein, the self-sacrificial templates of Co-MOF anchored on CC are controllably transformed into NiCo-LDH three-dimensional porous nanostructure by the method of ion etching/exchange reaction. Among them, the best reason for the improvement of electrochemical properties is the layered double metal hydroxides with ultrathin nanoflakes. Its advantage is that it is a battery electrode with a higher specific capacity, a high energy density of 27.39 W·h/kg, and a capacity retention rate of 93.5% after 5000 cycles.
1 Experiment 1.1 Preparation of CC/Co-MOFDuring this experiment, the chemicals were not used for further processing and were of analytical grade. The experimental water was deionized water. Co-MOFs were synthesized using a typical chemical solvent reaction. Firstly, Co(NO3)2·6H2O(50 mL) and 2-methylimidazole (0.4 mol/L) were dissolved in 40 mL of deionized water with a clean CC immersed in the solution. It was magnetically stirred for 4 h at room temperature, and then dried at 80 ℃ for 12 h.
1.2 Preparation of CC/NiCo-LDHTypically, 20 mg Ni(NO3)2·6H2O was dispersed in 30 mL of ethanol under continuous magnetic stirring for 10 min. Next, the solution and the CC coated with the Co-MOF were poured into a 50 mL Teflon-lined autoclave and heated at 120 ℃ for 2 h at the rate of 10℃/min. Subsequent to drying at 60 ℃ for 12 h, the mass loading is ~1mg/cm2.
1.3 CharacterizationThe crystalline and morphologies messages were characterized by X-ray diffraction (XRD, D/max TTR-III, Cu Kα), scanning electron microscope (SEM, SUPRA 40, German Zeiss) and transmission electron microscopy (TEM, Tecnai G2 F20). The element content was determined using X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific Esxalab 250Xi) with a spectrometer with a dual Al Kα X-ray source (1486.6 eV).
1.4 Electrochemical MeasurementThe electrochemical measurements of samples were conducted in 2 mol/L KOH with a three-electrode. Pt film and Ag/AgCl electrode were taken as counter electrode and reference electrode, respectively. The following Eq.(1) was used to calculate the specific capacity under different current densities[21]:
| $ C_s=(I \times \Delta t) /(3.6 \times m) $ | (1) |
where Cs, I and m are assigned to the specific capacity (mA·h/g), current (A) and the mass of the NiCo-LDH sample (g), respectively.
The following Eqs.(2)-(3) were employed to calculate the relevant energy and power density in the HSCs[22]:
| $ E=i \int V \mathrm{~d} t / M $ | (2) |
| $ P=E / \Delta t $ | (3) |
where M and Δt were employed to be on behalf of the total mass of two electrodes (g) and the discharge time (h), respectively.
2 Results and DiscussionFirst, the Co-MOF nanosheets were anchored on CC through the coordination of 2-methylimidazole with metal ions in aqueous solution according to the chemical aqueous reaction.
Second, the Co-MOF was changed over into NiCo-LDH three-dimensional nanostructures by two steps. In the process of transformation, protons produced by Ni2+ hydrolysis destroy the coordination bond of Co-MOF and release Co2+ ions, which are then oxidized to Co3+ by ethanol[23]. Then, the protons produced by Ni2+ hydrolysis continuously etch the Co-MOF template and the shaped Co2+/Co3+ co-precipitated with Ni2+ and OH- to develop into NiCo-LDH nanosheets, which were then assembled into 3D structures[24]. Finally, the three-dimensional hierarchical structure of NiCo-LDH was formed by assembling stacking.
The XRD images of NiCo-LDH converted from Co-MOF is depicted in Fig. 1(a). Clearly, the strong diffraction peak at 26.3° corresponds to the carbon cloth substrate. Otherwise, almost all the diffraction peaks of 11.6° and 46.9° correspond to NiCo-LDH (JCPDS No. 40-0216). In other words, the Co-MOF is successfully transformed into a porous NiCo-LDH nanostructure. In Fig. 1(b)-(c), it can be clearly seen that the dense triangular Co-MOF nanosheets are covered on the CC, and NiCo-LDH is obtained after the hydrothermal ion exchange reaction (Fig. 1(d) (e)). In addition, the enlarged view clearly reveals that NiCo-LDH is assembled with ultrathin nanosheets, as displayed in Fig. 1(f).

|
Fig.1 Synthesis and morphology of CC/Co-MOF and CC/NiCo-LDH: On the top is the schematic illustration of the synthesis process, (a) XRD, (b, c) SEM images of the CC/Co-MOF nanosheet arrays; (d, e, f) SEM images of CC/NiCo-LDH nanosheet arrays |
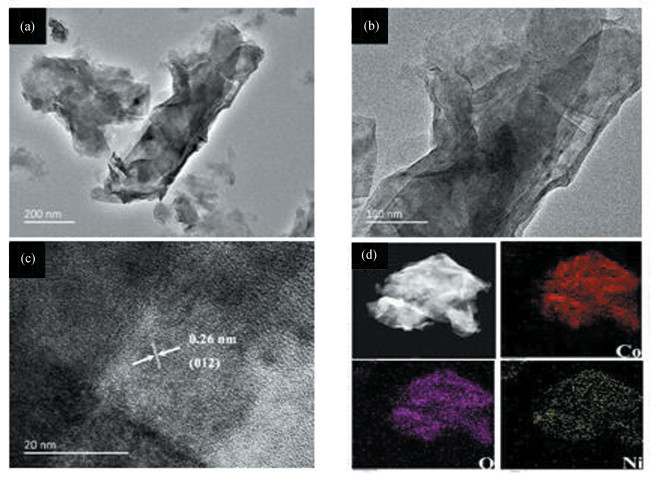
In Fig. 2(a)-(b), the porous structure of the CC/NiCo-LDH 2D nanosheet arrays can be clearly observed, which is due to the fact that the active nickel ions replace part of the cobalt ions on the CC/Co-MOF during the hydrothermal reaction [25]. Moreover, the thickness of the ultrathin nanosheet is ~20 nm. Images from the marginal region of HR-TEM distinctly show a plane spacing of 0.26 nm, which can be ascribed to the (012) plane of the CC/NiCo-LDH nanostructure arrays (Fig. 2(c)). In addition, the Co, Ni and O elements are uniformly present in the EDS mapping (Fig. 2(d)).
|
Fig.2 Internal structure analysis of CC/NiCo-LDH: (a, b) TEM, (c) HRTEM, (d) Elemental mapping images of CC/NiCo-LDH |
In Fig. 3(a), Ni, Co and O species are all present. As shown in Fig. 3(b), two convolution peaks are Ni 2p1/2 and Ni 2p3/2, which prove that both Ni2+ (856.43 and 873.91 eV) and Ni3+ (875.97 and 857.22 eV) co-exist in CC/Ni Co-LDH. Meanwhile, two peaks situated at 862.94 and 880.70 eV are attributed to the satellites (Sat.). Fig. 3(c) displays that the peaks at 782.68 and 798.22 eV for the Co 2p of CC/Ni Co-LDH are described to Co2+, in the meantime, the peaks situated at 782.63 and 796.94 eV are described to Co3+. In addition, the remaining two peaks are satellite peaks. In Fig. 3(d), three obvious diffraction peaks of O 1s can be observed, the lattice oxygen, oxygen vacancy and surface adsorbed oxygen correspond to 531.41, 532.19 and 533.60 eV, respectively.

|
Fig.3 XPS curves of (a) high-resolution XPS spectra, (b) Ni 2p, (c) Co 2p, (d) O 1s for CC/NiCo-LDH |
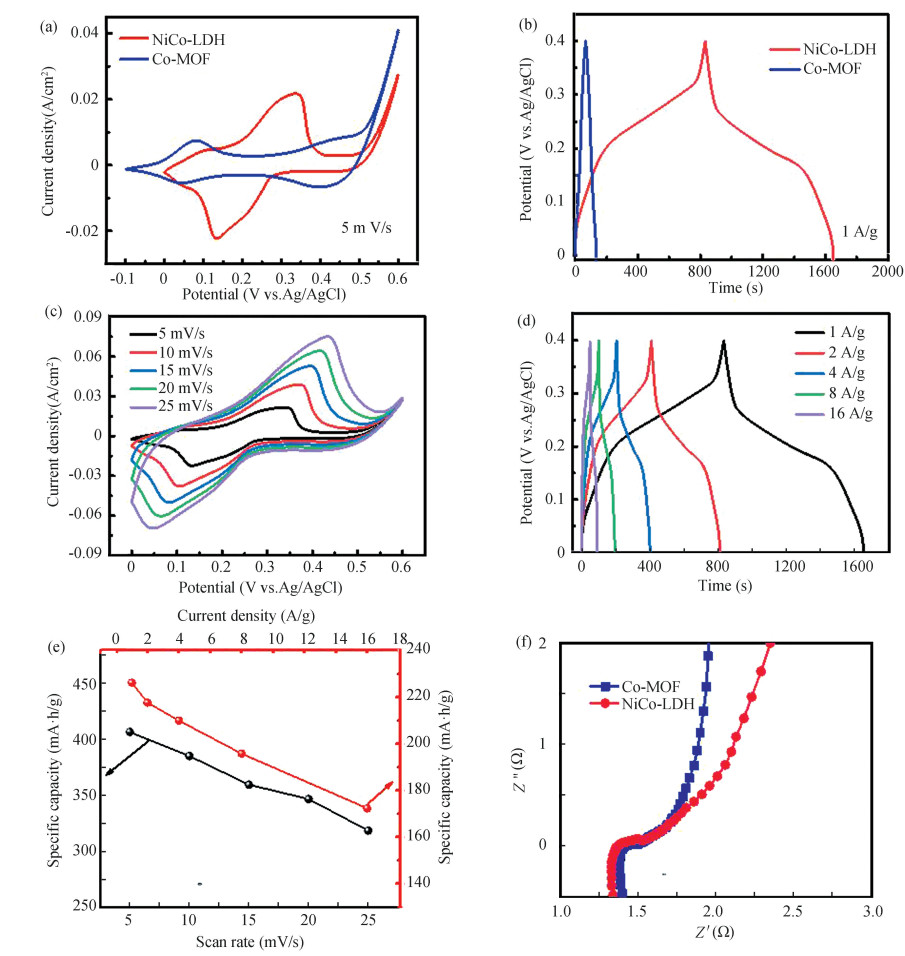
In Fig. 4(a), the range of study is the scanning rate of 5 mV/s. From this figure, the curves exhibited distinct and paired redox peaks showing that the two electrodes are battery-type. Meanwhile, the CV curve of CC/NiCo-LDH has a larger specific current in the same potential range, indicating the largest specific capacity. Moreover, the GCD curves of CC/Co-MOF and CC/NiCo-LDH electrodes at 1 A/g are exhibited in Fig. 4(b). The specific capacity of CC/NiCo-LDH electrode can reach 226.3 mA·h/g, whereas only 18.6 mA·h/g is obtained for CC/Co-MOF electrode.
|
Fig.4 Electrochemical process of the two samples: (a, c) CV curves, (b, d) GCD curves (e) Specific capacity of CC/NiCo-LDH as a function of scan rate, (f) Electrochemical impedance spectroscopy (EIS) as Nyquist plots of CC/Co-MOF and CC/NiCo-LDH |
In Fig. 4(c), the CV curves represent a proliferation manipulated electrochemical process and rapid ionic and electronic transport. The probable faradaic reactions are shown below:
| $ \mathrm{Ni}(\mathrm{OH})_2+\mathrm{OH}^{-} \leftrightarrow \mathrm{NiOOH}+\mathrm{H}_2 \mathrm{O}+\mathrm{e}^{-} $ | (4) |
| $ \mathrm{Co}(\mathrm{OH})_2+\mathrm{OH}^{-} \leftrightarrow \mathrm{CoOOH}+\mathrm{H}_2 \mathrm{O}+\mathrm{e}^{-} $ | (5) |
In addition, as displayed in Fig. 4(d), the GCD curves of this electrode are observed at different current densities from 1 to 16 A/g. The symmetric GCD curves provide a signature of high-velocity function with highly reversible and high-speed reply dynamics. Remarkably, the Cs obtained from the CC/NiCo-LDH electrode is 226.3 mA·h/g and 172.4 mA·h/g at current densities of 1 A/g and 16 A/g, indicating the excellent rate properties based on the porous hierarchical structure of NiCo-LDH (in Fig. 4(e)). In addition, EIS tests also confirmed lower RS impedance and faster kinetics on NiCo-LDH. As shown in Fig. 4(f), the equivalent series resistance (Rs) is the intersection with the real axis, and it includes electrolyte contact resistance, ion resistance and intrinsic resistance. Apparently, the Rs values are 1.41 and 1.36 Ω for CC/Co-MOF and CC/NiCo-LDH electrodes, respectively. It is worth noting that the CC/NiCo-LDH electrode shows high electronic conductivity, low resistance and rapid ion proliferation speed, revealing better charge transfer and ion pervasion dynamics behaviour.
The HSC device was assembled to prove the actual submission, where CC/ NiCo-LDH and AC are used as positive and negative electrodes, respectively (Fig. 5(a)). The mass ratio is calculated as follows:

|
Fig.5 Electrochemical behavior of the HSC devices: (a) Schematic illustration, (b, d) CV curves, (c, e) GCD curves, (f) Specific capacity calculated from GCD curves and CV curves |
| $ m_{+} / m_{-}=\left(C_s^{-} \times \Delta V_{-}\right) /\left(C_s^{+} \times \Delta V_{+}\right) $ | (6) |
where m corresponds to the mass (g), Cs refers to the specific capacity (mA·h/g), and ΔV denotes the voltage window.
It is noted that the stabilizing working voltage of the HSC device is 1.4 V in Fig. 5(b). When the voltage is greater than 1.5 V, the oxygen is freed and palpable polarization happens. In a similar way, Fig. 5(c) shows GCD curves of the HSCs at various voltages, denoting that the stabilizing working voltage is 1.4 V at 5 mA/cm2. Fig. 5(d) shows the CV curves of HSCs under various scan rates of 5-25 mV/s. The curves show a quasi-rectangular shape due to the reasonable matching of the positive and negative electrodes. Fig. 5(e) shows the GCD curves of the HSC, which depicts images at different current densities from 1 to 5 mV/s. The electrode had a high-capacity retention of 91.4%, which showed the excellent rate capability of our HSC device (Fig. 5(f)).
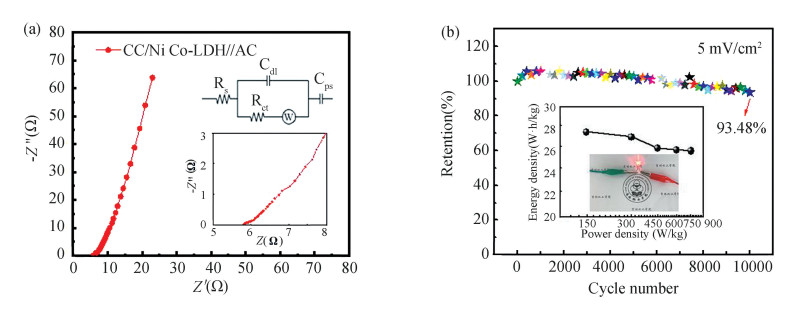
Fig. 6(a) summarizes the Nyquist diagrams, whose insets are the fitted equivalent circuit. It is worth nothing that relatively low intersection with the horizontal axis (Rs) and a slanted line in the low frequency (W) show the potential for energy storage. Certainly, the cycle function of HSC device is significant parameter for the actual submission. As shown in Fig. 6(b), the HSC device has excellent cycle stability after 5000 cycles, and the capacity retention rate reaches 93.5%, which reveals the outstanding stability. Ragone plot of HSC device is also detected for the functional application (inset in Fig. 6(b)). An energy density of CC/NiCo-LDH //AC device can be achieved to 27.39 W·h/kg (with a power density of 148.95 W/kg). It demonstrates that the HSC device composed of the layered structure CC/NiCo-LDH electrode has brilliant energy memory ability. Moreover, to verify the capacity for actual submission, two HSCs are connected in series to assemble a red light-emitting diode (LED) (inset in Fig. 6(b)).
|
Fig.6 EIS and cycling performance of HSC: (a) Nyquist plots curves, (b) Cycling performance.The Ragone plot related to energy and power densities (inset of b) |
3 Conclusions
In summary, we have successfully and controllably converted a self-sacrificial templated Co-MOF anchored on CC into a three-dimensional NiCo-LDH via an ion-etching/ exchange reaction method. Porous NiCo-LDH not only provides smooth channels for electrochemical reactions, but also offers high specific surface area and efficient kinetics. Therefore, CC/NiCo-LDH electrode exhibits excellent electrochemical performance. Moreover, the CC/NiCo-LDH//AC HSC device reaches a good cycle stability of 93.5%. This work comes up with a neoteric method for MOF-derived LDH electrodes with excellent electrochemical performance.
| [1] |
Wang X, Song H Y, Ma S L, et al. Template ion-exchange synthesis of Co-Ni composite hydroxides nanosheets for supercapacitor with unprecedented rate capability. Chemical Engineering Journal, 2021, 432: 134319. DOI:10.1016/j.cej.2021.134319 (  0) 0) |
| [2] |
Wang H Y, He Q Q, Liang S F, et al. Advances and perspectives of ZIFs-based materials for electrochemical energy storage: design of synthesis and crystal structure, evolution of mechanisms and electrochemical performance. Energy Storage Materials, 2021, 43: 531-578. DOI:10.1016/j.ensm.2021.09.023 (  0) 0) |
| [3] |
Abdelaal M M, Hung T C, Mohamed S G, et al. Two birds with one stone: hydrogel-derived hierarchical porous activated carbon toward the capacitive performance for symmetric supercapacitors and lithium-ion capacitors. ACS Sustainable Chemistry & Engineering, 2022, 10(14): 4717-4727. DOI:10.1021/acssuschemeng.2c00266 (  0) 0) |
| [4] |
Naveenkumar P, Maniyazagan M, Yesuraj J, et al. Electrodeposited MnS@Ni (OH)2 core-shell hybrids as an efficient electrode materials for symmetric supercapacitor applications. Electrochimica Acta, 2022, 412: 140138. DOI:10.1016/j.electacta.2022.140138 (  0) 0) |
| [5] |
Wei C Z, Yu Z J, Guo Z C, et al. Formation of carbon coated yolk-shelled Fe3O4-CeO2 hollow spheres toward remarkable performance supercapacitors. Journal of Energy Storage, 2022, 54: 105269. DOI:10.1016/j.est.2022.105269 (  0) 0) |
| [6] |
Zhou J J, Ji W X, Xu L, et al. Controllable transformation of CoNi-MOF-74 on Ni foam into hierarchical-porous Co (OH)2/Ni (OH)2 micro-rods with ultra-high specific surface area for energy storage. Chemical Engineering Journal, 2022, 428: 132123. DOI:10.1016/j.cej.2021.132123 (  0) 0) |
| [7] |
Li Y, Huang B, Zhao X, et al. Zeolitic imidazolate framework-L-assisted synthesis of inorganic and organic anion-intercalated hetero-trimetallic layered double hydroxide sheets as advanced electrode materials for aqueous asymmetric super-capacitor battery. Journal of Power Sources, 2022, 527: 231149. DOI:10.1016/j.jpowsour.2022.231149 (  0) 0) |
| [8] |
Zafar N, Yun S, Sun M L, et al. Cobalt-based incorporated metals in metal-organic framework-derived nitrogen-doped carbon as a robust catalyst for triiodide reduction in photovoltaics. ACS Catalysis, 2021, 11(21): 13680-13695. DOI:10.1021/acscatal.1c04286 (  0) 0) |
| [9] |
Ouyang Y, Zhang B, Wang C X, et al. Bimetallic metal-organic framework derived porous NiCo2 S4 nanosheets arrays as binder-free electrode for hybrid supercapacitor. Applied Surface Science, 2021, 542: 148621. DOI:10.1016/j.apsusc.2020.148621 (  0) 0) |
| [10] |
Lan M D, Wang X X, Zhao R J, et al. Metal-organic framework-derived porous MnNi2 O4 microflower as an advanced electrode material for high-performance supercapacitors. Journal of Alloys and Compounds, 2020, 821: 153546. (  0) 0) |
| [11] |
Zhou P, Wan J F, Wang X R, et al. Nickel and cobalt metal-organic-frameworks-derived hollow microspheres porous carbon assembled from nanorods and nanospheres for outstanding supercapacitors. Journal of Colloid and Interface Science, 2020, 575: 96-107. DOI:10.1016/j.jcis.2020.04.083 (  0) 0) |
| [12] |
Zhang C Y, Zhang L, Liu Q, et al. Enhanced interfacial electron transfer by constructing NiCo-LDH hollow nanocages decorated N-doped graphene quantum dots heterojunction for high-performance supercapacitors. Applied Surface Science, 2022, 602: 154352. DOI:10.1016/j.apsusc.2022.154352 (  0) 0) |
| [13] |
Liao F F, Yang G Y, Cheng Q H, et al. Rational design and facile synthesis of Ni-Co-Fe ternary LDH porous sheets for high-performance aqueous asymmetric supercapacitor. Electrochimica Acta, 2022, 428: 140939. DOI:10.1016/j.electacta.2022.140939 (  0) 0) |
| [14] |
Musella E, Gualandi I, Ferrari G, et al. Electrosynthesis of Ni/Al layered double hydroxide and reduced graphene oxide composites for the development of hybrid capacitors. Electrochimica Acta, 2021, 365: 137294. DOI:10.1016/j.electacta.2020.137294 (  0) 0) |
| [15] |
Wu S X, Hui K S, Hui K N, et al. Electrostatic-induced assembly of graphene-encapsulated-aluminum layered double hydroxide core-shell spheres hybrid structure for high-energy and high-power-density asymmetric supercapacitor. ACS Applied Materials&Interfaces, 2017, 9(2): 1395-1406. DOI:10.1021/acsami.6b09355 (  0) 0) |
| [16] |
Wang Y C, Liu Y Y, Chen Z, et al. In situ growth of hydrophilic nickel-cobalt layered double hydroxides nanosheets on biomass waste-derived porous carbon for high-performance hybrid supercapacitors. Green Chemical Engineering, 2022, 3(1): 55-63. DOI:10.1016/j.gce.2021.09.001 (  0) 0) |
| [17] |
Ran F T, Yang X B, Shao L, et al. Boosting the charge storage of layered double hydroxides derived from carbon nanotube-tailored metal organic frameworks. Electrochimica Acta, 2019, 301: 117-125. DOI:10.1016/j.electacta.2019.01.142 (  0) 0) |
| [18] |
Guo S Q, Xu X L, Liu J B, et al. In situ growth of Ni-doped Co-MOF-74 on Ni foam for high-performance electrochemical energy storage. Journal of The Electrochemical Society, 2020, 167(2): 020539. DOI:10.1149/1945-7111/ab6bbc (  0) 0) |
| [19] |
Zhou S Y, Wang S, Zhou S J, et al. An electrochromic supercapacitor based on an MOF derived hierarchical-porous NiO film. Nanoscale, 2020, 12(16): 8934-8941. DOI:10.1039/d0nr01152e (  0) 0) |
| [20] |
Lam D V, Sohail M, Kim J H, et al. Laser synthesis of MOF-derived Ni@Carbon for high-performance pseudocapacitors. ACS Applied Materials&Interfaces, 2020, 12(35): 39154-39162. DOI:10.1021/acsami.0c10235 (  0) 0) |
| [21] |
Xie L, Chen S X, Hu Y C, et al. Construction of phosphatized cobalt nickel-LDH nanosheet arrays as binder-free electrode for high-performance battery-like supercapacitor device. Journal of Alloys and Compounds, 2021, 858: 157652. DOI:10.1016/j.jallcom.2020.157652 (  0) 0) |
| [22] |
Zhang X T, Lu Q F, Guo E Y, et al. NiCo layer double hydroxide/biomass-derived interconnected porous carbon for hybrid supercapacitors. Journal of Energy Storage, 2021, 38: 102514. DOI:10.1016/j.est.2021.102514 (  0) 0) |
| [23] |
Yang Z, Cheng Q H, Li W W, et al. Construction of 2D ZIF-derived hierarchical and hollow NiCo-LDH "nanosheet-on-nano sheet" arrays on reduced graphene oxide/Ni foam for boosted electrochemical energy storage. Journal of Alloys and Compounds, 2021, 850: 156864. DOI:10.1016/j.jallcom.2020.156864 (  0) 0) |
| [24] |
Xue X L, Zhong J Y, Liu J H, et al. Hydrolysis of metal-organic framework towards three-dimensional nickel cobalt-layered double hydroxide for high performance supercapacitors. Journal of Energy Storage, 2020, 31: 101649. DOI:10.1016/j.est.2020.101649 (  0) 0) |
| [25] |
Hu Q, Chai Y R, Zhou X Y, et al. Electrochemical anion-exchanged synthesis of porous Ni/Co hydroxide nanosheets for ultrahigh-capacitance supercapacitors. Journal of Colloid and Interface Science, 2021, 600: 256-263. DOI:10.1016/j.jcis.2021.05.039 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


