2. Engineering Research Center of Advanced Glass Manufacturing Technology, Ministry of Education, Donghua University, Shanghai 201620, China;
3. Institute of Functional Materials, Donghua University, Shanghai 201620, China
Glass-ceramic (GC) fibers have been reported in the literature showing great potential in optical[1-4], bioactive[5], piezoelectrical[6], and refractory[7] systems. However, uncontrollable crystallization can be easily generated during the fiber drawing and heat treatment processes, which will lead to a catastrophic mechanical failure[8]. It is of great importance to prepare GC fibers to maintain certain mechanical strength, especially in the construction industry[9].
Basalt fiber (BF)is widely applied in construction industry to improve the mechanical properties of construction materials due to its excellent mechanical strength, corrosion durability[10-11] and high-temperature stability[12-13]. BF belongs to iron-rich aluminosilicate system which is easily crystallized at high temperatures[9, 14]. The nature crystallization process starts from the oxidation of Fe2+ initiated by a diffusion process of Ca2+ and Mg2+ and forming nano-crystalline layers on the surface of BF[15-20]. Researchers[21-22] have studied the influence of the extra addition of ZrO2 and Al2O3 on the crystallization behavior of BFs. For example, when the ZrO2 content was 7 wt%, pyroxene, hematite, plagioclase, and zirconia were crystalized simultaneously in BF, which will increase the thermal stability[21] of BF. BF with high alumina content tended to have a higher resistance to crystallization with augite as the major crystallization product[22]. To the best of our knowledge, few works have aimed to prepare GCBFs to maintain mechanical strength and examined their performance in seawater environments.
Continuous BFs can be prepared directly from natural rocks and have a great advantage in economics. Thus, in this work, the aim is to systematically study the crystallization kinetics of BFs and prepare glass-ceramic basalt fibers (GCBFs) maintaining tensile strength. Furthermore, the GCBFs will be tested in seawater environments and their application potential in marine industry will be explored. This work will pave the way to a better understanding of the fundamentals of GCBFs as well as benefit the development of the construction industry.
1 Experimental 1.1 Sample PreparationThe pristine basalt glass was prepared by melting the basalt rocks (Shandong Province, China) in a quartz crucible at 1550 ℃ for 2 h and quenched in water. The chemical composition of basalt glass, determined by X-ray fluorescence analysis, is 54.9% SiO2, 16.4% Al2O3, 9.2% Fe2O3, 8.1% CaO, 5.6% MgO, 3.3% Na2O and 2.5% others (wt%). The continuous basalt fibers (BFs) were drawen at temperatures of ~1350±10 ℃ from a laboratory monofilament fiber preparation system[23-24] with a platinum crucible.
Glass-ceramic basalt fibers (GCBFs) were prepared by following a two-step (nucleation and crystallization) method based on a thermal analysis (TGA/DSC 3+, Mettler) at a heating rate of 10 K/min. The glass transition temperature (Tg) of BFs is around 670±5 ℃, and the crystallization peak temperature (Tp) is 883±5 ℃. The BFs specimens were nucleated at 740 ℃, 760 ℃, and 780 ℃ (>50 ℃ higher than Tg) for 1, 2, and 3 h and crystallized (around crystallization peak Tc) at 830 ℃, 880 ℃, and 930 ℃ for 1, 2 and 3 h to obtain GCBFs. The experimental conditions are shown in Table 1.
| Table 1 Experimental conditions and properties of GCBFs |
1.2 Characterization
The crystalline phases of BFs samples were characterized by X-ray diffraction (XRD, Rigaku D/max 2500 PC) at a scan speed of 0.02 °/s. The Crystallinity Index [25] (CI%), which represents the volume fraction of the crystalline phases, was calculated by the following equation:
| $ C I \%=\left(\frac{A_c}{A_T}\right) \times 100 \% $ | (1) |
where Ac is the area of the crystalline phases, and AT (AT=amorphous+crystalline) represents the total area. The mean size of the crystallites (D) is calculated via Scherrer's equation[5, 26]:
| $ D=\frac{K \lambda}{\beta \cos \theta} $ | (2) |
where K represents a dimensionless shape factor, λ is the wavelength, β is the full width at the half-maximum intensity, and θ is the Bragg angle.
The tensile strength of GCBFs was measured via a monofilament dynamometer (YG005A, China). Each sample was measured independently 20 times to get an averaged tensile strength and a standard deviation. The surface morphology of GCBFs samples was characterized by a field emission scanning electron microscope (SEM, JSM-7500F, Japan) and the composition in a local area was measured by energy-dispersive spectroscopy (EDS).
The GCBFs samples were immersed in the seawater solution[9] at 80 ℃ to accelerate the corrosion process in the marine environment. The weights of GCBFs samples were measured by a balance (Precisa, Switzerland, ±0.0001 g) before and after the seawater aging for 24 h. Afterwards, 1 mL seawater solution was diluted 100 times and analyzed via an inductively coupled plasma spectroscopy (ICP, Prodigy, USA). The ion dissolution rate (IDR) can be calculated as:
| $ \mathrm{IDR}=\frac{w_{\text {seawater }}}{w_{\text {fiber }}} $ | (3) |
where wseawater refers to the weight of each element in seawater solution from ICP tests, and wfiber is the initial weight of each element in pristine BFs or GCBFs.
2 Results and Discussion 2.1 Crystallization KineticsThe crystallization kinetics of BFs was explored by heating the BFs to 1200 ℃ via DSC at different heating rates (e.g., 10 ℃/min, 20 ℃/min, 25 ℃/min, and 30 ℃/min). As can be seen in Fig. 1(b) that both the glass transition temperature (Tg, which is not the viscosity definition of the glass transition temperature.) and crystallization temperature (Tp) increase with the increase of heating rates. The crystallization activation energy (Ec, J/mol) is calculated by the Kissinger equation[27-28],
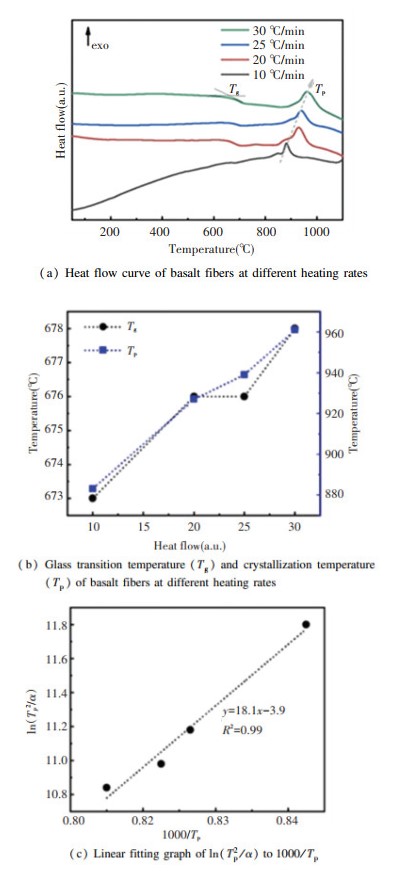
|
Fig.1 (a) Heat flow curve of basalt fibers at different heating rates; (b) Glass transition temperature (Tg) and crystallization temperature (Tp) of basalt fibers at different heating rates; (c) Linear fitting graph of ln(Tp2/α) to 1000/Tp |
| $ \ln \left(\frac{T_{\mathrm{p}}^2}{\alpha}\right)=\frac{E_{\mathrm{c}}}{R T_{\mathrm{p}}}-A $ | (4) |
where α represents the heating rates (K/s), R refers to the gas constant and A is a constant. The value of Ec/R equals the slope of the linear fitting in Fig. 1(c), thus, the Ec of BFs is 150.4 kJ/mol. To evaluate the crystallization mechanism, the Avrami parameter (n)[28] can be calculated by using the Augis-Bennett equation[29]:
| $ n=\frac{2.5 T_{\mathrm{p}}^2}{\Delta T \cdot E / R} $ | (5) |
where T corresponds to the temperature interval of the half-width of the crystallization peak. The n of the BFs sample is equal to 4.3±0.5 at heating rates from 10 K/min to 30 K/min, indicating that the main crystallization mechanism of BFs is three-dimensional crystal growth.
2.2 Crystalline Phases and MorphologyThe GCBFs samples were prepared based on the crystallization kinetics research and the XRD patterns of GCBFs are shown in Fig. 2. Compared with the amorphous structure of the pristine BFs, GCBFs own main crystalline phases of Diopside (PDF#89-0836) and Augite (PDF#78-1391). The main crystalline phases, as well as the crystallinity, are not changed significantly with different nucleation/ crystallization temperatures and times concerning a very high measurement uncertainty of CI around 46%±10%.

|
Fig.2 XRD patterns of GCBFs at different Tn, tn, Tc, tc |
The mean crystal size shows comparatively more significant change, especially as a function of crystallization time. It can be seen in Table 1, the GCBFs have the largest mean crystal size of 86±10 nm at Tn=760 ℃, tn=2 h, Tc=880 ℃, and tc=3 h, whereas, the one crystalizing for 1 h (Tn=760 ℃, tn=2 h, Tc=880 ℃) has the smallest crystal size of only 45±10 nm. In addition, the SEM morphology of nine different GCBFs samples is shown in Fig. 3. From the visual observation, all GCBFs samples show clear crystallization. Some of the samples seem to have very high crystallinities. However, the crystallites are not evenly distributed. The actual crystallinity for all the GCBFs is not changed significantly under different heat treatment conditions.
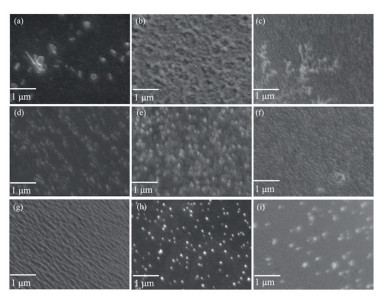
|
Fig.3 SEM images of GCBFs (a)-(i) corresponding to sample ID C1 to C9 |
2.3 Tensile Strength
Previous studies show that glass fibers will lose tensile strength after crystallization. However, in this work, the GCBFs samples retain up to 50% of tensile strength after heat treatment of up to 930 ℃ (the tensile strength of pristine BFs is ~736 MPa). As can be seen in Fig. 4(a), the tensile strength of GCBFs first increases from 252 MPa to 300 MPa with an increase in nucleation temperature from 740 ℃ to 760 ℃ and then decreases to 287 MPa when the nucleation temperature reaches 780 ℃. A similar phenomenon is detected as a function of nucleation time in Fig. 4(b), although the minimum tensile strength is 242 MPa at a holding time of 3 h. The influence of crystallization temperature and time is slightly different from the nucleation treatment. As shown in Fig. 4(c), the tensile strength of GCBFs continuously decreases from 367 MPa to 214 MPa with an increase in temperature from 830 ℃ to 930 ℃. Similarly, in Fig. 4(d), the tensile strength of GCBFs decreases from 347 MPa to 197 MPa with an increase in holding time from 1 h to 3 h. Overall, the GCBFs reach the highest tensile strength of 367 MPa at Tn=760 ℃, tn=2 h, Tc=830 ℃, and tc=2 h, and the lowest of 197 MPa at Tn=760 ℃, tn=2 h, Tc=880 ℃, and tc=tc=3 h. Combing all the results from tensile strength and mean crystal size, it is found that a larger mean crystal size tends to yield a lower tensile strength in GCBFs. It is generally agreed that the glass fracture process must first nucleate cavitation and then percolate through the bulk glass wall to generate a fracture[30]. It is perhaps reasonable to conclude that GCBFs with larger crystallites can easily generate cavitation under high tension, which will lead to comparatively lower tensile strength.

|
Fig.4 Tensile strength of GCBFs at different (a) nucleation temperatures (Tn); (b) nucleation time (tn); (c) crystallization temperatures (Tc); (d) crystallization time (tc).(Note: The error bars represent the standard deviations of tests on 20 samples) |
2.4 Anti-Seawater Corrosion
BFs are ideal alternatives to steel bars due to their excellent resistance to seawater corrosion. In this work, it is found that GCBFs have better anti-seawater corrosion than the pristine BFs and have the potential to be applied in marine industry. As can be seen in Fig. 5, the weight-loss ratio of GCBFs after soaking in seawater solution for 24 h at 80 ℃. In general, the overall weight-loss rate of GCBFs decreases with the increasing temperature of seawater, and the overall weight-loss rate of BFs increases with the extension of the nucleation and crystallization time. For example, the weight-loss rate of the pristine BFs sample after soaking at 80 ℃ seawater solution for 24 h is ~1.3%, but that of the GCBFs (Tn=760 ℃, tn=2 h, Tc=880 ℃, and tc=1 h) at the same conditions decreases to ~0.5% and it further increases to 0.3% when crystallization time extends to 3 h. A larger mean crystal size will enhance the anti-corrosion property of GCBFs due to the increasing probability for crystalline phases to directly contact seawater. It is generally believed that crystalline phases have better anti-corrosion performance than amorphous glass. The results from ICP tests also support this argument. As can be seen in Fig. 6, the main dissolution elements of pristine BFs after soaking at 80 ℃ seawater solution for 24 h are glass network formers, e.g. Si and Al, which are different from GCBFs. The breaking of glass network is much slower in GCBFs than in pristine BFs, thus, the ion dissolution rates of Si and Al are much lower in GCBFs than in pristine BFs. Moreover, the overall ion dissolution rates slightly decreases as a function of crystallization time. Therefore, GCBFs with largest mean crystal size has the best anti-corrosion performance in seawater environment.

|
Fig.5 Weight-loss ratio (weight-loss divided by the initial weight) of pristine BFs and GCBFs after soaking in seawater solution for 24 h at 80 ℃ |
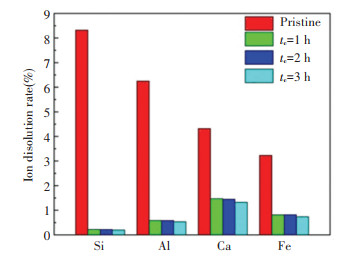
|
Fig.6 Ion dissolution rate of pristine BFs and GCBFs after soaking in seawater solution for 24 h at 80 ℃ |
3 Conclusions
Glass-ceramic basalt fibers were prepared via a two-step(nucleation and crystallization) heat-treatment process. The crystallization kinetics calculations prove that the main crystallization mechanism of BFs is three-dimensional crystal growth. Results from XRD and SEM show that, compared with the amorphous structure of the pristine BFs, GCBFs have main crystalline phases of Diopside and Augite. The main crystalline phases as well as the crystallinity are not changed significantly with different nucleation temperatures (740 ℃ to 780 ℃) and crystallization temperatures (830 ℃ to 930 ℃), as well as different times (1 h to 3 h), compared with the very high measurement uncertainty crystallinity of around ±10%. In particular, the GCBFs sample with the largest mean crystal size maintains the lowest tensile strength of ~197 MPa (compared with the pristine BFs of 737 MPa). Moreover, it is found that GCBFs have better anti-seawater corrosion performance than the pristine BFs and have the potential to be applied in marine industry.
| [1] |
Samson B N, Tick P A, Borrelli N F. Efficient neodymium-doped glass-ceramic fiber laser and amplifier. Optics Letters, 2001, 26(3): 145-147. DOI:10.1364/OL.26.000145 (  0) 0) |
| [2] |
Krishnaiah K V, Ledemi Y, Genevois C, et al. Ytterbium-doped oxyfluoride nano-glass-ceramic fibers for laser cooling. Optical Materials Express, 2017, 7(6): 1980-1994. DOI:10.1364/ome.7.001980 (  0) 0) |
| [3] |
Fang Z J, Xiao X S, Wang X, et al. Glass-ceramic optical fiber containing Ba2TiSi2O8 nanocrystals for frequency conversion of lasers. Scientific Reports, 2017, 7: 44456. DOI:10.1038/srep44456 (  0) 0) |
| [4] |
Fang Z J, Zheng S P, Peng W C, et al. Ni (2+) doped glass ceramic fiber fabricated by melt-in-tube method and successive heat treatment. Optics Express, 2015, 23(22): 28258-28263. DOI:10.1364/OE.23.028258 (  0) 0) |
| [5] |
Baranowska A, Lesniak M, Kochanowicz M, et al. Crystallization kinetics and structural properties of the 45S5 bioactive glass and glass-ceramic fiber doped with Eu (3). Materials, 2020, 13(6): 1281. DOI:10.3390/ma13061281 (  0) 0) |
| [6] |
Gerace K S, Mauro J C, Randall C A. Piezoelectric glass-ceramics: crystal chemistry, orientation mechanisms, and emerging applications. Journal of the American Ceramic Society, 2021, 104(5): 1915-1944. DOI:10.1111/jace.17680 (  0) 0) |
| [7] |
Utell M J, Maxim L D. Refractory ceramic fiber (RCF) toxicity and epidemiology: a review. Inhalation Toxicology, 2010, 22(6): 500-521. DOI:10.3109/08958370903521224 (  0) 0) |
| [8] |
Wang N, Hou S E, Jin H Y. Crystallization behavior of heat-treated basalt fiber. Advanced Materials Research, 2012, 560-561: 3-7. DOI:10.4028/www.scientific.net/AMR.560-561.3 (  0) 0) |
| [9] |
Wang Q W, Yan T, Ding L F. Effect of seawater environment on the structure and performance of basalt continuous fiber. Materials, 2021, 14(8): 1862. DOI:10.3390/ma14081862 (  0) 0) |
| [10] |
Li Z N, Shen A Q, Wang H, et al. Effect of basalt fiber on the low-temperature performance of an asphalt mixture in a heavily frozen area. Construction and Building Materials, 2020, 253: 119080. DOI:10.1016/j.conbuildmat.2020.119080 (  0) 0) |
| [11] |
Li Z W, Ma J X. Experimental study on mechanical properties of the sandwich composite structure reinforced by basalt fiber and nomex honeycomb. Materials, 2020, 13(8): 1870. DOI:10.3390/ma13081870 (  0) 0) |
| [12] |
Ahmad M R, Chen B. Microstructural characterization of basalt fiber reinforced magnesium phosphate cement supplemented by silica fume. Construction and Building Materials, 2020, 237: 117795. DOI:10.1016/j.conbuildmat.2019.117795 (  0) 0) |
| [13] |
Chen C, Liu X, Zhou F J, et al. Effect of basalt fiber on the microstructure and holding strength of sintered WC-based diamond composite. International Journal of Refractory Metals and Hard Materials, 2021, 99: 105600. DOI:10.1016/j.ijrmhm.2021.105600 (  0) 0) |
| [14] |
Pisciella P, Pelino M. FTIR spectroscopy investigation of the crystallisation process in an iron rich glass. Journal of the European Ceramic Society, 2005, 25(11): 1855-1861. DOI:10.1016/j.jeurceramsoc.2004.06.012 (  0) 0) |
| [15] |
Cook G B, Cooper R F, Wu T. Chemical diffusion and crystalline nucleation during oxidation of ferrous iron-bearing magnesium aluminosilicate glass. Journal of Non-Crystalline Solids, 1990, 120(1-3): 207-222. DOI:10.1016/0022-3093(90)90205-Z (  0) 0) |
| [16] |
Karamanov A, Ergul S, Akyildiz M, et al. Sinter-crystallization of a glass obtained from basaltic tuffs. Journal of Non-Crystalline Solids, 2008, 354(2-9): 290-295. DOI:10.1016/j.jnoncrysol.2007.07.040 (  0) 0) |
| [17] |
Karamanov A, Pisciella P, Pelino M. The crystallisation kinetics of iron rich glass in different atmospheres. Journal of the European Ceramic Society, 2000, 20(12): 2233-2237. DOI:10.1016/S0955-2219(00)00077-7 (  0) 0) |
| [18] |
Smedskjaer M, Solvang M, Yue Y Z. Crystallisation behaviour and high-temperature stability of stone wool fibres. Journal of the European Ceramic Society, 2010, 30(6): 1287-1295. DOI:10.1016/j.jeurceramsoc.2009.12.009 (  0) 0) |
| [19] |
Gutnikov S I, Manylov M S, Lipatov Y V, et al. Effect of the reduction treatment on the basalt continuous fiber crystallization properties. Journal of Non-Crystalline Solids, 2013, 368: 45-50. DOI:10.1016/j.jnoncrysol.2013.03.007 (  0) 0) |
| [20] |
Luo L D, Zhang Q C, Wang Q W, et al. Effect of the iron reduction index on the mechanical and chemical properties of continuous basalt fiber. Materials, 2019, 12(15): 2472. DOI:10.3390/ma12152472 (  0) 0) |
| [21] |
Lipatov Y V, Arkhangelsky I V, Dunaev A V, et al. Crystallization of zirconia doped basalt fibers. Thermochimica Acta, 2014, 575: 238-243. DOI:10.1016/j.tca.2013.11.002 (  0) 0) |
| [22] |
Gutnikov S I, Malakho A P, Lazoryak B I, et al. Influence of alumina on the properties of continuous basalt fibers. Russian Journal of Inorganic Chemistry, 2009, 54(2): 191-196. DOI:10.1134/s0036023609020041 (  0) 0) |
| [23] |
Gutnikov S I, Manylov M S, Lipatov Y V, et al. Effect of the reduction treatment on the basalt continuous fiber crystallization properties. Journal of Non-Crystalline Solids, 2013, 368: 45-50. DOI:10.1016/j.jnoncrysol.2013.03.007 (  0) 0) |
| [24] |
Wang Q W, Zhang Q C, Luo L D, et al. Effects of high-temperature treatment and iron reduction index on tensile strength of basalt continuous fiber. Journal of Non-Crystalline Solids, 2021, 564: 120836. DOI:10.1016/j.jnoncrysol.2021.120836 (  0) 0) |
| [25] |
Daguano J K M E, Strecker K, Ziemath E C, et al. Effect of partial crystallization on the mechanical properties and cytotoxicity of bioactive glass from the 3CaO center dot P2O5-SiO2-MgO system. Journal of the Mechanical Behavior of Biomedical Materials, 2012, 14: 78-88. DOI:10.1016/j.jmbbm.2012.04.024 (  0) 0) |
| [26] |
Muniz F T L, Miranda M A R, Morilla dos Santos C, et al. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallographica Section A, 2016, 72(3): 385-390. DOI:10.1107/S205327331600365X (  0) 0) |
| [27] |
He D F, Ma H, Zhong H. Effect of different nucleating agent ratios on the crystallization and properties of MAS glass ceramics. Journal of the European Ceramic Society, 2021, 41(16): 342-350. DOI:10.1016/j.jeurceramsoc.2021.09.034 (  0) 0) |
| [28] |
Avrami M. Kinetics of phase change (I): General theory. The Journal of Chemical Physics, 1939, 7(12): 1103-1112. DOI:10.1063/1.1750380 (  0) 0) |
| [29] |
Ercenk E, Guven B, Yilmaz S. Crystallization kinetics of machinable glass ceramics produced from volcanic basalt rock. Journal of Non-Crystalline Solids, 2018, 498: 262-271. DOI:10.1016/j.jnoncrysol.2018.06.041 (  0) 0) |
| [30] |
Varshneya A K. Stronger glass products: lessons learned and yet to be learned. International Journal of Applied Glass Science, 2018, 9(2): 140-155. DOI:10.1111/ijag.12341 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


