2. School of Physical Science and Technology, Soochow University, Suzhou 215006, Jiangsu, China
Exploiting cost-effective and eco-friendly energy storage devices with high reliability is important for extensive utilization of renewable energy[1-4]. Although non-aqueous lithium-ion batteries have been the dominant devices in electronics, their further practicalities in vehicles and renewable energy storage are limited by issues of safety, cost, and durability[5-7]. To handle these issues, research efforts have been focused on alternative battery chemistry and techniques[8-10]. Among the alternatives, aqueous zinc-ion batteries (ZIBs) have drawn booming interest since metallic zinc features a low redox potential of -0.76 V (vs. standard hydrogen electrode), high theoretical capacity of 5855 mA·h/cm3 and 820 mA·h/g, nontoxicity, and high reversibility of Zn/Zn2+ redox in mild aqueous media[11-13].
ZIBs share much in common with lithium-ion batteries in the energy storage mechanism. During charge and discharge, Zn ion shuttle between the anode and cathode, a "rocking chair" process. The profit of using aqueously compatible Zn anode brings about substantial convenience in material selection and electrode design. As a result, ZIBs are rising stars and would revolutionize the way we harness and store energy[14]. Moreover, the profitable studies on materials and technologies of zinc batteries have ushered in a prospect for multi-scale and multi-dimensional applications[15]. Recently, their multiple functionalities have been explored and developed to adapt for a specific purpose, as schematically shown in Fig. 1. In such a mini-review, we will report the state-of-the-art progress of multiple functionalities such as flexibility, self-healing, self-charging, integration, and miniaturization. The focus is on the engineering principles of why and how the functionalities can be realized through specific materials and structural engineering. The remaining challenges and research directions are also given at the end of this review.

|
Fig.1 Multiple functionalities of aqueous ZIBs |
1 Flexibility
The boom in wearable and smart electronic devices has triggered the growing need for reliable, nonflammable, cost-effective, and flexible energy storage. Therefore, aqueous ZIBs that sustain frequent mechanical deformations without sacrificing the electrochemical performances are deemed for powering these electronics. It is a necessity to achieve the flexibility of a battery by either using inherently soft and deformable materials or specially-designated structures. These structures, including origami, island-bridge, and wrinkled structures, are based on conventional rigid and fragile constituents[16]. To avoid delamination of active materials from flexible substrates under external stress, strong interfacial interaction is demanded. In this regard, robust, free-standing, and conductive electrodes are desired. On the other hand, gel electrolytes are free of leakage and more appropriate for flexible cells. Moreover, battery configuration is critical to electrochemical and mechanical behaviors. Currently, 2D sandwich-type and 1D cable-type configurations have been widely employed for flexible ZIBs.
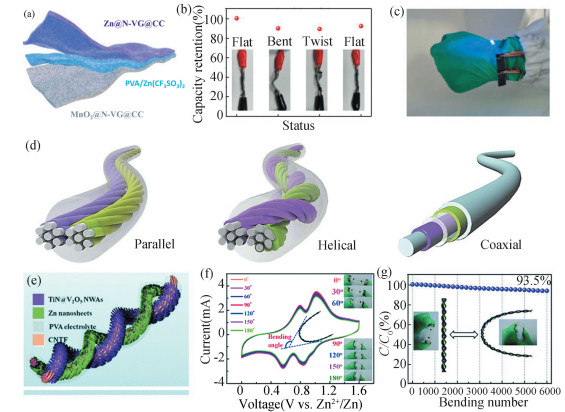
Sandwich-type ZIBs feature a laminated structure comprising a gel electrolyte layer between the anode and cathode (Fig. 2(a)). To impart flexibility, carbon matrixes such as carbon cloth, carbon nanotube, and graphene film are often utilized to load active materials[17-19]. A 3D architecture of nitrogen-doped vertical graphene grown on carbon cloth (N-VG@CC) has been constructed as a Zn scaffold for robust Zn anode[20]. Coupled with a MnO2@N-VG@CCcathode, the flexible ZIBs show an impressive capacity retention of 80% over 300 cycles and remarkable mechanical performance (Fig. 2(b), (c)). In addition to carbon-based substrates, metallic and polymeric scaffolds have also been applied in flexible zinc batteries[21-23].
|
Fig.2 Flexible zinc batteries. (a) Schematic diagram depicting the structure of a flexible ZIB with gel electrolyte. (b) The capacity retention of a flexible ZIB at various deformation stages. (c) Optical photos showing two flexible ZIBs connected in series. (Reproduced with permission[20]. Copyright 2021, Wiley-VCH.) (d) Schematic representation for the 1D cable-type configurations including the parallel, helical, and coaxial types. (Reproduced with permission[24]. Copyright 2020, Wiley-VCH.) (e) Illustration displaying a cable-type TiN@V2O5//Zn battery with helical configuration. (f) Cyclic voltammetry curves of the TiN@V2O5//Zn cell at different bending status. (g) The capacity retention of the assembled ZIB during 6000 cycles at a bending stage. (Reproduced with permission[25]. Copyright 2019, Royal Society of Chemistry) |
Another representative design for flexible ZIBs is the 1D cable configuration, which has been exploited in many charge storage devices[26]. Such configuration can maximize the device's flexibility and volumetric energy density. The cable-type configuration can be realized through three typical structures in ZIBs (Fig. 2(d)). The first is the parallel cable structure, where the cathode and Zn anode are parallel and separated. The second is the helical structure including single helix and double helix. The third is the coaxial cable structure, where the inner zinc wire is covered by separator and cathode layers. As demonstrated in Fig. 2(d)-(e), Yao et al. assembled a solid-state fiber ZIB with a twisted structure[25]. Fig. 2(f) presents negligible variations in the cyclic voltammetry curves measured at increasing bending angles from 0° to 180°, and 93.5% of the initial capacity can be maintained over 6000 bending cycles (Fig. 2(g)). Importantly, these linear shapes demonstrate superiorities of knittability and wearability. These are crucial for the integration with electronics such as smart clothes, intelligent watches, wireless sensors, and flexible displayers[27-29]. For example, coaxial fiber ZIBs can be woven into the textile to supply power for electronic devices, and the utilization of serial and parallel connections leads to extended operation potential and output current[30]. Compared with conventional sandwich-type design, cable-type configuration demonstrates several distinctive merits such as high flexibility, wearability, and integration. Thus, the attention should also be paid to the configuration design when developing flexible ZIBs.
2 Self-HealingSelf-healing is a long-term target of energy storage devices as they could undergo repeated deformations during utilization while autonomously repairing the damages. For developing self-healing ZIBs, self-healing materials are indispensable. Currently, self-healing research focuses on polymer electrolytes.
PVA-based gel electrolytes have been extensively employed for solid-state flexible ZIBs, which also possess the self-healing ability because of massive hydroxy side groups in polymer chains and the O-H…O hydrogen bonds[31-32]. With a freeze and thaw process, Huang et al. fabricated a hydrogel electrolyte of PVA/Zn(CF3SO3)2, which showed high ionic conductivity and desirable electrochemical performance[33]. Equipped with this electrolyte, an all-in-one integrated ZIB presents prominent self-healing behavior. After repeated cutting and healing cycles, the electrochemical performance is almost recovered. Similarly, a quasi-solid ZIB containing PVA/Zn(CH3COO)2/Mn(CH3COO)2 electrolyte has been reported[34]. This device displays both quick self-healing and flexible performances.
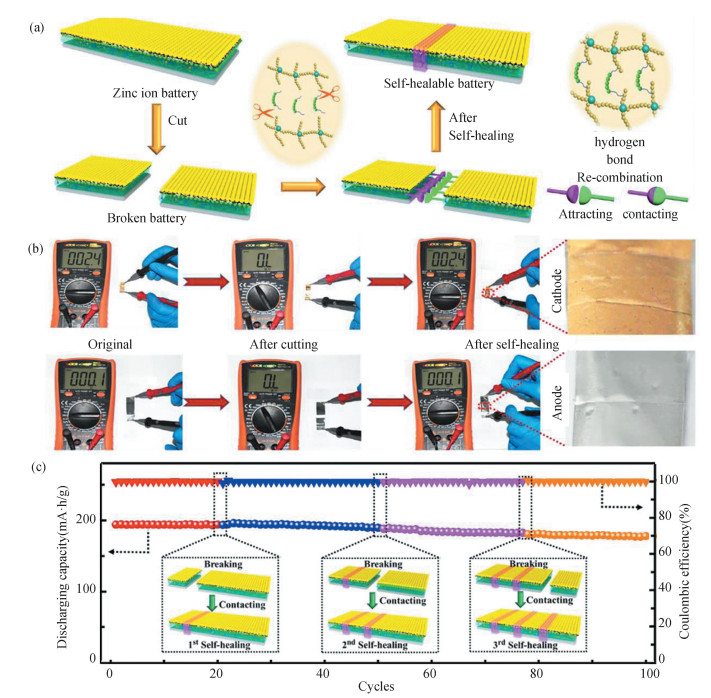
However, the self-healing function cannot be well retained at low temperatures (< 0 ℃) because water molecules in polymer electrolytes will be frozen. To mitigate this issue, Jin et al. designed a polyelectrolyte of acrylamide in water and ethylene glycol solvent[35]. It shows desirable anti-freezing and self-healing functions by suppressing the water icing and dynamically modulating the chemical interactions between water and polymer chains (Fig. 3). For the first time, ZIBs with this polyelectrolyte can work at -20 ℃, affording excellent self-healing stability over 100 cycles and capacity retention up to 90%. These encouraging findings would have a profound impact on exploiting aqueous self-healing batteries that can work in freezing environments.
|
Fig.3 Self-healing zinc batteries. (a) Schematic illustration of the self-healable ZIB. (b) The resistance variations of anode and cathode before cutting and after self-healing. (c) Cycle property of the ZIB at the initial state and after various cutting/self-healing times. (Reproduced with permission[35]. Copyright 2022, Elsevier) |
3 Self-Charging
Self-charging systems, consisting of both energy storage and energy harvesting units, have gained tremendous attention in the energy field. So far, diverse energy harvesting technologies, such as photovoltaic devices[36], triboelectric nanogenerators[37], piezoelectric and thermoelectric nanogenerators[38-39], have been integrated into batteries to realize self-charging. For example, a flexible ZIB combined with perovskite solar cells can capture light energy and ensure the operation of a commercial smart bracelet[40]. Notwithstanding, an integrated system is highly dependent on the design as many components are demanded. Hence, it is urgent to exploit self- charging ZIB systems that feature a simple configuration and are available in various environments.
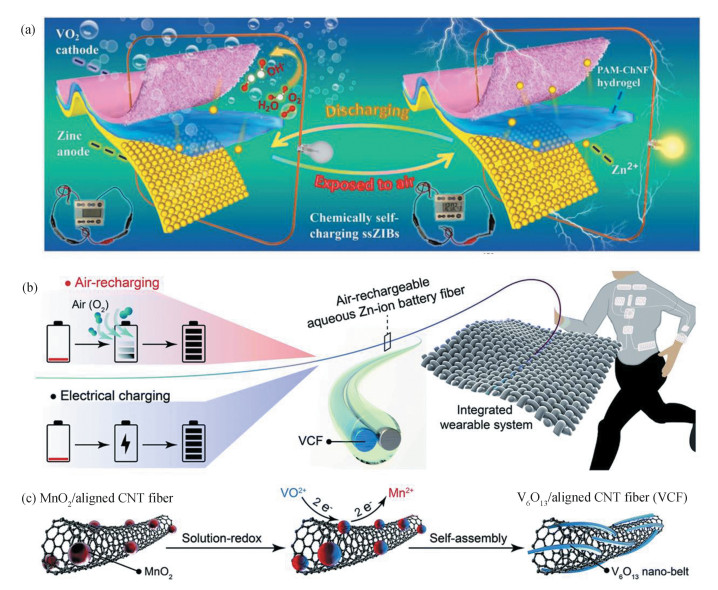
On the other hand, the chemical energy stored in molecules is a kind of energy source which can transform into electrical energy via spontaneous redox reactions[43-44]. As an abundant source of air, oxygen receives widespread interest in charge storage and conversion devices[45-46]. In this sense, a chemical self-charging system with a two-electrode configuration is reported[47]. Importantly, three components of energy harvesting, conversion, and storage have been integrated into a CaV6O16·3H2O cathode. The ZIB can be self-recharged when the cathode is exposed to air. Similarly, Liu et al. assembled a self-charging ZIB based on a V2O5 cathode with a polyacrylamide-chitin hydrogel electrolyte (Fig. 4(a))[41]. This ZIB exhibits a remarkable energy and power density of 231.9 W·h/kg and 139.0 W/kg, respectively. Also, a ZIB with ZnMn2O4 cathode can be self-charged to 1.5 V in an ambient environment, delivering a specific capacity of 176.8 mA·h/g at a current density of 0.2 A/g[48].
|
Fig.4 Self-charging zinc batteries. (a) Schematic diagram of self-charging and discharging processes of the solid-state ZIBs. (Reproduced with permission[41].Copyright 2020, Wiley-VCH.) (b) Schematic of the self-charging ZIB with air-recharging capability. (c) Schematic showing the synthesis of the freestanding VCF electrode in the ZIB fiber. (Reproduced with permission[42]. Copyright 2021, Royal Society of Chemistry) |
Surely, imparting air-recharging capability to fiber batteries will bring new chances for next-generation wearable electronics. Peng's group reported such a high-performance fiber ZIB, which is able to directly capture energy from ambient air without donating extra power supply (Fig. 4(b))[42]. As depicted in Fig. 4(c), the freestanding fiber cathode is a combination of aligned carbon nanotubes and nanostructured V6O13. The cathode can undergo spontaneous redox reactions in ambient environment at the discharge stage. Such a fiber battery affords a high capacity of 371 mA·h/g at a current density of 200 mA/g, which can be efficiently recharged to ~60% by simply exposing the cathode to air. As a demonstration, this battery is capable of powering the strain sensor in a wearable fingertip.
4 IntegrationTo develop free-standing and wireless electronics, self-charging systems composed of both energy storage and harvesting units can be further integrated into an electronic. A ZIB is such an energy storage unit. In an integrated device, zinc batteries offer basic energy or power for function units, but also store the energy harvested. Therefore, integration is an efficient route that further extends the functionalities of aqueous zinc batteries.
To date, diverse wireless electronics with integrated zinc batteries as energy units have been exploited. As an example, Kim et al. designed a ZIB containing a carbon fiber cathode coated by polyaniline and a laser micromachined Zn anode (Fig. 5(a)-(c))[49]. Batteries in different forms such as ring, square, circular H-, and other polygonal geometries, have been fabricated. Since they are stable against oxygen and moisture, the long lifespan of such batteries is achieved. Particularly, the integration of a ring-shaped battery device with photosensor and electronic components has been demonstrated, showing appealing performance.

|
Fig.5 Integration of zinc batteries. (a) SEM image of the carbon fiber coated with PANI. (b) Schematic diagram of a Zn-PANI battery with stacked electrodes. (c) Schematic of a wearable photosensor powered by a ring-shaped Zn-PANI battery. (Reproduced with permission[49]. Copyright 2018, American Chemical Society.) (d) SEM image of the cross-sectional Zn anode with Ni protective layer. Schematic illustration of the configuration design (e) and working mechanism (f) of the flexible photo-rechargeable system. (Reproduced with permission[50]. Copyright 2022, Elsevier) |
Bi et al.[50] designed flexible photo-rechargeable systems by integrating micro-ZIBs with perovskite solar cells (Fig. 5(d)-(f)). A Ni protective layer is used to stabilize device configuration and improve electrochemical property. The integration was readily constructed using inkjet printing and electrodeposition. Similarly, Li et al.[51] integrate zinc batteries with multifunctional fiber sensors. Without other energy supplies, this system is capable of detecting NO2 gas and human motions, holding promising potential in the application of wearable electronics.
5 MiniaturizationThe dramatic development in portable, smart, and integrated electronics calls for miniaturized energy storage units[52-54]. Miniaturization is a new research direction for batteries[55]. However, aqueous zinc microbatteries often face issues of low working voltage and limited lifespan. To mitigate these issues, Qu's group[56]employed laser carving and electrode-position to assemble microbatteries with an in-plane interdigitated pattern. Importantly, the microbatteries showed an extended voltage output of 1.74 V.
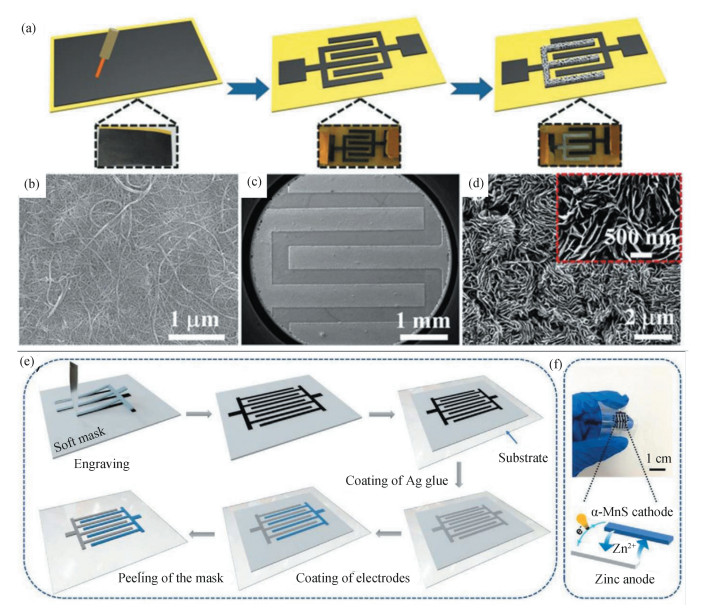
To simultaneously raise energy and power for miniaturized devices, Sun et al.[57]constructed a microscale device by integrating carbon nanotube cathode and a zinc anode. As shown in Fig. 6(a)-(d), the interdigital microelectrodes were prepared through a facile computer-controlled laser engraving system. Each microelectrode has a width of about 500 mm and the distance between neighboring fingers is 300 mm. Coupling with CNT micro-cathode and ZnSO4 electrolyte, the resultant microdevice afforded areal energy of 29.6 mW·h/cm2 at an areal power of 8 mW/cm2, surpassing most previously reported systems. Similarly, Jiang et al.[58] reported flexible microbatteries based on soft templates (Fig. 6(e), (f)). The microbatteries can be designed in arbitrary shapes on diverse substrates through an engraving method. This microbattery delivered an areal capacity of 178 μA·h/cm2. There is no doubt that the progress on miniaturized ZIBs with high energy and power density can stimulate the development of portable and wearable electronics.
|
Fig.6 Zinc microbatteries. (a) Schematic diagram showing the fabrication process of the ZmSC. The SEM images of the CNT paper (b), micro-electrode (c) and anode after plating (d). (Reproduced with permission[57]. Copyright 2018, Royal Society of Chemistry.) (e) Illustration of the fabrication process for ZIMB by soft template engraving. (f) Photograph and working principle of the fabricated interdigital configured ZIMB. (Reproduced with permission[58]. Copyright 2021, Wiley-VCH) |
6 Conclusions
In summary, aqueous zinc batteries have presented great promise thanks to their cost-effectiveness, eco-friendliness, high safety, and desirable electrochemical performance. To meet various application demands, multiple functionalities including flexibility, self-healing, self-charging, integration, and miniaturization have been extensively explored. Despite ongoing progress, study in this field is still in an early stage. There remain several issues that need to be timely addressed to meet future demands. Firstly, it is important to unveil the operation mechanism of zinc batteries with various functionalities. Apart from theoretical simulation, in situ characterization techniques should be developed to deepen the fundamental understanding. Secondly, it remains difficult to objectively compare the superiority of different functionalities. A reliable assessment system should be established for better comparison of overall battery performance. Thirdly, more efforts need to be devoted to the electrolyte as it is crucial to improve the battery performance and ensure the functionalities. Novel type electrolytes with prominent ionic conductivity, high mechanical strength, and favorable water-retaining property should be explored. Certainly, more challenges such as cell packing and function elevating should be met before these functionalities can be deployed in practice.
It is worth noting that the fulfillment of these functionalities has to meet the challenge of temperatures. At high temperatures, the water volatilization induces salt precipitation and an increase in battery internal pressure, and side reactions such as electrolyte decomposition may be significant[59]. A non-flammable and heat-stable solvent [60] and a coating of cathode materials [61] would be operative to protect the cell from damage. On the other hand, low temperature is also a challenge for ZIBs, as it may lead to the freezing of the electrolyte. Tuning the composition of electrolyte to decrease its freezing point has been proven effective [62-63].
| [1] |
Olabi A G. Renewable energy and energy storage systems. Energy, 2017, 136: 1-6. DOI:10.1016/j.energy.2017.07.054 (  0) 0) |
| [2] |
Gür T M. Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environmental Science, 2018, 11(10): 2696-2767. DOI:10.1039/C8EE01419A (  0) 0) |
| [3] |
Song Y, Pan Q, Lv H, et al. Ammonium-ion storage using electrodeposited manganese oxides. Angewandte Chemie International Edition, 2021, 60(11): 5718-22. DOI:10.1002/anie.202013110 (  0) 0) |
| [4] |
Lv H, Pan Q, Song Y, et al. A review on nano-/microstructured materials constructed by electrochemical technologies for supercapacitors. Nano-Micro Letters, 2020, 12(1): 118. DOI:10.1007/s40820-020-00451-z (  0) 0) |
| [5] |
Manthiram A. An outlook on lithium ion battery technology. ACS Central Science, 2017, 3(10): 1063-1069. DOI:10.1021/acscentsci.7b00288 (  0) 0) |
| [6] |
Whittingham M S. Special editorial perspective: beyond Li-ion battery chemistry. Chemical Reviews, 2020, 120(14): 6328-6330. DOI:10.1021/acs.chemrev.0c00438 (  0) 0) |
| [7] |
Kubota K, Dahbi M, Hosaka T, et al. Towards K-ion and Na-ion batteries as "Beyond Li-Ion". The Chemical Reccord, 2018, 18(4): 459-479. DOI:10.1002/tcr.201700057 (  0) 0) |
| [8] |
Jiang Y, Wang Y, Ni J, et al. Molybdenum-based materials for sodium-ion batteries. InfoMat, 2021, 3(4): 339-352. DOI:10.1002/inf2.12175 (  0) 0) |
| [9] |
Ni J, Li L. Cathode architectures for rechargeable ion batteries: progress and perspectives. Advanced Materials, 2020, 32(28): 2000288. DOI:10.1002/adma.202000288 (  0) 0) |
| [10] |
Wang Y, Chao D, Wang Z, et al. An energetic CuS-Cu battery system based on CuS nanosheet arrays. ACS Nano, 2021, 15(3): 5420-5427. DOI:10.1021/acsnano.1c00075 (  0) 0) |
| [11] |
He P, Chen Q, Yan M, et al. Building better zinc-ion batteries: A materials perspective. Energy Chem, 2019, 1(3): 100022. DOI:10.1016/j.enchem.2019.100022 (  0) 0) |
| [12] |
Fang G Z, Zhou J, Pan A Q, et al. Recent advances in aqueous zinc-ion batteries. ACS Energy Letters, 2018, 3(10): 2480-2501. DOI:10.1021/acsenergylett.8b01426 (  0) 0) |
| [13] |
Ruan P, Liang S, Lu B, et al. Design strategies for high-energy-density aqueous zinc batteries. Angewandte Chemie International Edition, 2022, 61(17): e202200598. DOI:10.1002/anie.202200598 (  0) 0) |
| [14] |
Meng J M, Song Y, Qin Z M, et al. Cobalt-nickel double hydroxide toward mild aqueous zinc-ion batteries. Advanced Functional Materials, 2022, 32(33): 2204026. DOI:10.1002/adfm.202204026 (  0) 0) |
| [15] |
Chen X H, Ruan P C, Wu X W, et al. Crystal structures, reaction mechanisms, and optimization strategies of MnO2 cathode for aqueous rechargeable zinc batteries. Acta Physico-Chimica Sinica, 2022, 38(11): 2111003. DOI:10.3866/PKU.WHXB202111003 (  0) 0) |
| [16] |
Zhu S, Sheng J, Chen Y, et al. Carbon nanotubes for flexible batteries: recent progress and future perspective. National Science Review, 2021, 8(5): nwaa261. DOI:10.1093/nsr/nwaa261 (  0) 0) |
| [17] |
Wu F F, Gao X B, Xu X L, et al. MnO2 nanosheet-assembled hollow polyhedron grown on carbon cloth for flexible aqueous zinc-ion batteries. Chem Sus Chem, 2020, 13(6): 1537-1545. DOI:10.1002/cssc.201903006 (  0) 0) |
| [18] |
Zeng Y X, Zhang X Y, Qin R F, et al. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Advanced Materials, 2019, 31(36): 1903675. DOI:10.1002/adma.201903675 (  0) 0) |
| [19] |
Lee Y-G, Lee J, An J H. Free-standing manganese oxide on flexible graphene films as advanced electrodes for stable, high energy-density solid-state zinc-ion batteries. Chemical Engineering Journal, 2021, 414: 128916. DOI:10.1016/j.cej.2021.128916 (  0) 0) |
| [20] |
Cao Q H, Gao H, Gao Y, et al. Regulating dendrite-free zinc deposition by 3D zincopilic nitrogen-doped vertical graphene for high-performance flexible Zn-ion batteries. Advanced Functional Materials, 2021, 31(37): 2103922. DOI:10.1002/adfm.202103922 (  0) 0) |
| [21] |
Zhao J, Ren H, Liang Q H, et al. High-performance flexible quasi-solid-state zinc-ion batteries with layer-expanded vanadium oxide cathode and zinc/stainless steel mesh composite anode. Nano Energy, 2019, 62: 94-102. (  0) 0) |
| [22] |
Jiang K, Zhou Z Y, Wen X, et al. Fabrications of high-performance planar zinc-ion microbatteries by engraved soft templates. Small, 2021, 17(12): 2007389. DOI:10.1002/smll.202007389 (  0) 0) |
| [23] |
Liu T, Chen X, Tervoort E, et al. Design and fabrication of transparent and stretchable zinc ion batteries. ACS Applied Energy Materials, 2021, 4(6): 6166-6179. DOI:10.1021/acsaem.1c00958 (  0) 0) |
| [24] |
Mo F, Liang G J, Huang Z D, et al. An overview of fiber-shaped batteries with a focus on multifunctionality, scalability, and technical difficulties. Advanced Materials, 2020, 32(5): 1902151. DOI:10.1002/adma.201902151 (  0) 0) |
| [25] |
Li Q L, Zhang Q C, Liu C L, et al. Anchoring V2O5 nanosheets on hierarchical titanium nitride nanowire arrays to form core-shell heterostructures as a superior cathode for high-performance wearable aqueous rechargeable zinc-ion batteries. Journal of Materials Chemistry A, 2019, 7(21): 12997-13006. DOI:10.1039/C9TA03330K (  0) 0) |
| [26] |
Zhu S, Ni J F, Li Y. Carbon nanotube-based electrodes for flexible supercapacitors. Nano Research, 2020, 13(7): 1825-1841. DOI:10.1007/s12274-020-2729-5 (  0) 0) |
| [27] |
Li H F, Liu Z X, Liang G J, et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano, 2018, 12(4): 3140-3148. DOI:10.1021/acsnano.7b09003 (  0) 0) |
| [28] |
Zhang X, Wang Q J, Peng B, et al. Pressure-driven and creep-enabled interface evolution in sodium metal batteries. ACS Applied Materials Interfaces, 2021, 13(22): 26533-26541. DOI:10.1021/acsami.0c22006 (  0) 0) |
| [29] |
He B, Zhang Q C, Man P, et al. Self-sacrificed synthesis of conductive vanadium-based metal-organic framework nanowire-bundle arrays as binder-free cathodes for high-rate and high-energy-density wearable Zn-ion batteries. Nano Energy, 2019, 64: 103935. DOI:10.1016/j.nanoen.2019.103935 (  0) 0) |
| [30] |
Zhang Q C, Li C W, Li Q L, et al. Flexible and high-voltage coaxial-fiber aqueous rechargeable zinc-ion battery. Nano Letters, 2019, 19(6): 4035-4042. DOI:10.1021/acs.nanolett.9b01403 (  0) 0) |
| [31] |
Holloway J L, Lowman A M, Palmese G R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter, 2013, 9(3): 826-833. DOI:10.1039/C2SM26763B (  0) 0) |
| [32] |
Chen D D, Wang D, Yang Y, et al. Self-healing materials for next-generation energy harvesting and storage devices. Advanced Energy Materials, 2017, 7(23): 1700890. DOI:10.1002/aenm.201700890 (  0) 0) |
| [33] |
Huang S, Wan F, Bi S S, et al. A self-healing integrated all-in-one zinc-ion battery. Angewandte Chemie International Edition, 2019, 131(13): 4357-4361. DOI:10.1002/anie.201814653 (  0) 0) |
| [34] |
Liu J, Long J, Shen Z, et al. A self-healing flexible quasi-solid zinc-ion battery using all-in-one electrodes. Advanced Science, 2021, 8(8): 2004689. DOI:10.1002/advs.202004689 (  0) 0) |
| [35] |
Jin X T, Song L, Dai C L, et al. A self-healing zinc ion battery under -20° C. Energy Storage Materials, 2022, 44: 517-526. DOI:10.1016/j.ensm.2021.11.004 (  0) 0) |
| [36] |
Paolella A, Faure C, Bertini G, et al. Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nature Communications, 2017, 8(1): 1-10. DOI:10.1038/ncomms14643 (  0) 0) |
| [37] |
Wang Z F, Ruan Z H, Ng W, et al. Integrating a triboelectric nanogenerator and a zinc-ion battery on a designed flexible 3D spacer fabric. Small Methods, 2018, 2(10): 1800150. DOI:10.1002/smtd.201800150 (  0) 0) |
| [38] |
Kim Y-S, Xie Y N, Wen X N, et al. Highly porous piezoelectric PVDF membrane as effective lithium ion transfer channels for enhanced self-charging power cell. Nano Energy, 2015, 14: 77-86. DOI:10.1016/j.nanoen.2015.01.006 (  0) 0) |
| [39] |
Yang Y, Lee S W, Ghasemi H, et al. Charging-free electrochemical system for harvesting low-grade thermal energy. Proceedings of the National Academy of Sciences, 2014, 111(48): 17011-17016. DOI:10.1073/pnas.1415097111 (  0) 0) |
| [40] |
Zhao J Q, Xu Z J, Zhou Z, et al. A safe flexible self-powered wristband system by integrating defective MnO2-x nanosheet-based zinc-ion batteries with perovskite solar cells. ACS Nano, 2021, 15(6): 10597-10608. DOI:10.1021/acsnano.1c03341 (  0) 0) |
| [41] |
Liu C Z, Xu W W, Mei C T, et al. A chemically self-charging flexible solid-state zinc-ion battery based on VO2 cathode and polyacrylamide-chitin nanofiber hydrogel electrolyte. Advanced Energy Materials, 2021, 11(25): 2003902. DOI:10.1002/aenm.202003902 (  0) 0) |
| [42] |
Liao M, Wang J W, Ye L, et al. A high-capacity aqueous zinc-ion battery fiber with air-recharging capability. Journal of Materials Chemistry A, 2021, 9(11): 6811-6818. DOI:10.1039/D1TA00803J (  0) 0) |
| [43] |
Lu X F, Zhang S L, Shangguan E, et al. Nitrogen-doped cobalt pyrite yolk-shell hollow spheres for long-life rechargeable Zn-air batteries. Advanced Science, 2020, 7(22): 2001178. DOI:10.1002/advs.202001178 (  0) 0) |
| [44] |
Luo Z L, Wang Y, Kou B D, et al. "Sweat-chargeable" on-skin supercapacitors for practical wearable energy applications. Energy Storage Materials, 2021, 38: 9-16. DOI:10.1016/j.ensm.2021.02.046 (  0) 0) |
| [45] |
Zhang C J, Huang K. A comprehensive review on the development of solid-state metal-air batteries operated on oxide-ion chemistry. Advanced Energy Materials, 2021, 11(2): 2000630. DOI:10.1002/aenm.202000630 (  0) 0) |
| [46] |
Lu X F, Fang Y J, Luan D Y, et al. Metal-organic frameworks derived functional materials for electrochemical energy storage and conversion: a mini review. Nano Letters, 2021, 21(4): 1555-1565. DOI:10.1021/acs.nanolett.0c04898 (  0) 0) |
| [47] |
Zhang Y, Wan F, Huang S, et al. A chemically self- charging aqueous zinc-ion battery. Nature Communications, 2020, 11(1): 1-10. DOI:10.1038/s41467-020-16039-5 (  0) 0) |
| [48] |
Qiu W D, Lin Z C, Xiao H B, et al. Construction of chemical self-charging zinc ion batteries based on defect coupled nitrogen modulation of zinc manganite vertical graphene arrays. Materials Advances, 2021, 2(20): 6694-6702. DOI:10.1039/D1MA00727K (  0) 0) |
| [49] |
Kim C, Ahn B Y, Wei T-S, et al. High-power aqueous zinc-ion batteries for customized electronic devices. ACS Nano, 2018, 12(12): 11838-11846. DOI:10.1021/acsnano.8b02744 (  0) 0) |
| [50] |
Bi J X, Zhang J, Giannakou P, et al. A Highly integrated flexible photo-rechargeable system based on stable ultrahigh-rate quasi-solid-state zinc-ion micro-batteries and perovskite solar cells. Energy Storage Materials, 2022, 51: 239-248. DOI:10.1016/j.ensm.2022.06.043 (  0) 0) |
| [51] |
Li Y P, Guan Q, Cheng J L, et al. Amorphous H0.82MoO3.26 cathodes based long cyclelife fiber-shaped Zn-ion battery for wearable sensors. Energy Storage Materials, 2022, 49: 227-235. DOI:10.1016/j.ensm.2022.04.001 (  0) 0) |
| [52] |
Ni J F, Li L. Self-supported 3D array electrodes for sodium microbatteries. Advanced Functional Materials, 2018, 28(3): 1704880. DOI:10.1002/adfm.201704880 (  0) 0) |
| [53] |
Ni J F, Dai A, Yuan Y F, et al. Three-dimensional microbatteries beyond lithium ion. Matter, 2020, 2(6): 1366-1376. DOI:10.1016/j.matt.2020.04.020 (  0) 0) |
| [54] |
Zhu S, Sheng J, Ni J F, et al. 3D vertical arrays of nanomaterials for microscaled energy storage devices. Accounts of Materials Research, 2021, 2(12): 1215-1226. DOI:10.1021/accountsmr.1c00175 (  0) 0) |
| [55] |
Lai W, Wang Y, Lei Z W, et al. High performance, environmentally benign and integratable Zn//MnO2 microbatteries. Journal of Materials Chemistry A, 2018, 6(9): 3933-3940. DOI:10.1039/C7TA10936A (  0) 0) |
| [56] |
Sun G Q, Jin X T, Yang H S, et al. An aqueous Zn-MnO2 rechargeable microbattery. Journal of Materials Chemistry A, 2018, 6(23): 10926-10931. DOI:10.1039/C8TA02747A (  0) 0) |
| [57] |
Sun G Q, Yang H S, Zhang G F, et al. A capacity recoverable zinc-ion micro-supercapacitor. Energy Environmental Science, 2018, 11(12): 3367-3374. DOI:10.1039/C8EE02567C (  0) 0) |
| [58] |
Jiang K, Zhou Z Y, Wen X, et al. Fabrications of high-performance planar zinc-ion microbatteries by engraved soft templates. Small, 2021, 17(12): 2007389. DOI:10.1002/smll.202007389 (  0) 0) |
| [59] |
Chen M H, Xie S A, Zhao X Y, et al. Aqueous zinc-ion batteries at extreme temperature: Mechanisms, challenges, and strategies. Energy Storage Materials, 2022, 51: 683-718. DOI:10.1016/j.ensm.2022.06.052 (  0) 0) |
| [60] |
Zeng Z Q, Jiang X Y, Li R, et al. A safer sodium-ion battery based on nonflammable organic phosphate electrolyte. Advanced Science, 2016, 3(9): 1600066. DOI:10.1002/advs.201600066 (  0) 0) |
| [61] |
Han M, Zhi J, Hoang T K A, et al. Artificial solid electrolyte interphase for thermally stable rechargeable aqueous zinc batteries. Journal of Power Sources, 2019, 441: 227171. DOI:10.1016/j.jpowsour.2019.227171 (  0) 0) |
| [62] |
Reber D, Kuehnel R-S, Battaglia C. Suppressing crystallization of water-in-salt electrolytes by asymmetric anions enables low-temperature operation of high-voltage aqueous batteries. ACS Materials Letters, 2019, 1(1): 44-51. DOI:10.1021/acsmaterialslett.9b00043 (  0) 0) |
| [63] |
Zhang Q, Ma Y L, Lu Y, et al. Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nature Communications, 2020, 11(1): 1-10. DOI:10.1038/s41467-020-18284-0 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


