As an energy carrier, Green hydrogen is primarily derived from water and can address issues of environmental sustainability, carbon emissions, and energy security. Electrochemical hydrogen evolution reaction (HER) and oxygen reduction reaction (ORR) represent two essential electrochemical processes that drive the hydrogen fuel cells and water electrolyzers, respectively[1-3]. The energy efficiency relies on the performance of electrocatalyst. Platinum (Pt) has the lion's share of the electrocatalyst market since Pt locates well at the apex of the well-established activity volcano plot and possesses good corrosion resistance[4-9]. However, the global reserves of Pt will hardly meet the demand once hydrogen energy replaces fossil fuels in the terawatt scale. Palladium (Pd) as a major by-product of platinum production is an attractive source. The ratios of Pd/Pt in the Merensky and Chromitite ores in South Africa are around 1.0/2.4 and 1.0/1.2[10], respectively. Nonetheless, the performance gap between Pd and Pt is large. The binding strength of reaction intermediating on Pd is not as optimized as that on Pt, resulting in the inferior intrinsic activity and sluggish kinetics[11-15]. Also, many attempts to utilize Pd in fuel cells or electrolyzers are hampered by its instability due to the propensity of oxide formation as well as corrosion under comparable potentials applied on Pt.
Two general strategies including alloying and heterostructuring have been frequently deployed in improving the electrocatalytic performance of Pd. Alloying with foreign elements (e.g. Au, Ag, Co and Sn)[16-20] alters the crystal lattice constants and electronic properties of Pd. The ligand effect together with crystal strains can effectively tune the binding energies of reaction intermediates thereby enhancing performance. Recently, the alloying of Pd with non-metal elements such as boron and phosphorus aroused particular interests[21-22]. The insertion of P, which is rich in valence electrons, into crystalline Pd can effectively change the bonding and electronic configuration of the catalyst. Mu's group[23-25] found that the H* chemisorption and liberation strength on the PdP2 surface was just right. The Gibbs free energy of hydrogen adsorption (ΔGH*) is as low as 0.012 eV. Moreover, due to the high enthalpy of material alloying, metal phosphides usually have superior corrosion resistance over the metal counterparts. The P can be oxidized into phosphate in harsh electrochemical conditions. The leached metal ions can coordinate with phosphate and generate an insoluble surface metal phosphate that can prevent further corrosion[26-28]. On the other hand, coupling Pd with different material phases can also enhance the activity by taking advantage of the synergistic structural and electronic interaction in heterostructures[29-30]. Li et al.[31] reported a Ni@Pd heterostructure, in which the electron flow from Ni to the surface of Pd promoted the HER activity. Lu et al. [32] fabricated a Pd/W18O49 nanosheet electrocatalyst. The strong structural interaction and electronic interaction between the two components improve the ORR activity and stability of the catalyst. Although significant advances have been made based on a single strategy, there is plenty of room to further improve performance. A combination of both strategies may improve the electrocatalytic performance or even extend the catalytic functionality, but has rarely been reported. This is possibly due to the lack of a facile synthetic approach that simultaneously achieves structural and composition engineering.
As a new emerging porous material, conjugated microporous polymers (CMPs) [33-38] feature facile synthesis, large surface area, extended conjugation, and excellent chemical stability. The CMPs have recently attracted increasing attention as self-sacrificed templates and/or precursors to fabricate porous carbon materials owing to their cross-linked network structure and intrinsic porosity. By carefully selecting the building blocks and the synthetic routes, heteroatom-enriched porous carbon can be readily obtained via simple pyrolysis of the corresponding CMPs. Although several heteroatom-doped carbons derived from CMPs have been reported [39-42], a simple method to convert CMP into heterostructured electrocatalyst with multifunctionality is so far not available.
This study takes advantage of the holey framework of CMPs and develop CMP-guest chemistry that can construct nitrogen- and phosphide-doped porous carbon (NPC) electrocatalyst consisting of palladium phosphide heterostructures via one-step pyrolysis. Fig. 1 shows the schematic preparation process. Amine- and pyridine-based conjugated microporous polymer (AP-CMP) was prepared through Buchwald-Hartwig (BH) coupling [43-46] of amines and aryl halides catalyzed by bis(dibenzylideneacetone)palladium(0) (Pd(dba)2). The strong coordinative capacity of N functional groups at the porous skeleton of AP-CMP can not only bind Pd complex but also allow loading phytic acid. AP-CMP as host transformed into porous carbon with homogeneous N doping during pyrolysis, while phytic acid as guest served as P source diffusing to the Pd/C interface, resulting in the formation of the PdP2@Pd heterostructure. Compared with the Pd control sample without phosphating, the as-prepared electrocatalyst demonstrated significantly enhanced electrocatalytic performances, including promising HER activity (58 mV @ 10 mA/cm2), and Pd-like ORR activity together with nice long-term stability. Our work illustrates the intriguing power of CMP-guest chemistry in heterostructure engineering.
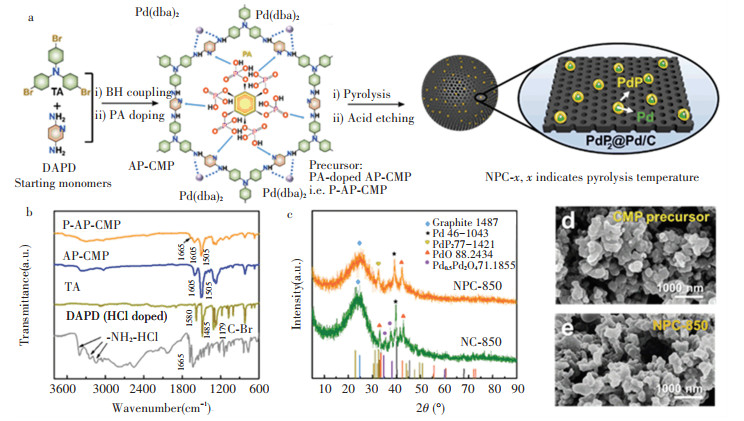
|
Fig.1 (a) Schematic synthesis process of P-AP-CMP and NPC- x; (b) Fourier transform infrared (FT-IR) spectra of P-AP-CMP, AP-CMP, TA and DAPD (HCl doped); (c) X-ray diffraction (XRD) patterns of NPC-850 and NC-850; Scanning electron microscopy (SEM) images of (d) CMP precursor and (e) NPC-850 |
1 Results and Discussion
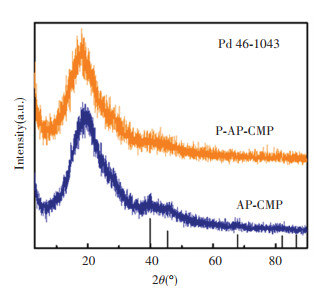
The schematic synthesis process is illustrated in Fig. 1(a). The synthesis of AP-CMP started with mixing 2, 5-diaminopyridine dihydrochloride (2, 5-DAPD) and tris(4-bromophenyl)amine (TA) in the toluene under an N2 atmosphere. The BH coupling was triggered at an elevated temperature of 110 ℃ catalyzed by the Pd(dba)2. AP-CMP was obtained after reaction for 24 h. The X-ray diffraction (XRD) pattern of AP-CMP was recorded (Fig. S1). A broad characteristic peak centered at ~20 ° was observed, which is indicative of the amorphous nature of the AP-CMP. There is also a small peak located at ~39 ° corresponding to the characteristic peak of metallic Pd. This suggests that partial decomposition of Pd(dba)2 is probably due to the heat treatment during the sample drying process. Fourier transform infrared (FT-IR) spectrum identified bands of the primary amine group of 2, 5-DAPD monomer at 3406 and 3242 cm-1 (-NH2 stretching) as well as the aryl C-Br groups of the TA monomer at 1179 cm-1 are absent or strongly attenuated in the FT-IR spectrum of the AP-CMP (Fig. 1(b)). Solid-state 13C cross-polarization magic angle spinning nuclear magnetic resonance (CP/MAS NMR) spectrum of the AP-CMP shows five main resonances at ~147×10-6, ~140×10-6, ~130×10-6, ~121×10-6, and ~112×10-6 (Fig. S2), originating from the aryl carbons of the polymer matrix. The X-ray photoelectron spectrum (XPS) survey spectrum indicates the presence of Pd, N and C in AP-CMP (Fig. S3a). The N 1s core-level XPS spectrum indicates two main binding energies at ~399.7 and ~398.7 eV attributed to C-N bond of amine unit and C=N bond of pyridine unit (Fig. S3c), respectively. The above results confirm the polymer structure of AP-CMP as displayed in Fig. 1(a). Phytic acid (PA) was introduced into the porous framework of AP-CMP by a solvent impregnation method forming phosphide-doped polymer i.e. P-AP-CMP. The dark blue color of AP-CMP turned into dark green after loading PA (Fig.S4). The XRD pattern of P-AP-CMP shows that the amorphous feature remained unchanged after impregnating PA (Fig. S1). Thermogravimetric analyses (TGA) confirm that ~21 wt% PA as the dopant was introduced into AP-CMP (Fig.S5). The FT-IR spectrum of P-AP-CMP shows a new band centered at ~1665 cm-1 (Fig. 1(b)), which can be ascribed to the presence of quaternary N+ in P-AP-CMP due to protonation of pyridine units upon acid doping. The XPS survey spectrum confirms the successful incorporation of PA in the P-AP-CMP (Fig.S3). The P content is measured to be 2.45 wt%.
The N and P co-doped porous carbon (NPC-850) was obtained after thermal pyrolysis of P-AP-CMP at 850 ℃. As for control, N doped porous carbon (NC-850) was prepared by pyrolyzing AP-CMP at the same temperature. Fig. 1(c) shows the XRD patterns of NPC-850 and NC-850. Both samples have a similar broad peak locating at ~25°, which is attributed to the (002) plane of polycrystalline graphite. The XRD pattern of NPC-850 shows the main contributions from the diffraction peaks of PdP2 (PDF#77-1421) and Pd face-centered cubic (fcc) phase (PDF#46-1043). An additional peak centered at ~42° was also observed, which can be attributed to the diffraction peak of PdO (PDF#88-2434). In contrast, the XRD pattern of NC-850 shows mixed contributions from the diffraction peaks of metallic Pd and Pd oxide including PdO (PDF#88-2434) and Pd0.5Pd3O4 (PDF#88-2434). Such difference indicated that the PA dopant in the framework of P-AP-CMP led to the formation of PdP2 alloy.
SEM images (Figs. 1(d) and 1(e)) show that the as-prepared NPC-850 shared similar irregular granular morphologies with the polymer precursor. Transmission electron microscopy (TEM) characterization was applied to investigate the morphology and crystalline structure in detail. Figs. 2(a) and 2(b) show the TEM image of NPC-850 with a size distribution histogram. Pd-based nanoparticles are homogeneously embedded in the porous carbon support, and their average diameter was determined to be 25.8 ± 8.6 nm. High-resolution TEM inspected different areas of nanoparticles. The interspacing of lattice fringes in the core region (Fig. 2(d)) was measured to be ~2.27 Å, corresponding to the (111) plane of fcc metallic Pd. In contrast, the lattice spacing at the outer surface of the nanoparticle (Fig. 2 (e) and 2(f)) is larger (2.44 Å), implying the formation of PdP2@Pd/C heterostructure. EDS mapping was employed to visualize the element distribution (Fig. 3). N was homogeneously doped throughout the carbon support. However, P was not evenly distributed. The position of the intensified signal of P coincides with that of Pd, which corroborates the formation of PdP2@Pd/C heterostructure. Of note, PdO particles were not found during TEM characterization. This might be caused by the uneven distribution of PdO. The PdO is possibly the product of the impurity metallic Pd phase in the polymer precursor that underwent oxidation rather than phosphorization during pyrolysis. TEM characterization was also applied to characterize the NC-850(Fig.S6). EDS mapping confirmed the presence of Pd. The size distribution of Pd-based particles in NC-850 is not as uniform as that in NPC-850.
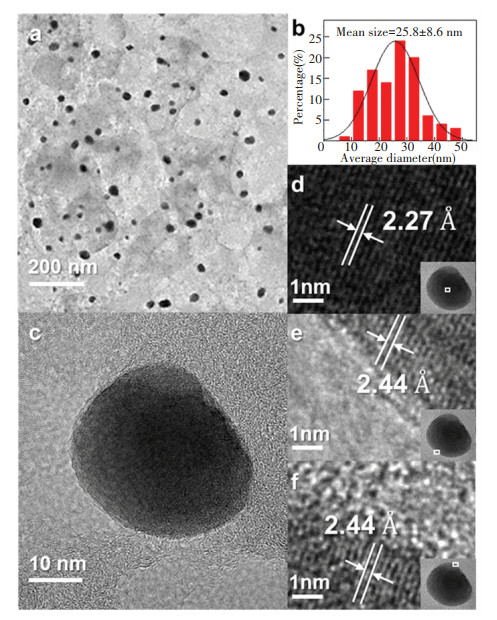
|
Fig.2 (a) TEM image of NPC-850 and (b) the statistic histogram of Pd-based nanoparticles; (c) HRTEM image of NPC-850;(d), (e) and (f) HRTEM images of areas marked in Fig. 2(c) |
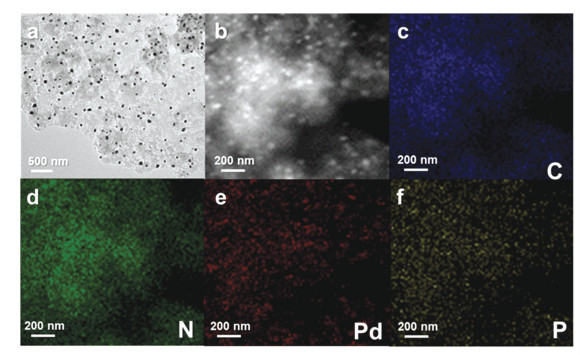
|
Fig.3 (a) TEM, (b) STEM images of NPC-850;(c), (d), (e) and (f)The corresponding C, N, Pd and P elemental EDS mapping spectra |
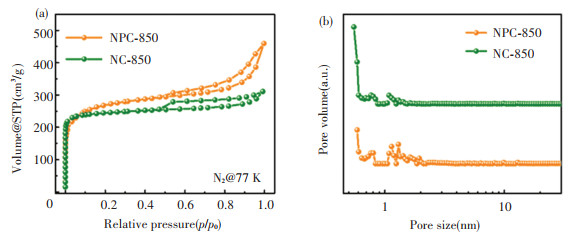
Raman spectra (Fig. 4(a)) show two distinctive peaks at 1343 cm-1 (D band) and 1592 cm-1(G band). The NC-850 and NPC-850 show a comparable ID/IG ratio (0.86 vs. 0.87), implying that the phytic acid doping did not affect the graphitization. The mass loadings of Pd in NPC-850 and NC-850 are ~4.0 and 3.8 wt%, respectively, as determined by the inductively coupled plasma optical emission spectroscopy (ICP-OES). Based on N2 isotherms (Fig. S7a), the surface areas of NC-850 and NPC-850 were calculated to be 744 and 849 m2/g, respectively. Both two materials show a small pore size of 0.55-0.60 nm (Fig. S7b), suggesting that the carbonized product well inherited the microporous nature of the polymer precursor without structure collapse.
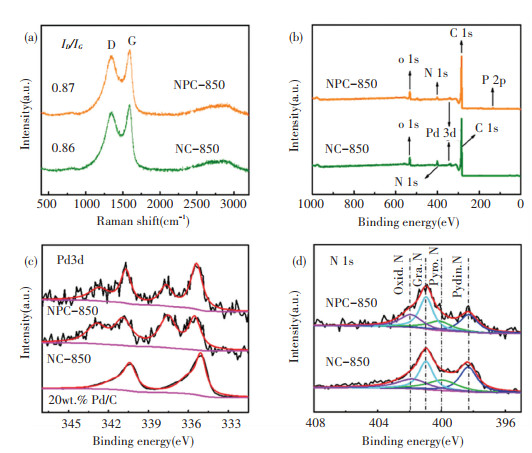
|
Fig.4 (a) Raman spectra of NPC-850 and NC-850; (b) XPS survey spectra of NPC-850 and NC-850;XPS spectra of (c) Pd 3d, (d) N 1s for NPC-850, NC-850 and 20 wt.% Pd/C |
X-ray photoelectron spectroscopy (XPS) measurements were performed to probe the composition and chemical state of constituent elements. The XPS survey spectrum confirmed the presence of Pd, P, N, O and C in NPC-850. NC-850 has the same composition except for P. The element contents are listed in Table S1. NPC-850 and NC-800 have similar N contents (~5 wt%). The measured Pd/P ratio is much smaller than 2, suggesting part of P was doped into the framework of carbon support of NPC-850. Fig. 4(c) shows the high-resolution Pd 3d spectrum. As listed in Table S1, the binding energy (BE) of the peak position of NPC-850 and NC-850 is higher than that of 20 wt% Pd/C, indicating the oxidative nature of Pd species in both samples. Of note, the Pd 3d spectrum of NPC-850 shifted ~0.2 eV toward lower BE than that of NC-850, which is caused by different bonding strengths of Pd-P and Pd-O. Fig. 4(d) shows the high-resolution N 1s spectrum. Deconvolution of N 1s spectrum of NPC-850 and NC-850 yielded similar N species including oxidized N, graphitic N, pyrrolic N, and pyridinic N, and their contents are listed in Table S2. The addition of PA has an insignificant influence on the nitrogen species. The P 2p spectrum indicates three P species including P-O, P-C, and Pd-P (Fig. S8). The content of Pd-P is smaller than that of P-O and P-C since partial P was doped into carbon support rather than alloying with Pd.
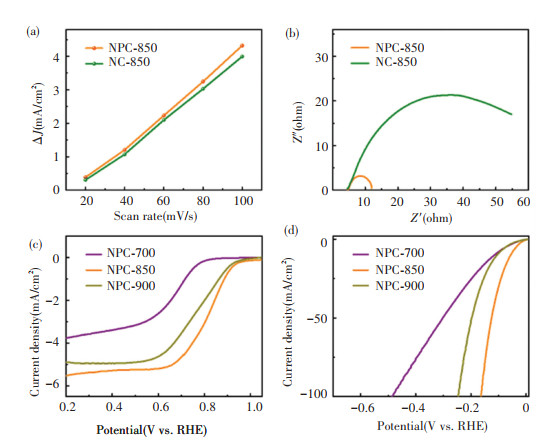
In order to assess the HER activity, the as-prepared samples were loaded on the glass carbon (GC) to be used as the working electrode in a three-electrode cell in N2 saturated 0.5 mol/L H2SO4 electrolyte with a graphite rod and an Ag/AgCl as the counter and reference electrodes, respectively. The linear sweep voltammetry (LSV) curves (Fig. 5(a)) indicate that the NPC-850 requires an overpotential (η) of 58 mV to reach the current density of 10 mA/cm2, which is much lower than that of NC-850 (η10 = 72 mV). Comparably, the Pd/C exhibits an overpotential of 62 mV at 10 mA/cm2. From Tafel plots (Fig. 5(b)) analysis, the measured value for NC-850 is 84 mV/dec. NPC-850 exhibits a smaller Tafel slope of 56 mV/dec, indicating better reaction kinetic, which can be attributed to the effect of surface phosphorization. Notably, NPC-850 displays excellent electrochemical stability. After 6000 cycles, the HER polarization curve shows a slight difference from the initial (Fig. 5(c)). The chronoamperometry measurement with a constant voltage of 0.7 V indicates the NPC-850 exhibits good stability for at least 10 h (Fig. 5(d)).

|
Fig.5 HER performance: (a) Polarization curves and (b) corresponding Tafel plots of 20 wt% Pt/C, 20 wt% Pd/C, NPC-850 and NC-850 obtained in 0.5 M H2SO4, (c) Polarization curves of NPC-850 before and after 6000 cycles, (d) Time dependence of the current density of NPC-850 acquired at 58 mV vs. reversible hydrogen electrode RHE |
The oxygen reduction reaction (ORR) activity of NPC-850 was examined via a rotating disk electrode (RDE) to further explore the functionality. Fig. 6(a) shows the ORR polarization curves recorded at 1600 r/min in O2 saturated 0.1 mol/L KOH. As shown in Fig. 6(b), NPC-850 demonstrates a substantial improvement in ORR activity upon surface phosphorization. Specifically, NPC-850 exhibits an onset potential (Eonset = 0.94 V), a half-wave potential (E1/2=0.85 V) and a diffusion-limited current density (JL= -5.5 mA/cm2), which are higher than those of NC-850 (Eonset=0.85 V, E1/2=0.74 V, JL=-3.5 mA/cm2). The activity of NPC-850 is similar to that of commercially available 20 wt% Pd/C (Eonset=0.94V, E1/2=0.87 V, JL=-5.3 mA/cm2) and approaches that of 20 wt% Pt/C (Eonset=0.96 V, E1/2=0.87 V, JL=-6.2 mA/cm2). The calculated kinetic current density of NPC-850 approaches that of 20 wt% Pd/C. To get insight into the reaction pathway, rotating ring disk electrode (RRDE) measurements were performed. As shown in Figs. 6(c), 6(d), the electron transfer number (n) was calculated to be 3.8-4.0 in the potential range of 0.4-0.9 V. The peroxide yield on the surface of NPC-850 is observed to be less than 15 %. Figs. 6(e) and 6(f) show that NPC-850 demonstrates good stability for 5000 cycles and strong tolerance to methanol poison. In contrast, 20 wt% Pd/C shows substantial performance degradation in both E1/2 and JL. The results highlight the power of surface phosphorization in improving the electrocatalytic performance of Pd.

|
Fig.6 ORR performance: (a) LSV curves of 20 wt% Pt/C, 20 wt% Pd/C, NPC-850 and NC-850 in oxygen-saturated 0.1 mol/L KOH measured by RDE at the rotating speed of 1600 r/min; (b) Comparison of half-wave potential (E1/2), and kinetic current density (at 0.7 V) of 20 wt% Pt/C, 20 wt% Pd/C, NPC-850 and NC-850; (c) LSV curves of NPC-850 measured by RDE and RRDE; (d) The electron transfer number and the H2O2 yield of NPC-850; (e) Comparison of the methanol-resistance performance of NPC-850 and 20 wt% Pd/C as an inset; (f) LSV of NPC-850 and 20 wt% Pd/C upon 5000 cycles |
To understand the promotive role of phosphorization, capacitance and impedance spectroscopy measurements were carried out. Cyclic voltammetry curves were obtained at different scan rates. Fig. 7(a) shows the recorded current densities as a function of scanning rate. Evidently, the NPC-850 possessed nearly the same electrochemical double-layer capacitance as NC-850, which suggests that both samples hold similar electrochemical surface areas for electrochemical reaction. This means the intrinsic activity of Pd significantly improved after phosphorization. Fig. 7(b) shows the Nyquist plots. NPC-850 displayed much smaller charge transfer resistance compared with NC-850. This implies much faster charge transfer kinetics on the PdP2 surface than that on Pd. To exclude the interfering factors such as variation of N content, and P doping in the carbon framework, control samples pyrolyzed at 700 and 900 ℃ were also prepared. Pd species in the NPC-700 and -900 existed in the same form as that in the NPC-700 as evidenced by XRD measurements (Fig. S9a). XPS survey spectra also revealed the presence of N and P dopants in the carbon framework of NPC-700 and -900 (Fig. S9b). Both control samples show much worse HER and ORR performance than that of NPC-850 (Figs. 7(c) and 7(d)). NPC-700 has much higher N and P content than that of NPC-850 (9.93 vs. 4.95 wt% and 2.65 vs. 1.42 wt%) as shown in Table S1. The low pyrolysis temperature of NPC-700 decreased the graphitization degree of the carbon support as evidenced by the Raman results (Fig. S10), thereby reducing the electron conductivity and affecting the final electrocatalytic performance. The above results unambiguously suggest that surface phosphorization markedly enhances the electrochemical activity of Pd supported by porous carbon.
|
Fig.7 (a) Dependence of capacitive current density of NPC-850 and NC-850 on the scan rate; (b) Nyquist plots of NPC-850 and NC-850; (c) ORR and (d) HER polarization curves of NPC-700, -850 and -900 |
2 Conclusions
In summary, we reported the construction of Pd-based heterostructures (PdP2@Pd/C) for electrocatalysis pyrolyzed from an amine- and pyridine-containing conjugated microporous polymer (AP-CMP) synthesized by Buchwald-Hartwig (BH) coupling method. In the pyrolysis step, the AP-CMP as a host transformed into porous carbon with uniform N doping. Meanwhile, the guest materials including phytic acid and Pd(dba)2 was converted into PdP2@Pd structure. Compared with Pd/C, PdP2@Pd/C demonstrated greatly improved electrocatalytic performances including a promising HER activity (58 mV @ 10 mA/cm2), Pd-like ORR activity and excellent long-term stability. Our work illustrates the intriguing power of CMP-guest chemistry in heterostructure engineering.
Supplement Materials Appendix A 1 Experimental 1.1 MaterialsAll chemicals were purchased from commercial sources and used as received, which include 2, 5-Diaminopyridine dihydrochloride (2, 5-DAPD, >98%), tris(4-bromophenyl)-amine (TA, >98%), bis(dibenzylideneacetone)palladium(0) (Pd(dba)2, 18-24% Pd), dicyclohexylphosphino-2', 4', 6'-triisopropylbiphenyl (XPhos, 98%), sodium tert-butoxide (NaOtBu, >98%), and aqueous phytic acid (PA, 1 mol/L). All chemicals were purchased from commercial sources and used as received.
1.2 Synthesis of AP-CMP and P-AP-CMP PrecursorsA Schlenk tube was charged with 2, 5-DAPD (0.75 mol), TA (0.5 mol), Pd(dba)2 (0.03 mol), XPhos (0.045 mol), and NaOtBu (1.95 mol) placed in a N2 atmosphere. Anhydrous toluene (40 mL) was added and the reaction mixture was heated by stirring at 110 ℃. After 24 h, the reaction was cooled to room temperature and solvents were then removed by centrifugation. The remaining solids were washed by chloroform, hot MQ water, and methanol (3×200 mL, each solvent), and then dried at 50 ℃ for 72 h in a vacuum oven to yield corresponding amine and pyridine-based conjugated microporous polymer (AP-CMP) as a black blue powder. Anal. Calcd. For AP-CMP: C, 75.96.1; N, 18.12; H, 5.92. Found: C, 73.53; N, 16.53; H, 5.22. The AP-CMP (200 mg) was dispersed in 4 mL acetone with the help of sonication for 10 min. Then aqueous PA (2 g) was added. After stirring for 6 h, filtration and dry processes offered a PA-doped AP-CMP i.e. P-AP-CMP.
1.3 Synthesis of Heteroatom-Doped Porous CarbonsAP-CMP or P-AP-CMP was heated to 700, 800, 900 and 1000 ℃ under an argon atmosphere with a heating rate of 3 ℃/min and then kept at a constant temperature for 3 h, yielding nitrogen-doped pours carbons i.e. NC-x or nitrogen and phosphorus-doped porous carbons i.e. NPC-x, where x indicates carbonization temperature.
1.4 CharacterizationsFT-IR spectra were obtained on a Nicolet 670 spectrometer. Solid-state 13C cross-polarization magic angle spinning nuclear magnetic resonance (CP/MAS NMR) spectra were obtained on an AVANCE400 spectrometer. X-ray photoelectron spectra (XPS) was recorded on a Thermo Fisher Scientific ESCALAB 250Xi spectrometer. Thermogravimetric analysis (TGA) was carried out on a Mettler Toledo TGA 1 instrument in the air in a temperature range of 30-900 ℃ (heating rate 10 ℃ /min). Elemental analyses (EA) were conducted using a Euro Vector EuroEA3000 Elemental Analyzer. Powder X-ray diffraction (PXRD) patterns were obtained on a Bruker D8 Advance diffractometer (40 kV, 30 Ma) using Cu K α radiation (2θ=3-90°). Raman spectra were obtained on an inVia Reflex Raman Microscope with an argon-ion laser (excitation wavelength: 532 nm). Scanning electron microscopy (SEM) on a HITACHI S-4800 and transmission electron microscopy (TEM) images were taken on a JEM-2100 TEM microscope. The metal compositions were analyzed using a Leeman Labs Prodigy ICP/MS along with XPS and elemental analyses. Both nitrogen (N2) and carbon dioxide (CO2) adsorption/desorption measurements were conducted to obtain the pore information of the samples. The Brunauer-Emmett-Teller (BET) surface areas were calculated from nitrogen adsorption/desorption isotherms at 77.4 K after degassing the samples under high vacuum at 80 ℃ for 12 h, which were obtained by a Micromeritics ASAP 2460 surface area analyzer. The non-local density functional theory (NLDFT) model was used to calculate the pore size distribution. CO2 adsorption/desorption measurements were performed at 273 K.
1.5 Electrochemical Measurements:1) Electrode preparation.
To prepare the electrodes, 5 mg of porous carbon was dispersed in a 100 μ L 5 wt% Nafion solution (Sigma Aldrich) and 400 μ L ethanol and then sonicated to obtain a homogeneous ink after further stirring for 6 h. A catalyst ink (4 μ L) was drop-casted on a polished glassy carbon rotating disk electrode (RDE, Pine, USA, diameter: 5 mm; geometric area: 0.196 cm2; catalyst loading: ~0.3 mg/cm2) and dried in 50 ℃ oven, affording the working electrodes. The ring electrode was also used for comparison.
2) Electrochemical HER measurements.
All the electrochemical measurements were performed in a three-electrode system on a Gamry Interface 1100E Potentiostat at room temperature. The as-synthesized catalyst dispersed onto a glass carbon ring disc electrode (RDE) was used as a working electrode, a silver/silver chloride (Ag/AgCl) electrode was used as the reference electrode, and a graphite rod was served as the counter electrode.
The HER tests were performed in 0.5 mol/L H2SO4 with iR compensation. Linear sweep voltammetry (LSV) scans were conducted with a rate of 5 mV/s after purging N2 into the electrolyte solutions for half an hour to ensure the complete elimination of dissolved oxygen. During the measurements, the electrochemical cell was purged with N2. Electrochemical impedance spectroscopy (EIS) Nyquist plots were acquired by carrying out the measurements in the same configuration at η=0.15 V from 105-0.1 Hz with an AC voltage of 5 mV. The stability of the catalysts was tested by continuously cycling the electrodes in a potential range between +0.1 and -0.5 V vs. RHE at a scan rate of 50 mV/s while the time-dependent stability was operated at a constant current density of 20 mA/cm2. All the potentials were converted according to the reversible hydrogen electrode (RHE) based on ERHE=0.242+0.059 pH. The Tafel slope was calculated from polarization curves based on the Tafel equation: η=a+b log j, where η is the overpotential (mV), b is the Tafel slope, j is the current density (mA/cm2). Moreover, the exchange current density (j0) was obtained by extrapolation of Tafel plots (η = 0, j0= 10(-a/b)). Double-layer capacitance (Cdl) was obtained by cyclic voltammetry measurements in the potential range of 0.1 to 0.3 V vs. RHE with scan rates from 20 to 100 mV/s. The capacitive currents of ΔJ|Ja-Jc|/2 at 0.2 V vs. RHE were plotted against the scan rates.
3) Electrochemical ORR measurements
The ORR tests were conducted in 0.1 KOH. The measurements of LSV polarization curves were conducted between the potentials of 0.2-1.1 V with a rate of 5 mV/s at different r/min (400, 625, 900, 1225, and 1600) after purging O2 into the electrolyte solutions for 30 min to ensure an O2-saturated 0.1 mol/L KOH electrolyte. During the measurements, the electrochemical cell was continuously purged with O2. For comparison, the commercial 20 wt% Pd/C was measured under the same conditions. For the ORR at an RDE, the electron transfer number was calculated according to Koutecky-Levich (K-L) equation:
| $ 1 / j=1 / j_k+1 / B \omega^{1 / 2} $ |
where jk is the kinetic current and ω is the electrode rotating rate. B is the slope determined from the K-L plots according to the Levich equation:
| $ B=0.2 n F\left(D_{\mathrm{O}_2}\right)^{2 / 3} \nu-{ }^{1 / 6} C_{\mathrm{O}_2} $ |
where n represents the transferred electron number per oxygen molecule. F is the Faraday constant (96485 C mol-1). DO2 is the diffusion coefficient of O2 in 0.1 mol/L KOH (1.9×10-5 cm2/s). ν is the kinetic viscosity (0.01 cm2/s). CO2 is the bulk concentration of O2 (1.2 ×10-6 mol/cm3). The constant 0.2 is adopted when the rotation speed is expressed in r/min.
For the rotating RDE, the ring potential was kept at a constant potential of 1.3 V vs. RHE. The HO2-% and transferred electron number per oxygen molecule (n) were calculated by the following equation:
| $ \begin{aligned} \mathrm{HO}_2{ }^{-} & =200\left(I_{\mathrm{r}} / N\right) /\left(I_{\mathrm{d}}+I_{\mathrm{r}} / N\right) \\ n & =4 I_{\mathrm{d}} /\left(I_{\mathrm{d}}+I_{\mathrm{r}} / N\right) \end{aligned} $ |
where Id is the disk current, Ir is the ring current, and N is the current collection efficiency of the Pt ring (0.4).
The onset potential and half-wave-potential E1/2 values of all catalysts were obtained when the current density reached 10% and 50% of the limited current density of each catalyst at 0.2 V, respectively.
Appendix B
|
S1 XRD Spectra of AP-CMP and P-AP-CMP |

|
S2 13CNMR Spectrum of AP-CMP |

|
S3 (a) XPS survey spectra of P-AP-CMP and AP-CMP; XPS core-level spectra of (b) Pd 3d and (c) N 1s for P-AP-CMP and AP-CMP; (d) XPS core-level spectrum of P 2p for P-AP-CMP |
|
S4 Photograph of (a) AP-CMP, (b)P-AP-CMP and (c) NPC-x |

|
S5 TGA spectra of AP-CMP and P-AP-CMP |

|
S6 (a) TEM image; (b) SEAD pattern; (c) STEM image and statistic histogram of Pd based nanoparticles; (d) C, (e) N and (f) Pd elemental EDS mapping of NC-850 |
|
S7 (a) Nitrogen adsorption/desorption isotherms and (b) pore size distribution of NPC-850 and NC-850 |
| Table S1 XPS results of NPC-700, NPC-850, NPC-900, NC-850 and 20 wt% Pd/C |
| Table S2 Percentage of each nitrogen species for NPC-850 and NC-850 |

|
S8 P 2p XPS spectrum of NPC-850 |

|
S9 (a) XRD spectra of NPC-700 and NC-900; (b) XPS survey spectra of NPC-700 and NC-900 |

|
S10 Raman spectra of NPC-700, NPC-850, NPC-900 and NC-850 |
| [1] |
Carmo M, Fritz D L, Mergel J, et al. A comprehensive review on PEM water electrolysis. International Journal of Hydrogen Energy, 2013, 38(12): 4901-4934. DOI:10.1016/j.ijhydene.2013.01.151 (  0) 0) |
| [2] |
Chen Z W, Higgins D, Yu A P, et al. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy and Environmental Science, 2011, 4: 3167-3192. DOI:10.1039/C0EE00558D (  0) 0) |
| [3] |
Lin R H, Zhao Y Y, Wu B D. Toward a hydrogen society: Hydrogen and smart grid integration. Internaltional Journal of Hydrogen Energy, 2020, 45: 20164-20175. DOI:10.1016/j.ijhydene.2020.01.047 (  0) 0) |
| [4] |
Fang D H, Tang X, Yang L, et al. Facile synthesis of Pt-decorated Ir black as a bifunctional oxygen catalyst for oxygen reduction and evolution reactions. Nanoscale, 2019, 11: 9091-9102. DOI:10.1039/C9NR00279K (  0) 0) |
| [5] |
Nie Y, Li L, Wei Z D, et al. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chemical Society Reviews, 2015, 44: 2168-2201. DOI:10.1039/c4cs00484a (  0) 0) |
| [6] |
Nørskov J K, Rossmeisl J, Logadottir A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. The Journal of Physical Chemistry B, 2004, 108: 17886-17892. DOI:10.1021/jp047349j (  0) 0) |
| [7] |
Sui S, Wang X Y, Zhou X T, et al. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: nanostructure, activity, mechanism and carbon support in PEM fuel cells. Journal of Materials Chemistry A, 2017, 5: 1808-1825. DOI:10.1039/C6TA08580F (  0) 0) |
| [8] |
Tian X L, Zhao X, Su Y Q, et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science, 2019, 366: 850-857. DOI:10.1126/science.aaw749 (  0) 0) |
| [9] |
Wang J, Long W Y, Wang L L, et al. Unlocking the door to highly active ORR catalysts for PEMFC applications: polyhedron-engineered Pt-based nanocrystals. Energy and Environmental Science, 2018, 11: 258-275. DOI:10.1039/C7EE02444D (  0) 0) |
| [10] |
Vesborg P C K, Jaramillo T F. Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy. RSC Advances, 2012, 2: 7933-7947. DOI:10.1039/C2RA20839C (  0) 0) |
| [11] |
Liu Q M, Peng Y, Li Q Z, et al. Atomic dispersion and surface enrichment of palladium in nitrogen-doped porous carbon cages lead to high-performance electrocatalytic reduction of oxygen. ACS Applied Materials and Interfaces, 2020, 12: 17641-17650. DOI:10.1021/acsami.0c03415 (  0) 0) |
| [12] |
Rebuffi L, Mukherjee B, Siboni B, et al. Surface softening in palladium nanoparticles: effects of a capping agent on vibrational properties. Nanoscale, 2020, 12: 5876-5887. DOI:10.1039/D0NR00182A (  0) 0) |
| [13] |
Weber M, Tuleushova N, Zgheib J, et al. Enhanced electrocatalytic performance triggered by atomically bridged boron nitride between palladium nanoparticles and carbon fibers in gas-diffusion electrodes. Applied Catalysis B: Environmental, 2019, 257: 117917-117926. DOI:10.1016/j.apcatb.2019.117917 (  0) 0) |
| [14] |
Xiao W P, Cordeiro A M L, Gong M L, et al. Optimizing the ORR activity of Pd based nanocatalysts by tuning their strain and particle size. Journal of Materials Chemistry A, 2017, 5: 9867-9872. DOI:10.1039/C7TA02479G (  0) 0) |
| [15] |
Zhu W X, Yuan H, Liao F, et al. Strain engineering for Janus palladium-gold bimetallic nanoparticles: enhanced electrocatalytic performance for oxygen reduction reaction and zinc-air battery. Chemistry Engineering Journal, 2020, 389: 124240-124246. DOI:10.1016/j.cej.2020.124240 (  0) 0) |
| [16] |
Seo M H, Choi S M, Seo J K, et al. The graphene-supported palladium and palladium-yttrium nanoparticles for the oxygen reduction and ethanol oxidation reactions: Experimental measurement and computational validation. Applied Catalysis B: Environmental, 2013, 129: 163-171. DOI:10.1016/j.apcatb.2012.09.005 (  0) 0) |
| [17] |
Tzorbatzoglou F, Brouzgou A, Tsiakaras P. Electrocatalytic activity of Vulcan-XC-72 supported Pd, Rh and PdxRhy toward HOR and ORR. Applied Catalysis B: Environmental, 2015(174-175): 203-211. DOI:10.1016/j.apcatb.2015.03.002 (  0) 0) |
| [18] |
Kariuki N N, Wang X, Mawdsley J R, et al. Colloidal synthesis and characterization of carbon-supported Pd-Cu nanoparticle oxygen reduction electrocatalysts. Chemistry of Materials, 2010, 22: 4144-4152. DOI:10.1021/CM100155Z (  0) 0) |
| [19] |
Erikson H, Sarapuu A, Kozlova J, et al. Oxygen electroreduction on electrodeposited PdAu nanoalloys. Electrocatalysis, 2015, 6: 77-85. DOI:10.1007/s12678-014-0222-1 (  0) 0) |
| [20] |
Yan Y C, Zhan F W, Du S, et al. Kinetically-controlled growth of cubic and octahedral Rh-Pd alloy oxygen reduction electrocatalysts with high activity and durability. Nanoscale, 2015, 7: 301-307. DOI:10.1039/C4NR04942J (  0) 0) |
| [21] |
Sarkar S, Patel S, Sampath S. Efficient oxygen reduction activity on layered palladium phosphosulphide and its application in alkaline fuel cells. Journal of Power Sources, 2020, 445: 227280-227285. DOI:10.1016/j.jpowsour.2019.227280 (  0) 0) |
| [22] |
Zhang C, Yu S S, Xie Y Y, et al. Suppressing the Pd-C interaction through B-doping for highly efficient oxygen reduction. Carbon, 2019, 149: 370-379. DOI:10.1016/j.carbon.2019.04.060 (  0) 0) |
| [23] |
Luo F, Zhang Q, Yu X X, et al. Palladium phosphide as a stable and efficient electrocatalyst for overall water splitting. Angewandte Chemie International Edition, 2018, 57: 14862-14867. DOI:10.1002/anie.201810102 (  0) 0) |
| [24] |
Ji P X, Jin H H, Xia H L, et al. Double metal diphosphide pair nanocages coupled with P-doped carbon for accelerated oxygen and hydrogen evolution kinetics. ACS Applied Materials and Interfaces, 2020, 12: 727-733. DOI:10.1021/acsami.9b17960 (  0) 0) |
| [25] |
Lin C, Wang P Y, Jin H H, et al. An iron-doped cobalt phosphide nano-electrocatalyst derived from a metal-organic framework for efficient water splitting. Dalton Transactions, 2019, 48: 16555-16561. DOI:10.1039/C9DT03619A (  0) 0) |
| [26] |
Lou M, Wang R, Zhang J, et al. Optimized synthesis of nitrogen and phosphorus dual-doped coal-based carbon fiber supported Pd catalyst with enhanced activities for formic acid electrooxidation. ACS Applied Materials and Interfaces, 2019, 11: 6431-6441. DOI:10.1021/acsami.8b20736 (  0) 0) |
| [27] |
Li J D, Tian Q F, Jiang S Y, et al. Electrocatalytic performances of phosphorus doped carbon supported Pd towards formic acid oxidation. Electrochimica Acta, 2016, 213: 21-30. DOI:10.1016/j.electacta.2016.06.041 (  0) 0) |
| [28] |
Kucernak A R J, Fahy K F, Sundaram V N N. Facile synthesis of palladium phosphide electrocatalysts and their activity for the hydrogen oxidation, hydrogen evolutions, oxygen reduction and formic acid oxidation reactions. Catalysis Today, 2016, 262: 48-56. DOI:10.1016/j.cattod.2015.09.031 (  0) 0) |
| [29] |
Wang J, Teschner D, Huang X, et al. Nanosized palladium on holey graphene sheets incorporating PxOy for effective formic acid oxidation. Electrochemistry Communications, 2017, 74: 24-27. DOI:10.1016/j.elecom.2016.11.012 (  0) 0) |
| [30] |
Yao Y, Rubino S, Gates B D, et al. In situ X-ray absorption spectroscopic studies of magnetic Fe@FexOy/Pd nanoparticle catalysts for hydrogenation reactions. Catalysis Today, 2017, 291: 180-186. DOI:10.1016/j.cattod.2017.02.049 (  0) 0) |
| [31] |
Li J, Zhou P P, Li F, et al. Ni@Pd/PEI-rGO stack structures with controllable Pd shell thickness as advanced electrodes for efficient hydrogen evolution. Journal of Materials Chemistry A, 2015, 3: 11261-11268. DOI:10.1039/C5TA01805F (  0) 0) |
| [32] |
Lu Y Z, Jiang Y Y, Gao X H, et al. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. Journal of the American Chemical Society, 2014, 136: 11687-11697. DOI:10.1021/ja5041094 (  0) 0) |
| [33] |
Liao Y Z, Weber J, Mills B M, et al. Highly efficient and reversible iodine capture in hexaphenylbenzene-based conjugated microporous polymers. Macromolecules, 2016, 49: 6322-6333. DOI:10.1021/acs.macromol.6b00901 (  0) 0) |
| [34] |
Lee J-S M, Cooper A I. Advances in conjugated microporous polymers. Chemical Reviews, 2020, 120: 2171-2214. DOI:10.1021/acs.chemrev.9b00399 (  0) 0) |
| [35] |
Liu W B, Wang K, Wang C, et al. Mixed phthalocyanine-porphyrin-based conjugated microporous polymers towards unveiling the activity origin of Fe-N4 catalysts for the oxygen reduction reaction. Journal of Materials Chemistry A, 2018, 6: 22851-22857. DOI:10.1039/C8TA08173E (  0) 0) |
| [36] |
Marco A B, Cortizo-Lacalle D, Perez-Miqueo I, et al. Twisted aromatic frameworks: readily exfoliable and solution-processable two-dimensional conjugated microporous polymers. Angewandte Chemie International Edition, 2017, 56: 6946-6951. DOI:10.1002/anie.201700271 (  0) 0) |
| [37] |
Roy S, Bandyopadhyay A, Das M, et al. Redox-active and semi-conducting donor-acceptor conjugated microporous polymers as metal-free ORR catalysts. Journal of Materials Chemistry A, 2018, 6: 5587-5591. DOI:10.1039/C8TA00099A (  0) 0) |
| [38] |
Zhao W, Jiao Y Z, Li J J, et al. One-pot synthesis of conjugated microporous polymers loaded with superfine nano-palladium and their micropore-confinement effect on heterogeneously catalytic reduction. Journal of Catalysis, 2019, 378: 42-50. DOI:10.1016/j.jcat.2019.07.056 (  0) 0) |
| [39] |
He Y F, Gehrig D, Zhang F, et al. Highly efficient electrocatalysts for oxygen reduction reaction based on 1D ternary doped porous carbons derived from carbon nanotube directed conjugated microporous polymers. Advanced Functional Materials, 2016, 26: 8255-8265. DOI:10.1002/adfm.201603693 (  0) 0) |
| [40] |
Jiao R, Zhang W L, Sun H X, et al. N- and S-doped nanoporous carbon framework derived from conjugated microporous polymers incorporation with ionic liquids for efficient oxygen reduction reaction. Materials Today Energy, 2020, 16: 100382-100393. DOI:10.1016/j.mtener.2020.100382 (  0) 0) |
| [41] |
Li Q, Shao Q, Wu Q, et al. In situ anchoring of metal nanoparticles in the N-doped carbon framework derived from conjugated microporous polymers towards an efficient oxygen reduction reaction. Catalysis Science & Technology, 2018, 8: 3572-3579. DOI:10.1039/C8CY00483H (  0) 0) |
| [42] |
Zhou Y B, Zhan Z P. Conjugated microporous polymers for heterogeneous catalysis. Chemistry, an Asian Journal, 2018, 13: 9-19. DOI:10.1002/asia.201701107 (  0) 0) |
| [43] |
Liao Y Z, Wang H G, Zhu M F, et al. Efficient supercapacitor energy storage using conjugated microporous polymer networks synthesized from buchwald-hartwig coupling. Advanced Materials, 2018, 30: 1705710-1705719. DOI:10.1002/adma.201705710 (  0) 0) |
| [44] |
Wang H G, Hou B, Yang Y, et al. Cobalt nanocrystals encapsulated in heteroatom-rich porous carbons derived from conjugated microporous polymers for efficient electrocatalytic hydrogen evolution. Small, 2018, 14: 1803232-1803237. DOI:10.1002/smll.201803232 (  0) 0) |
| [45] |
Li H, Lyu W, Liao Y Z. Engineering redox activity in conjugated microporous polytriphenylamine networks using pyridyl building blocks toward efficient supercapacitors. Macromolecular Rapid Communications, 2019, 40: 1900455-1190461. DOI:10.1002/marc.201900455 (  0) 0) |
| [46] |
Hu X W, Wang H G, Faul C F J, et al. A crosslinking alkylation strategy to construct nitrogen-enriched tetraphenylmethane-based porous organic polymers as efficient carbon dioxide and iodine adsorbents. Chemical Engineering Journal, 2020, 382: 122998-123005. DOI:10.1016/j.cej.2019.122998 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


