2. Laboratory of Physical Chemistry, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, Eindhoven 5600 MB, The Netherlands;
3. Institute for Complex Molecular Systems (ICMS), Eindhoven University of Technology, Eindhoven 5600 MB, The Netherlands
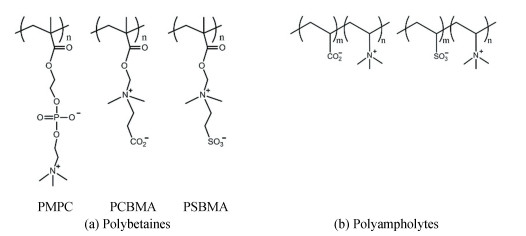
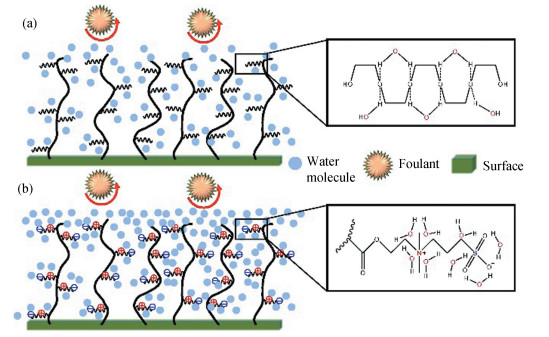
Recently, zwitterion-based materials have attracted more and more attention due to their many distinctive properties for the fabrication of functional coatings. Zwitterion-based materials are commonly regarded as biomimetic materials because they can be found in cell membrane [1-2], proteins and osmolytes[3]. Since the first successful synthesis of zwitterion-based materials reported in the 1950s [4], many more new zwitterionic structures have emerged and continuously evolved. There are two main types of zwitterion-based materials: polybetaines that exhibit both positive and negative charges on the same monomer unit, and polyampholytes with positive and negative charges on different monomer units [5]. Specifically, polybetaines can be further divided into sulfobetaine (SB), phosphobetaine (PB) and carboxybetaine (CB). Polyampholyte polymers, also known as pseudo-zwitterionic polymers, consist of two different monomers bearing the positive and negative charges separately [6-7] (Fig. 1). Despite the characteristics of exhibiting high dipole moments and highly charged groups, zwitterion-based materials remain charge-neutral. Due to the simultaneous presence of positive and negative moieties, zwitterion-based materials can bond the surrounding water molecules by electrostatic interaction to form a hydration layer, which is more stable than the hydrogen bonded water layer provided by other common types of hydrophilic materials such as poly(ethylene glycol) (PEG) [8-9]. Moreover, considering that each repeating unit of PEG-based polymers contains one oxygen atom, it can bond only one water molecule, while zwitterion-based materials can bond up to eight water molecules (Fig. 2) [7].
|
Fig.1 Two typical chemical structures of zwitterion-based materials. (a) Polybetaines, (b) Polyampholytes (Reproduced with permission[7], Copyright 2016, Elsevier) |
|
Fig.2 Schematic showing the difference of the formation of hydration layer between (a) PEG-based polymers and (b) zwitterion-based polymers (Reproduced with permission[7], Copyright 2016, Elsevier) |
Due to this special structure and property, zwitterion-based materials have many potential applications. They could be utilized in marine antifouling (AF) coatings to resist pollutants in water media such as protein, bacteria, diatom and mussels[10]. They have also been demonstrated to be potentially beneficial to medical applications, by preventing the generation of dense collagenous capsules which can block the mass transport and/or electric communication between the biomedical devices implanted in vivo and the body of mice [11], as well as reduce the nonspecific protein adsorption in blood serum [12]. Moreover, they can increase the stability of enzymes in urea while maintaining the bioactivity [13] and cause no immunological response in vivo in blood circulation[14].
In general, a large proportion of reports showed the potential of zwitterion-based materials for fabrication of functional coatings[10, 15-16]. Some of them involve the synthesis of monomeric/polymeric zwitterion-based materials and modification of pre-made coatings with zwitterions, others focus on the working mechanism at molecular level and the comprehensive performance under laboratory and field circumstances[17-19]. This review will mainly report the progress on the fabrication strategies of zwitterion-based functional coatings (zwitterion-bearing binder route, zwitterion-bearing additive route, post-generation of the coatings containing zwitterionic precursors, etc.), and the application field of zwitterion-based functional coatings (medical implants, marine AF, oil-resistant, etc.). The advantages and disadvantages of different fabrication methods and their impact on coating performance (wettability, mechanical properties, antifouling, etc.) will be particularly addressed. Perspective and advice for the current and future developments of zwitterion-based functional coatings will also be briefly discussed.
1 Fabrication MethodsZwitterionic surfaces can be obtained by surface grafting, monolayer-assembly, casting of zwitterion-based coatings, post-treatment generation, etc. Herein, we only focus on zwitterion-based functional coatings which could be fabricated using zwitterion-bearing binders, additives and fillers, or further combined with post-treatment, regardless of their specific application scenarios. The characteristics of different fabrication methods and their comparison with each other will be discussed in detail.
1.1 Zwitterion-Bearing Binder's RouteIncorporating zwitterionic components into polymeric binders is the most common method to fabricate zwitterion-based functional coatings. In this case, zwitterion-based components are chemically attached to polymer chains in advance and act as part of the binders, and also provide the zwitterionic functionality. Generally, this route requires rational molecular structure design and synthesis and the zwitterionic-component should have reactive groups to covalently bind to the film-forming resins.
As mentioned before, zwitterion-based materials are generally regarded as biomimetic materials and harmless to the human body, thus can be used to fabricate implant materials. On the other hand, polydimethylsiloxane(PDMS) is also known as a common kind of biocompatible material and its additional characteristics of being elastic, transparent, gas-permeable and having conformal contact with surfaces, makes it also suitable as human implant material[20]. However, PDMS materials generally exhibit extremely low surface energy and unreactive surfaces which may be in some cases less favorable for (biomedical or marine) applications. Therefore, there are numerous reports proposing the utilization of zwitterion-based materials to improve the overall performance of PDMS.
Fouling-release coatings (FRCs) mainly composed of silicone-based elastomers (i.e., PDMS) are regarded as good alternatives to traditional biocide-based marine AF coatings. The eco-benign FRCs exhibit a low adhesion to biofouling organisms because of their low surface energy and elastic modulus, so that the contaminants can be easily detached under hydrodynamic shear conditions [21-22]. Nevertheless, PDMS suffers from a weak static AF capability because the detachment of adhered contaminants can only be achieved under certain hydrodynamic forces[22]. Besides, PDMS has a poor inhibition capability towards marine slime layers, which mainly consist of bacteria, diatoms (unicellular algae), etc, that facilitate macrofouling colonization[21]. In this case, the intrinsic hydrophobicity of PDMS is responsible for slime attachment. Extensive research has been paid on incorporating zwitterionic materials into PDMS matrices. The coatings are expected to achieve fouling-release capabilities under hydrodynamic situations because of the PDMS segment, and meanwhile fouling-resistant capabilities under static conditions, because of the zwitterion segments. In this regard, we would like to specifically introduce the fabrication of functional coatings by the combination of zwitterion-based materials and PDMS.
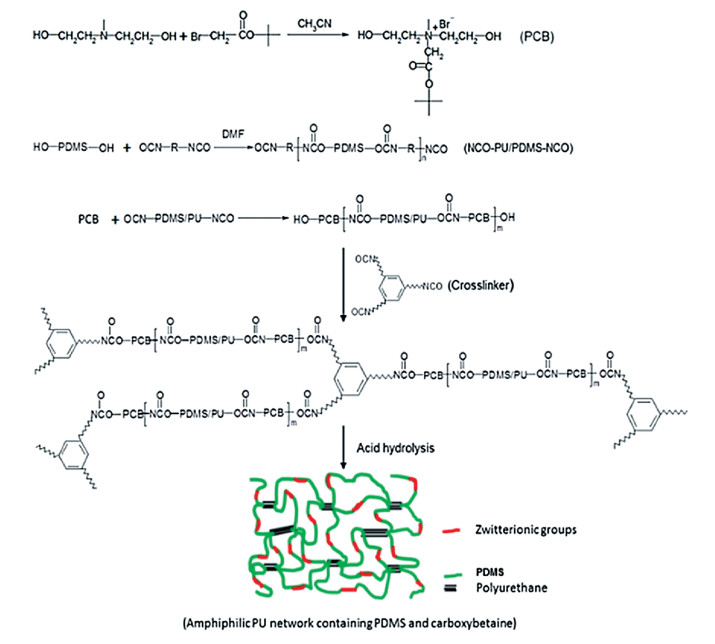
Jiang et al. fabricated zwitterion-based amphiphilic PDMS-polyurethane (PU) AF coatings from a cationic carboxybetaine diol[23] (Fig. 3). Briefly, the PDMS-PU network tethered with cationic carboxybetaine groups was synthesized by the cross-linking of a siloxane diol, a di/triisocyanate and a carboxybetaine-based diol. Then, the zwitterionic structure was achieved by the hydrolysis of carboxybetaine ester groups. Their excellent AF performance was a result of the following three synergistic effects: fouling release by PDMS, antibacterial ability by cationic quaternary amine group, and fouling resistance by the zwitterionic carboxybetaine segment. In another work, Hu et al. grafted a telomer of tertiary carboxybetaine dodecafluoroheptyl ester ethyl acrylate and 3-mercaptopropyltriethoxysilane to hydroxyl-terminated PDMS (PDMS-OH) molecules to fabricate a silicone elastomer with zwitterionic pendant chains[24]. Typically, the fluorocarbon groups drive the orientation of the telomer onto the upper coating surface during the film formation process, and at the same time, the groups of tertiary carboxybetaine dodecafluoroheptyl ester ethyl acrylate were prone to hydrolyses generating an upper coating surface enriched in a zwitterionic structure. AF assays towards marine bacteria (Pseudomonas sp.) and diatoms (Navicula incerta) showed that the cooperation of telomer contributed to the improvement of AF capability. Among the coatings with the telomer content of 10, 20 and 30 wt %, the one with 30 wt % telomer displayed the best AF capability, i.e., 95% decrease in bacteria adsorption and 81% decrease in diatom adhesion. This strategy could effectively avoid the polarity difference between zwitterions and silicones alone and provide a facile and effective way of fabricating silicone elastomer-based coatings with both fouling release and fouling resistance ability.
|
Fig.3 Structure and fabrication of carboxybetaine-based zwitterionic amphiphilic PDMS-PU network for marine AF coatings (Reproduced with permission[23], Copyright 2017, Elsevier) |
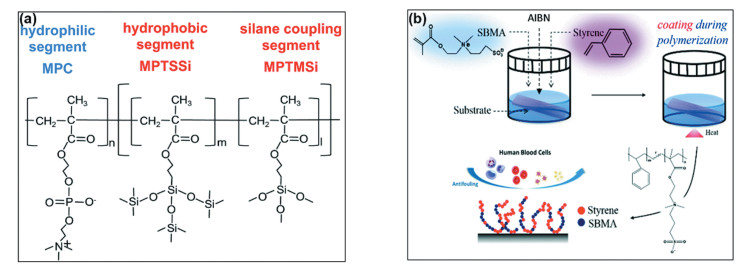
Besides PDMS, zwitterions have also been incorporated into the molecular chains of other film-forming binders of the functional coatings. Using commercially available poly(styrene-co-allyl alcohol) copolymer, Seetho et al. synthesized a phosphorylcholine-containing copolymer and further fabricated zwitterionic amphiphilic anti-biofouling coatings by the ring opening reaction of phosphotriester units in the presence of tertiary amine, i.e., N, N, N′, N′-tetramethyl-1, 4-butane-diamine[25]. In detail, different amount of N, N, N′, N′-tetramethyl-1, 4-butane-diamine was mixed with the solution of phosphorylcholine-containing copolymer. The mixture was then casted onto the silanized glass slides and later immersed in the solution of N, N-dimethylformamide containing excess trimethylamine. Nominally 0, 25% and 50% cross-linked amphiphilic coatings, that is, the maximum amount of cross-linking between the amines and phosphotriester units based upon their stoichiometries, were fabricated to adjust the surface properties of the AF coatings. Among them, 25% cross-linked coatings displayed the highest surface roughness, while 0 and 50% coatings showed lower surface roughness and hence slightly better AF ability towards protein. However, because of the highest surface hydrophilicity, the 0 cross-linked coatings showed the best resistance towards zoospore settlement. Noguchi et al.[26] developed a series of block copolymers from 3-methacryloxypropyl trimethoxysilane (MPTMSi), 3-(methacryloyloxy)propyl tris(tri(methylsilyloxy))silane (MPTSSi), and poly(2-methacryloyloxyethyl phosphorylcholine) (MPC) units (Fig. 4(a)). The block copolymers could be applied on glass substrates to form homogeneous films. Longer hydrophilic MPC segments led to higher hydrophilicity of the coatings in their dry form, and better AF performance without hydration pretreatment. Moreover, the coatings showed protein resistance and durability in the pH range of 2-9. This strategy had potential application not only in marine AF, but also in other fields such as public healthcare, sanitary, and medical field, etc., in which it is hard to apply a hydration pretreatment. Chou et al. proposed a versatile strategy to achieve surface zwitterionization using a so-called combination of copolymerization and self-assembling process between hydrophobic styrene and hydrophilic sulfobetaine methacrylate units [27]. This novel in situ self-assembling coating (ISC) method was capable of overcoming the issues often observed in zwitterion-based AF coatings, regarding the dissolution of the zwitterion-based amphiphilic copolymers (Fig. 4(b)). The as-prepared ISC showed not only ultralow protein adsorption but also resistance towards bacteria, leukocytes, erythrocytes, tissue cells and human blood platelets. In addition, the proposed versatile ISC strategy has also been proven to be effectively applied on hydrophobic substrates, such as poly(tetrafluoroethylene), polypropylene and PDMS.
|
Fig.4 Two kinds of zwitterion-based amphiphilic marine AF coatings. (a) Chemical structures of the block copolymers composed of silane coupling segments and hydrophilic block segments bound on a random copolymer structure (Reproduced with permission[26], Copyright 2020, RSC); (b) Scheme diagram showing the fabrication of ISC: polymerization of styrene and sulfobetaine methacrylate units and corresponding simultaneous assembly to form an AF coating(Reproduced with permission[27], Copyright 2018, Royal Society of Chemistry) |
Considering that degradable polymers are of high relevance for fields such as human implants and marine AF coatings, there have been many reports on introducing zwitterions into the backbone of degradable polymers to fabricate functional coatings. The basic structure of degradable zwitterionic coatings consists of degradable main chains and/or hydrolysable side chains. Considering the polymeric main chains, degradable zwitterionic coatings can be divided into polyester-, polypeptide- and natural polysaccharide-based (such as cellulose, chitosan and starch) ones. Typically, the degradation occurs due to the presence of 2-methylene-1, 3-dioxepane (MDO)[28], methacrylic anhydride (MAAH)[29], isocyanate-terminated polylactic acid(PLA)[30] or poly(e-caprolactone) (PCL)[31], etc. Zwitterionic segments can be incorporated into degradable polymers in the form of a polymer chain or small moieties. Generally, the degradable polymers modified with polymeric zwitterions are partially degradable, while those modified with zwitterionic small moieties are completely degradable. In some cases, additionally to the hydrolysis of the polymeric main chains, the side chains of the polymers can also hydrolyze, contributing to the generation of zwitterionic structures. For example, these cases include the hydrolysis of tertiary carboxybetaine ester (TCB)[32] and ethylcarboxybetaine ester analog methacrylate (ECBEMA)[33].
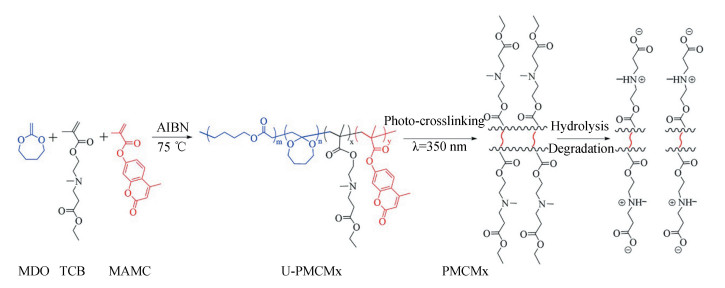
2-methylene-1, 3-dioxepane (MDO) is one of the most widely used degradable units for marine AF coatings due to its facile synthesis and multifarious ways to integrate with other components. It has been reported that the degradation rate of the copolymers with MDO segment can be easily adjusted by tuning the type and fraction of the non-degradable segments. Xie et al. synthesized a series of biodegradable copolymers (PMCM) using MDO, TCB and 7-methacryloyloxy-4-methylcoumarin as monomers [34](Fig. 5). The coating based on the copolymer had sufficient adhesion to a glass fiber-reinforced epoxy resin substrate. The degradation rate and water adsorption in artificial seawater (ASW) are adjustable through the variation of the copolymer composition. Moreover, because the hydrolysis to generate zwitterionic component is limited to the upper surface of the polymeric coatings only, the coating retained sufficient mechanical strength and adhesion to the substrate during service.
|
Fig.5 Schematic diagram of the synthesis and hydrolysis mechanism of the biodegradablecopolymer (Reproduced with permission[34], Copyright 2018, American Chemical Society) |
Inspired by the self-renewing properties of silyl acrylate polymers (i.e. self-regeneration of new surface by dissolution of the polymer with the increased hydrophilicity due to the transformation of trialkylsilyl into carboxyl groups upon hydrolysis) and fouling resistance ability of zwitterion-based materials, Dai et al. firstly synthesized a hydrolysis-induced zwitterionic monomer, namely, tertiary carboxybetaine triisopropylsilyl ester ethyl acrylate (TCBSA) and then copolymerized it with methyl methacrylate (MMA)[28]. The as-obtained copolymer could quickly self-generate zwitterionic components via hydrolysis in seawater. Further, they employed TCBSA, MMA, and MDO to produce the degradable copolymer in seawater. Quantitative analysis indicated that the degradation of the copolymer is controllable, and the degradation rate increased with the external enzyme (i.e., lipase from Pseudomonas cepacia) concentration in the seawater.
Degradable polymers with other kinds of main chains have also been reported for applications in marine AF. Ye et al. developed poly(ester sulfobetaine) urethane urea without/with carboxyl groups (PESBUU /PESBUU-COOH) and poly(ester sulfobetaine) urethane with carboxyl group (PESBU-COOH)[33] (Fig. 6). It was found that the incorporation of carboxyl groups increased the degradation rate of both poly(estersulfobetaine)urethane (PESBU) and PESBUU.

|
Fig.6 Schematic diagram of the synthesis route of SB-bearing biodegradable PU and polyurethane urea with/without carboxyl groups(Reproduced with permission[33], Copyright 2019, American Chemical Society) |
1.2 Zwitterion-Bearing Additive Route
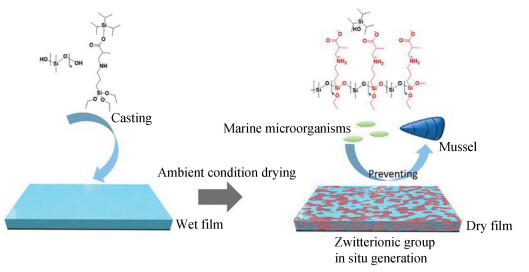
Apart from the zwitterion-bearing binder route, zwitterions have also been introduced into functional coatings in the form of additives, i.e., zwitterions are not included in the polymer binder in advance. Wang et al.[17] proposed an in-situ generation strategy to fabricate zwitterion-based amphiphilic marine AF coatings (Fig. 7). In this work, a new seawater-responsive silane, i.e., triisopropylsilyl 2-methyl-3-((3-(triethoxysilyl)propyl) amino) propanoate (TMAP) was facilely synthesized via aza-Michael addition between triisopropylsilyl methacrylate and (3-aminopropyl) triethoxysilane. The TMAP was mixed with PDMS-OH to get ambient-curable coatings. The zwitterionic structure could be generated in situ from the TMAP under ASW conditions. After examining the antifouling performance, both in a laboratory and marine field exposure, the PDMS-TMAP coatings exhibited better overall fouling resistance than the control PMDS coatings. Particularly, the coatings with 50 wt.% TMAP could completely resist mussels' adhesion.
|
Fig.7 Structure and fabrication of PDMS-TMAP zwitterion-based amphiphilic marine AF coatings based on the seawater-responsive silane TMAP (Reproduced with permission[17], Copyright 2021, Elsevier) |
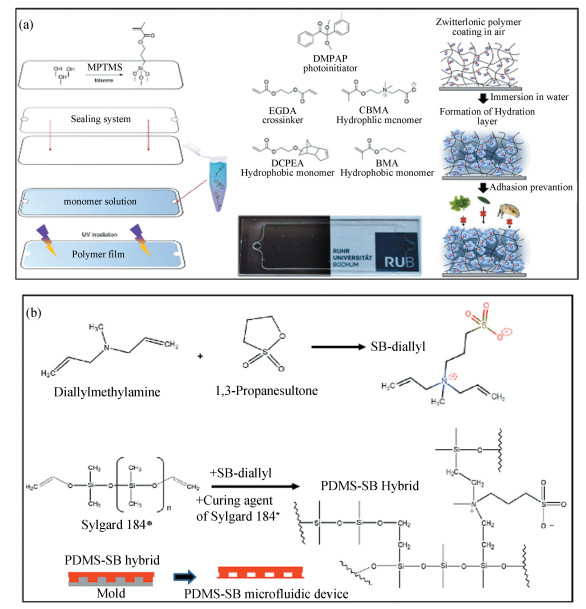
Koschitzki et al.[35] incorporated a small amount (5 wt%) of zwitterionic carboxybetaine methacrylate (CBMA) into UV-curable coatings being composed of hydrophobic ethylene glycol dicyclopentenyl ether acrylate (DCPEA) and ethylene glycol diacrylate (EGDA) to fabricate zwitterion-based amphiphilic AF coatings (Fig. 8(a)). The as-obtained coatings exhibited fouling resistance and good durability in marine field exposure trials for 5 days. Though the pure CBMA-based coatings also showed ultralow fouling in the laboratory assays, they failed to survive in the marine field exposure trials because of their relatively poor mechanical strength and high hydrophilicity.
|
Fig.8 (a) Scheme representing the fabrication of zwitterion-based amphiphilic polymer coatings containing the main building blocks of DCPEA, CBMA, small amount of EGDA as cross-linker and DMPAP as photoinitiator, as well as the AF mechanism of the coatings(Reproduced with permission[35], Copyright 2020, American Chemical Society) (b) Synthesis of monomeric zwitterion of SB-Diallyl and its cooperation with commercial PDMS (Sylgard 184) to fabricate antifouling microfluidic device(Reproduced with permission[36], Copyright 2022, American Chemical Society) |
For a different type of application in the biomedical field, Mercader et al.[36] firstly synthesized a diallyl-terminated monomeric sulfobetaine molecule (SB-diallyl) from diallylmethylamine and 1, 3-propanesultone. The SB-diallyl was then directly incorporated into commercial vinyl-terminated polydimethylsiloxane (PDMS, Sylgard 184) to prepare zwitterion-PDMS hybrid without the need for complex or post-modification procedures (Fig. 8(b)). After the incorporation of the zwitterionic SB-diallyl, the surface of the hybrid material showed an obvious reduction of platelet deposition, indicating that it was promising to be used in the field of medical implant coatings. The zwitterion-PDMS hybrid was further used to fabricate microfluidic devices by the molding method, showing improved blood compatibility and reduced channel occlusion due to clot formation. CO2 transfer tests proved that the zwitterion-PDMS hybrid membrane had comparable gas transfer performance to that without zwitterionic SB-diallyl.
1.3 Post-Generation of the Coatings Containing Zwitterionic PrecursorsIn recent years, there have been an increasing number of reports on the incorporation of zwitterion-based precursors into coatings followed by the in situ generations of zwitterionic groups at their surface, since this procedure can reduce the poor compatibility between the zwitterions and the film-forming polymers.
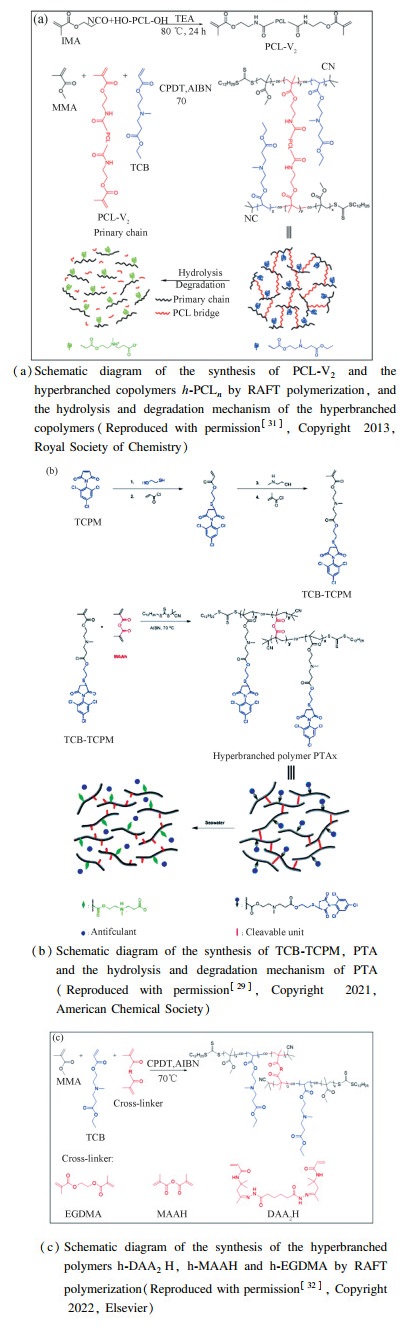
Particularly, TCB is commonly used in this strategy because it can easily generate the zwitterion-based structure via hydrolysis. Mei et al. synthesized a surface-fragmenting hyperbranched copolymer (i.e., h-PCLn, where n = 11, 32, 53 and refer to the weight percentage of PCL segment) with TCB as primary chains and PCL as bridged chains[31] (Fig. 9(a)). The degradation rate of the copolymer was tunable by the content of PCL bridged chains. Dai et al. proposed a single "kill-resist-renew trinity" polymeric AF coatings that simultaneously had fouling resistant, fouling killing, and fouling releasing properties[29] (Fig. 9(b)). N-(2, 4, 6-trichlorophenyl)maleimide(TCPM)-bearing TCB monomer (TCB-TCPM) was synthesized via sequential reaction of TCPM with 2-mercaptoethanol, methacryloyl chloride, 2-methylaminoethanol, and methacryloyl chloride in the presence of catalyst (Fig. 9(b)), and then copolymerized with MAAH by reversible addition-fragmentation chain transfer (RAFT) polymerization to yield a degradable hyperbranched copolymer (denoted as PTA). The triple AF mechanism of these copolymer coatings could be depicted as follows: the upper surface of the coatings degraded into short segments to form a dynamic surface to realize fouling releasing. The hydrolysis of the copolymer generated antifouling TCPM and zwitterionic groups to realize fouling killing and fouling resistance, respectively. Moreover, the degradable rate of these copolymer coatings could be well controlled by the degrees of branching. Pan et al. reported a degradable zwitterionic hyperbranched polymer for AF coatings which were synthesized from TCB, MMA, and divinyl monomers (i.e., N, N′-adipic bis(diacetone acrylamide hydrazone) (DAA2H), MAAH and ethylene glycol dimethacrylate (EGDMA)) by RAFT polymerization (denoted as h-DAA2H, h-MAAH and h-EGDMA, respectively) [32] (Fig. 9(c)). Its degradation rate could be adjusted by the content or type of divinyl monomer. Higher MAAH content caused faster degradation rate. The impact of the type of divinyl monomer on the degradation rate was: DAA2H > MAAH > EGDMA. Similarly, Dai et al.[28]prepared two copolymers, i.e., poly(TCBSA-co-MMA) and poly(MDO-co-TCBSA-co-MMA), and demonstrated that they could generate a zwitterionic surface after hydrolysis.
|
Fig.9 Some TCB-based copolymers for degradable zwitterionic marine AF coatings |
1.4 Other Routes
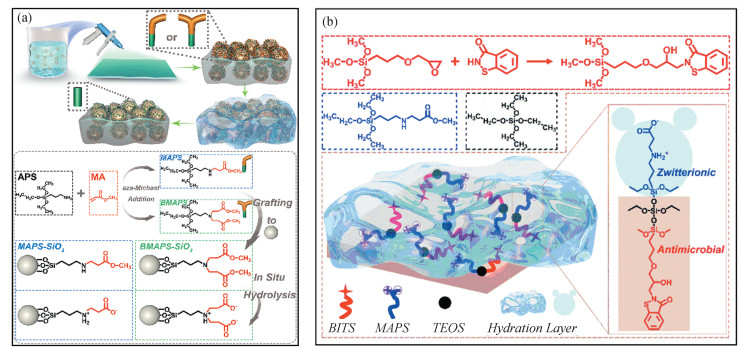
The aforementioned reports focus on the anchoring of the zwitterionic component into the polymeric chains. However, this usually requires complex and tedious grafting procedures or reaction processes, such as RAFT or atom transfer radical polymerization, which limits its large-scale fabrication and practical applications. Recently, grafting of zwitterionic group to nanoparticles has been proposed. Tan et al. synthesized two new analogues of seawater-responsive silanes, (N-methoxyacylethyl)-3-aminopropyltriethoxysilane (MAPS) and bis(N-methoxyacylethyl)-3-aminopropyltriethoxysilane (BMAPS) via aza-Michael addition reaction. These seawater-responsive silanes were further grafted to SiO2 nanoparticles by silanization to achieve modified-SiO2 (MAPS-SiO2 and BMAPS-SiO2)[18] (Fig. 10(a)).
|
Fig.10 (a) Schematic diagram for the fabrication process of PDMS/MAPS-SiO2 and PDMS/BMAPS-SiO2, BMAPS and hydrolysis of MAPS-SiO2 and BMAPS-SiO2(Reproduced with permission[18], Copyright 2012, Royal Society of Chemistry); (b) Schematic diagram of the synthesis of BITS and the hard marine AF coatings prepared with BITS, MAPS and TEOS via sol-gel method(Reproduced with permission[19], Copyright 2013, Royal Society of Chemistry) |
After the incorporation of MAPS-SiO2 or BMAPS-SiO2 into the commercial PDMS silicone elastomer, a biomimetic micro/nanostructured surface could be achieved, providing the coatings with superwettability. Interestingly, the original coating surface showed superhydrophobicity in air, while it transformed into underwater superoleophobicity after being immersed in ASW for 3 days. The reason was that the seawater-responsive silanes on the surface of MAPS-SiO2 or BMAPS-SiO2 could hydrolyze and generate the zwitterionic component, which greatly increased the hydrophilicity of the coatings. Since the hydrolysis of MAPS-SiO2 or BMAPS-SiO2 is limited at the upper surface of the coatings, it can effectively avoid serious swelling and deterioration of the mechanical strength. Tan et al. further employed MAPS, tetraethoxysilane (TEOS), and 2-(2-hydroxy-3-(3-(trimethoxysilyl)propoxy)propyl)benzo[d]isothiazol-3(2H)-one (BITS) to fabricate sol-gel derived AF coatings[19] (Fig. 10(b)). These coatings could reach pencil hardness of 5 H at MAPS/TEOS/BTIS molar ratio of 1∶2∶1 and slightly deteriorated to pencil hardness 4 H after 9 months of immersion in artificial seawater. Generally, higher TEOS content led to higher hardness and better AF performance. Even with the same biofilm formation after field assay, coatings with higher TEOS content exhibited better resistance to mussel settlement. A slight synergistic effect was revealed between MAPS and BITS. This hard coating could be potentially used as the marine AF coating for underwater robot cleaning applications.
Zwitterion-functionalized SiO2 nanoparticles with AF performance were also reported by Knowles et al.[37]. They deposited zwitterionic SB onto SiO2 nanoparticles and investigated the impact of the pH condition on the grafting of SB. Besides the resistance towards protein bovine serum albumin, the particle coatings performed the reduction of the adhesion towards fungal spores Epicoccum nigrum and bacteria Escherichia coli by up to 87% and 96%, respectively. On the other hand, Dundua et al. utilized a two-step process to fabricate PDMS-Poly(zwitterion) interpenetrating polymer network (IPN) [38]. Firstly, the IPN was formed between PDMS matrix and 2-dimethylaminoethyl methacrylate (DMAEMA). Secondly, the N-alkylation of tertiary amino groups in the DMAEMA segments generated zwitterionic structure to afford PDMS-Poly(zwitterion) IPN. The strategy of post-treatment to generate zwitterionic structure prevents the incompatibility between hydrophobic PDMS matrix and hydrophilic zwitterion. The as-obtained IPN presented an obvious positive relationship between surface hydrophilicity and protein (fibrinogen) resistance.
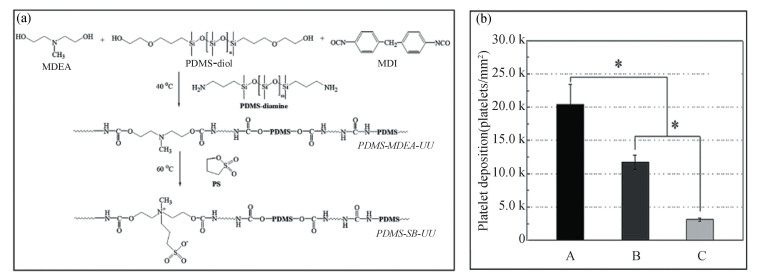
2 Applications 2.1 Medical Implant CoatingsKim et al.[39] fabricated a biostable PDMS-based polyurethane-urea bearing zwitterion sulfobetaine for potential use in the coating of blood-contacting devices (artificial lungs, conduits, continuous glucose monitors, and microfluidic devices, etc.) (Fig. 11(a), (b)). The zwitterion-based PDMS was demonstrated to possess in vitro stability against lipase and 30% H2O2 for 8 weeks, as well as, displayed much higher resistance towards platelet deposition and fibrinogen adsorption compared to the control PDMS.
|
Fig.11 (a) Schematic diagram of the synthesis of zwitterionic PDMS-based polyurethane-urea coatings for blood-contacting devices; (b) Deposited (blood) platelet number acquired by lactate dehydrogenase activity assay (n = 3). ((a) and (b) Reproduced with permission[39], Copyright 2020, Royal Society of Chemistry) |
At early stages of the implantation of continuous glucose monitors (CGMs), the signal during the initial recording period contains much noise, which then requires frequent recalibration via finger-prick tests. Considering this limitation, Xie et al. applied a combinatorial chemistry approach to fabricate zwitterionic polymers to act as functional coatings for CGMs [40]. The modified CGM was found to show less noise and better performance than the one reported by Kim et al.[39]. Moreover, the modified CGM has been tested in mice and healthy/diabetic non-human primates. The results showed that the CGM with polymer zwitterionic coatings could not only record glucose levels without any recalibration, but also largely retracted immune responses.
Although there are many reports on fabricating AF coatings for bio-inert materials, their instability remained to be addressed. For example, poly(sulfobetaine methacrylate) was found to lose its AF property after exposure to hot humid air, which is present during steam sterilization of biomedical devices in clinical uses. To solve this problem, Venault et al.[41] synthesized the zwitterionic biomaterial, poly(4-vinylpyridine propylsulfobetaine) (4VPPS). The 4VPPS was found to show similar resistance to leukocyte, erythrocyte, thrombocyte, whole blood and various bacteria compared with poly(sulfobetaine methacrylate). More importantly, the 4VPPS coatings could also be applied on various substrates such as stainless steel, titanium and silicon and maintained their AF performance after steam sterilization. Zwitterion-based bio-inert coatings provide an excellent alternative for biomedical devices that require steam sterilization, and are used in a continuous or repeated way.
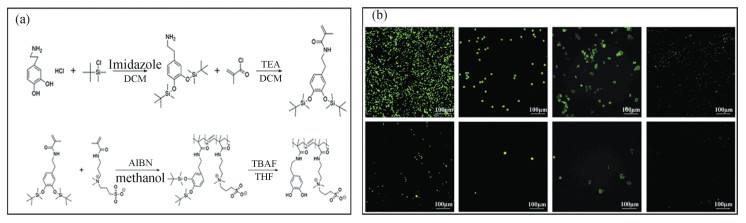
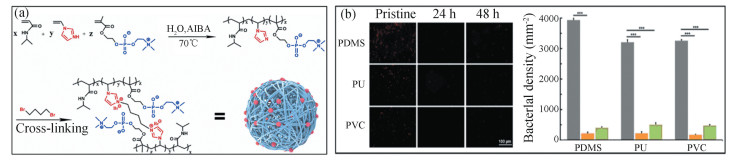
Moreover, zwitterion-based materials have been integrated with other functional materials or technologies to achieve multi-function implant materials. Bio-inert biomaterials are essential for medical implants, biosensors, drug delivery systems, etc. Considering that most of the modifiers used in bio-inert biomaterials were specific to the substrate (regarding adhesion), Dizon et al. synthesized a zwitterion-based copolymer containing catechol groups that had non-substrate dependent anchoring ability. This strategy allows using a copolymer with both AF ability and strong adhesion to universal substrates[42] (Fig. 12(a), (b)). The coatings were assessed in contact with human blood after being applied on ceramic, metallic and polymeric substrates. The AF assays confirmed that the zwitterion-based coatings possessed resistance towards protein, bacteria, thrombocyte, erythrocyte and cell tissues. In order to modify the biomaterial surfaces of medical implants, Shen et al.[43] proposed a one-step modification method to fabricate bactericidal and cell-friendly functional coatings. They prepared poly(N-isopropylacrylamide) microgels (ZQP) containing both zwitterionic component (Z) and quaternary ammonium salt component (Q), to obtain AF and bactericidal properties, respectively (Fig. 13(a), (b)). By adjusting the ratio between Z and Q, they found that the microgel coatings properties, such as particle size and distribution, charge and film-forming ability, could be tuned to adjust the AF performance. When the molar ratio of Q/Z was 15, the microgel coating exhibited a good balance between anti-bacterial and bactericidal capabilities. The microgel coatings could easily be applied to the substrates such as PDMS, PU and PVC, showing their universal applications.
|
Fig.12 (a) Synthesis route of the zwitterion-based copolymer containing catechol groups; (b) Fluorescent images of thrombocyte, erythrocyte, HT1080 cells, and GFP E. coli on a silicon wafer without (above) and with (below) the zwitterion-based copolymer coatings((a) and (b) Reproduced with permission[42], Copyright 2018, Elsevier) |
|
Fig.13 (a) Schematic diagram for the preparation process of ZQP microgels. (b) Left: fluorescence images of the pristine PDMS, PU and poly(vinyl chloride) substrates and the corresponding ones after being coated with ZQP microgels and incubated in bacterial suspensions for 24 and 48 h, at 37 ℃. Right: The corresponding bacterial density of different samples (n = 3). ((a) and (b) Reproduced with permission[43], Copyright 2022, Elsevier) |
Zwitterion-based functional coatings have also been applied in implantable organic bioelectronics, which is a rising area combined with material science, biomedical science and electronic engineering science. Xu et al.[44] have tried to solve the fouling issues of conjugated polymers typically used in biomedical applications, which may result in short lifetime and even failure of the bioelectronics. They synthesized zwitterion-based liquid crystalline polymers called PCBTh-CBC10, which combined three segments, that is, a conjugated polythiophene backbone, a mesogenic unit and a multifunctional zwitterionic side chain[44]. The polymer displayed resistance towards protein adsorption, bacterial adhesion and cell settlement. Moreover, the polymer possessed conductivity so it had the potential for being used as AF functional coatings for implantable bioelectronic devices.
2.2 Marine Antifouling CoatingsThere are numerous reports on the application of zwitterion-based materials in marine AF coatings. Some of them involve the synthesis of monomeric/polymeric zwitterion-based materials, and modification of the traditional coatings with zwitterions, some focus on the AF mechanism at molecular level and the AF performance under laboratory and marine field exposure. A large number of zwitterion-based coatings for marine antifouling applications have been introduced in Section 1, Fabrication Methods.
Due to the different adhesion mechanisms of biomolecules, an exclusively hydrophilic or hydrophobic coating usually fails to resist a broad-spectrum of fouling adhesion. On the contrary, zwitterion-based amphiphilic materials/coatings have both hydrophobic and hydrophilic segments, endowing them with fouling resistance and fouling detachment at the same time.
Zwitterion-based amphiphilic materials with covalently bonded cationic and anionic groups exhibit electrically neutral properties but extremely high polarity. More importantly, the charge density of zwitterion-based amphiphilic materials can be adjusted easily and reversibly by varying the pH values[45]. Furthermore, since the zwitterion-based amphiphilic materials possess the characteristic of hydration, self-association, electric neutrality, high polarity and synergistic effect with electrolytes, they have inherently special features which are known to be advantageous for marine AF coatings[46]. Finally, they are not easily prone to migration under potential gradients in the medium, have inherent environmental or stimuli-responsiveness, show easy functionalization, etc.
Apart from the previously mentioned zwitterion-based amphiphilic coatings fabricated with PDMS as hydrophobic segment, there have been other reports considering various hydrophobic polymers. Ruiz-Sanchez et al.[47] fabricated zwitterion-based amphiphilic AF coatings using perfluoropolyether (PFPE). Different from the polymers based on pendant perfluorooctanoic acid that cause bioaccumulation in tissues, PFPE is considered as biodegradable as their degradation products should not accumulate or be harmful to the environment. The amphiphilic PFPE-zwitterion coatings showed near-zero settlement of two barnacle species (Balanus amphitrite and Balanus improvises), very low diatom adhesion strength and largely reduced bacterial adhesion densities.
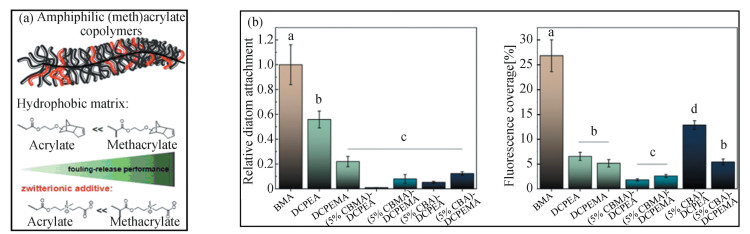
In another report from Koschitzki et al., they compared the surface topography and AF performance of a UV-curable carboxybetaine acrylate (CBA) based amphiphilic coating, with those of CBMA-based one [48] (Fig. 14(a), (b)). The AF performances were evaluated against the settlement of diatom Navicula perminuta and under a short-term dynamic field exposure experiment. Interestingly, the incorporation of both CBA and CBMA moieties could greatly reduce the diatom adhesion of the coatings in the laboratory assays, when compared with a hydrophobic coating control. However, CBMA could endow the UV-curable coatings with better AF performance under marine field exposure, because CBMA-based coatings exhibited much lower surface roughness (higher surface roughness commonly exerting detrimental effects on fouling resistance[49-50]) and weaker phase variation compared with CBA-based coatings. The different surface roughness was resulted from their different reactivity with hydrophobic monomers.
|
Fig.14 (a) Scheme of the component of amphiphilic zwitterionic acrylate/methacrylate copolymers for marine AF coatings. (b) Left: the diatom density on the zwitterion-based amphiphilic coatings compared with the hydrophobic control. Right: Fluorescence coverage of the surface of zwitterion-based amphiphilic coatings after being immersed in the ocean. ((a) and (b) Reproduced with permission[48], Copyright 2021, American Chemical Society) |
Walker et al.[51] synthesized random copolymers with DMAEMA and propargyl methacrylate. The DMAEMA segment of the copolymers were then betainized to produce zwitterionic structure. The copolymers were cast on polystyrene substrates to form a film with thickness around 100 nm and then cross-linked under UV-light irradiation in the presence of 2 wt % of photoactive benzophenone. In nonspecific protein (bovine serum albumin) adsorption tests, the betainized network coating possessed better AF performance than its original counterpart due to a high degree of swelling in aqueous mediums, which is attributed to entropic shielding and size exclusion factors.
Despite the unique advantages of zwitterion-based AF coatings, they still exhibit some limitations which need to be addressed. Particularly, the zwitterion-based silicone AF coatings suffer poor mechanical strength, especially under marine field conditions. More efforts are expected to be spent on fabricating zwitterion-based amphiphilic AF coatings with better comprehensive performance, namely improved mechanical properties. Another problem is the decline of their AF performance in time, because they are prone to be covered by dead biomolecules in biomedical environments or by inorganic and organic pollutants in marine field environments. Besides, zwitterion-AF coatings will be swollen under aqueous environment so their mechanical strength and adhesion to the substrates also deteriorate in time[52].
Recent studies tried to incorporate degradable polymers with zwitterions to fabricate "dynamic AF surfaces", in which the degradable polymers realize self-renewing of the surface, and the zwitterions realize the AF capability. In addition to the zwitterion-based degradable functional coatings fabricated with MDO units, there have been other reports using alternative degradable units. Ma et al. prepared hydrolysis-induced zwitterionic biodegradable materials with self-healing ability to combat long-term biofilm formation via a two-step strategy[30].The materials displayed excellent long-term anti-bacterial performance, with the Gram-positive bacteria and Gram-negative bacteria resistance of more than 14 and 6 days, respectively. Quartz crystal microbalance with dissipation (QCM-D) tests showed that the surface could degrade gradually to renew itself. Though the coating could gradually generate a zwitterionic structure, the coatings did not show obvious swelling.
Some critical issues for the current degradable zwitterionic marine AF coatings remain, however, to be answered such as the controversy between AF performance and degradation rate in actual service environment. A faster degradation rate can provide marine AF coatings with better AF efficiency while it also leads to shorter service life. Moreover, little research work has been carried out to examine the practical reliability and durability of the degradable zwitterionic marine AF coatings in live field exposure tests.
2.3 Oil-Resistant CoatingsDue to their hydrophilicity, zwitterion-based materials have also been used to fabricate oil-resistant coatings. To achieve gravity-driven oil-water separation, three-dimensional-printed membranes have been fabricated by stereolithography (SLA) which need to be hydrophilic as well as oleophobic. To solve this issue, Song et al.[53] fabricated a zwitterion-based hydrogel coating on SLA-based oil-water separation membranes. The oil-water separation tests showed that the modified membrane had high efficiency even in 31 repeating cycles. This was attributed to the high water absorption rate of the zwitterionic hydrogel-coated membrane. In addition, after each oil-water separation cycle, significant water loss would be solved by adsorbing water in the next separation test, which assures its long-term usage in water-oil separation.
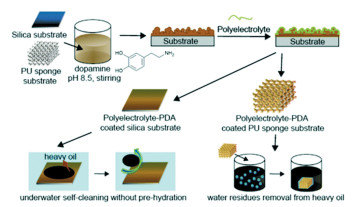
He et al.[54] fabricated a self-cleaning coating by grafting zwitterionic poly(2-methacryloyloxylethyl phosphorylcholine) (PMPC) onto the solid substrate. Besides its oil-resistance in the water-wetted state (i.e., underwater superoleophobicity), it could also clean the oil fouled on the dry surfaces by the presence of water alone. The self-cleaning characteristic of PMPC surface was derived from the zwitterionic phosphorylcholine groups that exhibited strong binding affinity to water even when they are already wetted by oil. They further applied the PMPC coating on the steel meshes to produce oil-water separation membranes, which also showed self-cleaning of oil contamination by simply rinsing with water. Zhang et al.[55]proposed a facile and scalable strategy to gain substrate-independent zwitterion-based coatings (Fig. 15). Firstly, the substrates were coated with polydopamine (PDA), followed by the grafting of poly(3-[dimethyl(2-methacryloyloxyethyl)ammonium]propanesulfonate) (PMAPS). The modified membranes showed underwater self-cleaning of asphaltenes-containing oil without the need for prewetting for the first time. Moreover, the zwitterion-based PMAPS-PDA coating kept its underwater superoleophobicity after more than 10 repeating cycles, without prewetting and under a 2 to 11 pH range. Further on, the zwitterion-based PMAPS-PDA coating was applied onto a PU sponge, which achieved nice separation efficiency of water residues from asphaltenes-in-toluene solution, and its performance could be easily regenerated by washing with water.
|
Fig.15 Fabrication of zwitterion-based PMAPS-PDA coating on silica and PU sponge and their corresponding applications (Reproduced with permission[55], Copyright 2018, Royal Society of Chemistry) |
Huang et al.[56]fabricated superhydrophilic and underwater superoleophobic coatings on silica glass and stainless steel wire mesh for antifogging, self-cleaning and oil-water separation, by the silanization of zwitterionic SB silanes on the modified substrates. The coatings displayed superhydrophilic properties with the water contact angle of less than 5°, and showed long-term stability under exposure to heat and UV irradiation. After being coated the stainless steel wire meshes with the SB silanes, it could achieve an oil recovery rate of more than 99.5% and a water flux of 6.5×107 L/m2·h bar, offering gravity-driven and energy-saving oil-water separation performance.
3 SummaryCurrently, most of the zwitterion-based functional coatings are fabricated by zwitterion-bearing binder and additive routes. Because of the high polarity of zwitterion, these two routes generally suffer from the poor compatibility of zwitterion-bearing components with other components of the coatings. As an alternative, post-generation of coatings containing zwitterionic precursors is a preferred strategy to avoid the compatibility issue, which makes it promising for the fabrication of zwitterion-based functional coatings. Nevertheless, post-generation strategy needs various types of zwitterionic precursors, in order to meet their application in various coatings as well as the environments of the coatings served.
Since zwitterions resemble biomimetic materials, they are non-toxic and harmless to the human body, so they have been widely utilized to fabricate medical implant coatings. Due to the strong binding energy with water molecules to form a hydration layer which blocks the foulants, zwitterion-based materials have been widely used to fabricate marine AF coatings and oil-resistant coatings. There is no doubt that the existing literatures contributed a lot to the development of zwitterion-based functional coatings, however, some issues remain to be addressed. For example, the limited stability of the zwitterionic layer structure and the high-water affinity of zwitterions may deteriorate the mechanical strength of the coatings reducing their service lifetime. Endowing the coatings with self-healing ability[18, 30] or in situ generation of zwitterions[19] may be the feasible solutions to address this issue. In addition, by using zwitterions only, it is difficult to completely meet the requirements of the diverse and complex problems of fouling, namely in marine or biomedical environments.
In our opinion, in order to fully take advantage of zwitterion-based materials to fabricate high-performance functional coatings, the relationship between chemical structure (surface chemical composition and topography) and the properties (stability underwater, water affinity, antifouling property, etc.) of zwitterion have to be further studied in depth, to find relations which may enable the fabrication of even more efficient AF coatings. As for the fabrication methods, the zwitterion-bearing additive route has received so far less attention than zwitterion-bearing binder route, but it should have a high potential for large-scale production because of its higher versatility in preparing coating formulations. In addition, to meet the needs of practical applications, especially in marine AF coatings, zwitterions have to be combined with other strategies to fully accomplish the mission of avoiding the settlement and growth of undesired contaminants and organisms on surfaces in contact with water and oils of body fluids.
| [1] |
Zwaal R F, Comfurius P, Van Deenen L L. Membrane asymmetry and blood coagulation. Nature, 1977, 268(5618): 358-360. DOI:10.1038/268358a0 (  0) 0) |
| [2] |
Bretscher M, Raff M C. Mammalian plasma membranes. Nature, 1975, 258(5530): 43-49. DOI:10.1038/258043a0 (  0) 0) |
| [3] |
Yancey P H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. Journal of Experimental Biology, 2005, 208(Pt 15): 2819-2830. DOI:10.1242/jeb.01730 (  0) 0) |
| [4] |
Alfrey T, Morawetz H, Fitzgerald E B, et al. Synthetic electrical analog of proteins. Journal of the American Chemical Society, 1950, 72(4): 1864. DOI:10.1021/ja01160a532 (  0) 0) |
| [5] |
Chen S F, Li L Y, Zhao C, et al. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer, 2010, 51: 5283-5293. DOI:10.1016/j.polymer.2010.08.022 (  0) 0) |
| [6] |
Bernards M, He Y. Polyampholyte polymers as a versatile zwitterionic biomaterial platform. Journal of Biomaterials Science, Polymer Edition, 2014, 25: 1479-1488. DOI:10.1080/09205063.2014.938976 (  0) 0) |
| [7] |
He M R, Gao K, Zhou L J, et al. Zwitterionic materials for antifouling membrane surface construction. Acta Biomaterialia, 2016, 40: 142-152. DOI:10.1016/j.actbio.2016.03.038 (  0) 0) |
| [8] |
Chen S F, Zheng J, Li L Y, et al. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. Journal of the American Chemical Society, 2005, 127(41): 14473-14478. DOI:10.1021/ja054169u (  0) 0) |
| [9] |
He Y, Hower J, Chen S F, et al. Molecular simulation studies of protein interactions with zwitterionic phosphorylcholine self-assembled monolayers in the presence of water. Langmuir, 2008, 24(18): 10358-10364. DOI:10.1021/la8013046 (  0) 0) |
| [10] |
Schlenoff J B. Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir, 2014, 30(32): 9625-9636. DOI:10.1021/la500057j (  0) 0) |
| [11] |
Zhang L, Cao Z Q, Bai T, et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nature Biotechnology, 2013, 31(6): 553-556. DOI:10.1038/nbt.2580 (  0) 0) |
| [12] |
Yang W, Xue H, Li W, et al. Pursuing'zero'protein adsorption of Poly (Carboxybetaine) from undiluted blood serum and plasma. Langmuir, 2009, 25(9): 11911-11916. DOI:10.1021/la901578811911 (  0) 0) |
| [13] |
Keefe A J, Jiang S. Poly (zwitterionic) protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nature Chemistry, 2012, 4: 59-63. DOI:10.1038/nchem.1213 (  0) 0) |
| [14] |
Shao Q, White A D, Jiang S. Difference of carboxybetaine and oligo (ethylene glycol) moieties in altering hydrophobic interactions: A molecular simulation study. The Journal of Physical Chemistry B, 2014, 118(1): 189-194. DOI:10.1021/jp410224w (  0) 0) |
| [15] |
Chen X, Yang D. Functional zwitterionic biomaterials for administration of insulin. Biomaterials Science, 2020, 8(18): 4906-4919. DOI:10.1039/D0BM00986E (  0) 0) |
| [16] |
Saha P, Ganguly R, Li X, et al. Zwitterionic nanogels and microgels: an overview on their synthesis and applications. Macromolecular Rapid Communications, 2021, 42(13): Article number 2100112. DOI: 10.1002/marc.202100112.
(  0) 0) |
| [17] |
Wang D H, Xu J K, Tan J Y, et al. In situ generation of amphiphilic coatings based on a self-catalytic zwitterionic precursor and their antifouling performance. Chemical Engineering Journal, 2021, 422: 130115. DOI:10.1016/j.cej.2021.130115 (  0) 0) |
| [18] |
Tan J, Xu J, Wang D, et al. Seawater-responsive SiO2 nanoparticles for in situ generation of zwitterionic polydimethylsiloxane antifouling coatings with underwater superoleophobicity. Journal of Materials Chemistry A, 2020, 8: 24086-24097. DOI:10.1039/D0TA07617A (  0) 0) |
| [19] |
Tan J, Liang X, Yang J, et al. Sol-gel-derived hard coatings from tetraethoxysilane and organoalkoxysilanes bearing zwitterionic and isothiazolinone groups and their antifouling behaviors. Journal of Materials Chemistry B, 2022, 10: 406-417. DOI:10.1039/D1TB02069B (  0) 0) |
| [20] |
Wolf M P, Salieb-Beugelaar G B, Hunziker P. PDMS with designer functionalities-Properties, modifications strategies, and applications. Progress in Polymer Science, 2018, 83: 97-134. DOI:10.1016/j.progpolymsci.2018.06.001 (  0) 0) |
| [21] |
Lejars M, Margaillan A, Bressy C. Fouling release coatings: a nontoxic alternative to biocidal antifouling coatings. Chem. Rev., 2012, 112(8): 4347-4390. DOI:10.1021/cr200350v (  0) 0) |
| [22] |
Banerjee I, Pangule R C, Kane R S, et al. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Advanced Materials, 2011, 23(6): 690-718. DOI:10.1002/adma.201001215 (  0) 0) |
| [23] |
Jiang J X, Fu Y C, Zhang Q H, et al. Novel amphiphilic poy (dimethylsiloxane) based polyurethane networks tethered with carboxybetaine and their combined antibacterial and anti-adhesive property. Applied Surface Science, 2017, 412: 1-9. DOI:10.1016/j.apsusc.2017.03.117 (  0) 0) |
| [24] |
Hu P, Zeng H, Zhou H, et al. Silicone elastomer with self-generating zwitterions for antifouling coatings. Langmuir, 2021, 37(27): 8253-8260. DOI:10.1021/acs.langmuir.1c00984 (  0) 0) |
| [25] |
Seetho K, Zhang S, Pollack K A, et al. Facile synthesis of a phosphorylcholine-based zwitterionic amphiphilic copolymer for anti-biofouling coatings. ACS Macro Letters, 2015, 4(5): 505-510. DOI:10.1021/mz500818c (  0) 0) |
| [26] |
Noguchi A, Masuda T, Chen C, et al. Hydrophilic surfaces from simple dip-coating method: amphiphilic block copolymers with zwitterionic group form antifouling coatings under atmospheric conditions. Mater. Adv., 2020, 1: 2737-2744. DOI:10.1039/D0MA00184H (  0) 0) |
| [27] |
Chou Y-N, Venault A, Wang Y-H, et al. Surface zwitterionization on versatile hydrophobic interfaces via a combined copolymerization/self-assembling process. Journal of Materials Chemistry B, 2018, 6: 4909-4919. DOI:10.1039/C8TB01054D (  0) 0) |
| [28] |
Dai G X, Xie Q Y, Ai X Q, et al. Self-Generating and Self-Renewing Zwitterionic Polymer Surfaces for Marine Anti-Biofouling. ACS Applied Materials&Interfaces, 2019, 11(44): 41750-41757. (  0) 0) |
| [29] |
Dai G X, Ai X Q, Mei L Q, et al. Kill-Resist-Renew Trinity: Hyperbranched Polymer with Self-Regenerating Attack and Defense for Antifouling Coatings. ACS Applied Materials & Interfaces, 2021, 13: 13735-13743. DOI:10.1021/acsami.1c02273 (  0) 0) |
| [30] |
Ma J, Lin W F, Xu L B, et al. Resistance to long-term bacterial biofilm formation based on hydrolysis-induced zwitterion material with biodegradable and self-healing properties. Langmuir, 2020, 36(12): 3251-3259. DOI:10.1021/acs.langmuir.0c00006 (  0) 0) |
| [31] |
Mei L Q, Ai X Q, Ma C F, et al. Surface-fragmenting hyperbranched copolymers with hydrolysis-generating zwitterions for antifouling coatings. Journal of Materials Chemistry B, 2020, 8: 5434-5440. DOI:10.1039/D0TB00886A (  0) 0) |
| [32] |
Pan J, Mei L, Zhou H, et al. Self-regenerating zwitterionic hyperbranched polymer with tunable degradation for anti-biofouling coatings. Progress in Organic Coatings, 2022, 163: 106674. DOI:10.1016/j.porgcoat.2021.106674 (  0) 0) |
| [33] |
Ye S.-H, Chen Y Q, Mao Z W, et al. Biodegradable zwitterionic polymer coatings for magnesium alloy stents. Langmuir, 2019, 35: 1421-1429. DOI:10.1021/acs.langmuir.8b01623 (  0) 0) |
| [34] |
Xie Q Y, Xie Q N, Pan J S, et al. Biodegradable polymer with hydrolysis-induced zwitterions for antibiofouling. Applied Materials, 2018, 10: 11213-11220. (  0) 0) |
| [35] |
Koschitzki F, Wanka R, Sobota L, et al. Amphiphilic dicyclopentenyl/carboxybetaine-containing copolymers for marine fouling-release applications. Applied Materials, 2020, 12: 34148-34160. (  0) 0) |
| [36] |
Mercader A, Ye S-H., Kim S, et al. PDMS-Zwitterionic hybrid for facile, antifouling microfluidic device fabrication. Langmuir, 2022, 38: 3775-3784. DOI:10.1021/acs.langmuir.1c03375 (  0) 0) |
| [37] |
Knowles B R, Wagner P, Maclaughlin S, et al. Silica nanoparticles functionalized with zwitterionic sulfobetaine siloxane for application as a versatile antifouling coating system. ACS Applied Materials and Interfaces, 2017, 9(22): 18584-18594. DOI:10.1021/acsami.7b04840 (  0) 0) |
| [38] |
Dundua A, Franzka S, Ulbricht M. Macromol. Improved Antifouling Properties of Polydimethylsiloxane Films via Formation of Polysiloxane/Polyzwitterion Interpenetrating Networks. Macromolecular Rapid Communications, 2016, 37: 2030-2036. DOI:10.1002/marc.201600473 (  0) 0) |
| [39] |
Kim S, Ye S-H, Adamo A, et al. A biostable, anti-fouling zwitterionic polyurethane-urea based on PDMS for use in blood-contacting medical devices. Journal of Materials Chemistry B, 2020, 8(36): 8305-8314. DOI:10.1039/d0tb01220c (  0) 0) |
| [40] |
Xie X, Doloff J C, Yesilyurt V, et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nature Biomedical Engineering, 2018, 2: 894-906. DOI:10.1038/s41551-018-0273-3 (  0) 0) |
| [41] |
Venault A, Lai M.-W, Jhong J.-F, et al. Superior bioinert capability of zwitterionic Poly (4-vinylpyridine propylsulfobetaine) withstanding clinical sterilization for extended medical applications. ACS Applied Materials&Interfaces, 2018, 10(21): 17771-17783. DOI:10.1021/acsami.8b05466 (  0) 0) |
| [42] |
Dizon G V, Chou Y-N, Yeh L-C, et al. Bio-inert interfaces via biomimetic anchoring of a zwitterionic copolymer on versatile substrates. Journal of Colloid and Interface Science, 2018, 529: 77-89. DOI:10.1016/j.jcis.2018.05.073 (  0) 0) |
| [43] |
Shen J, Chen R, Wang J, et al. One-step surface modification strategy with composition-tunable microgels: From bactericidal surface to cell-friendly surface. Colloids and Surfaces B: Biointerfaces, 2022, 212: 112372. DOI:10.1016/j.colsurfb.2022.112372 (  0) 0) |
| [44] |
Xu J J, Xu J, Moon H, et al. Zwitterionic liquid crystalline polythiophene as an antibiofouling biomaterial. Journal of Materials Chemistry B, 2021, 9(2): 349-356. DOI:10.1039/D0TB02264K (  0) 0) |
| [45] |
Wu A, Gao Y, Zheng L, et al. Zwitterionic amphiphiles: their aggregation behavior and applications. Green Chemistry, 2019, 21(16): 4290-4312. DOI:10.1039/C9GC01808E (  0) 0) |
| [46] |
Nurioglu A G, Esteves A C C, de With G, et al. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. Journal of Materials Chemistry B, 2015, 3(32): 6547-6570. DOI:10.1039/c5tb00232j (  0) 0) |
| [47] |
Ruiz-Sanchez A J, Guerin A J, El-Zubir O, et al. Preparation and evaluation of fouling-release properties of amphiphilic perfluoropolyether-zwitterion cross-linked polymer films. Progress in Organic Coatings, 2020, 140: 105524. DOI:10.1016/j.porgcoat.2019.105524 (  0) 0) |
| [48] |
Koschitzki F, Wanka R, Sobota L, et al. Amphiphilic zwitterionic acrylate/methacrylate copolymers for marine fouling-release coatings. Langmuir, 2021, 37(18): 5591-5600. DOI:10.1021/acs.langmuir.1c00428 (  0) 0) |
| [49] |
Scardino A J, Harvey E, De Nys R. Testing attachment point theory: diatom attachment on microtextured polyimide biomimics. Biofouling, 2006, 22(1-2): 55-60. DOI:10.1080/08927010500506094 (  0) 0) |
| [50] |
Cao X, Pettitt M E, Wode F, et al. Interaction of zoospores of the green alga ulva with bioinspired micro-and nanostructured surfaces prepared by polyelectrolyte layer-by-layer self-assembly. Advanced Functional Materials, 2010, 20(12): 1984-1993. DOI:10.1002/adfm.201000242 (  0) 0) |
| [51] |
Walker Jr. E J, Pandiyarajan C K, Efimenko K, et al. Generating surface-anchored zwitterionic networks and studying their resistance to bovine serum albumin adsorption. ACS Applied Polymer Materials, 2019, 1(12): 3323-3333. DOI:10.1021/acsapm.9b00772 (  0) 0) |
| [52] |
Ma J, Ma C, Zhang G, et al. Degradable polymer with protein resistance in a marine environment. Langmuir, 2015, 31(23): 6471-6478. DOI:10.1021/acs.langmuir.5b01720 (  0) 0) |
| [53] |
Song Y, Wang B, Altemose P, et al. 3D-printed membranes with a zwitterionic hydrogel coating for more robust oil-water separation. Industrial & Engineering Chemistry Research, 2020, 59(48): 21058-21065. DOI:10.1021/acs.iecr.0c04436 (  0) 0) |
| [54] |
He K, Duan H, Chen G Y, et al. Cleaning of oil fouling with water enabled by zwitterionic polyelectrolyte coatings: overcoming the imperative challenge of oil-water separation membranes. ACS Nano, 2015, 9(9): 9188-9198. DOI:10.1021/acsnano.5b03791 (  0) 0) |
| [55] |
Zhang J W, Zhang L, Cui X W, et al. Scalable polyzwitterion-polydopamine coating for regenerable oil/water separation and underwater self-cleaning of stubborn heavy oil fouling without pre-hydration. Chemical Communications, 2018, 54(70): 9734-9737. DOI:10.1039/C8CC04611E (  0) 0) |
| [56] |
Huang K-T, Yeh S-B, Huang C-J. Surface modification for superhydrophilicity and underwater superoleophobicity: applications in antifog, underwater self-cleaning, and oil-water separation. ACS Applied Materials & Interfaces, 2015, 7(38): 21021-21029. DOI:10.1021/acsami.5b07362 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


