Carbon materials such as fullerenes (0D), carbon nanotubes (1D), graphene (2D) and diamond (3D), have attracted great attention owing to their unique properties of large specific surface area, high richness, stable chemical and mechanical performance[1-5]. Additionally, carbon materials also exist in forms of carbon spheres, carbon fibers, fibrous membranes, whiskers and graphite polyhedral crystals[6]. Due to their diverse structures and unique properties, these carbon materials have been widely used in various fields like pharmaceutical engineering, electronics, energy storage and environmental remediation[7]. Numerous methods have been developed to prepare carbon composites for optimizing their structures and properties, such as chemical vapor deposition (CVD) and arc discharge technique. Carbonaceous gas (e.g., CH4, C2H2, CO) is usually acted as carbon source during CVD, while the arc discharge technique generally employs graphite electrode as the carbon resource in the presence of metal catalyst.
Carbon materials can also be derived from carbonizable precursors with abundant resources. For example, biomass compounds such as lignin, folic acid and cellulose, are commonly used as sustainable sources to produce carbon materials[8-9]. These renewable resources normally exist in fruits and vegetables with advantages of environmental protection, low cost, a wide range of sources and abundant surface groups[10]. In addition, coordination complexes like metal-organic frameworks (MOFs), covalent organic framework (COFs) and infinite coordination polymers (ICPs), are also employed to make porous carbon materials due to the features of high specific surface area, tailorable structures and large amounts of organic struts[11-14]. Polymeric compounds are another big type of resources for carbon materials, such as polyimide, phenol formaldehyde, resin and polyacrylonitrile[15-18]. Among these precursors, polyacrylonitrile (PAN) has been considered as one of the most effective precursors, because of its high carbon content, low cost, perfect mechanical properties and superior stability[19]. Compared with the coordination complexes (e.g., MOFs, COFs, ICPs) and other polymers, PAN as a carbon precursor has its prominent advantages: 1) multiple applicable polymerization methods; 2) easy processing and lower cost; 3) variable structure and higher carbon content; 4) good mechanical properties and superior stability. Hence, even compared with graphene and carbon nanotube, the obtained carbon materials may exist in a variety of forms and are used in a diverse range of applications. In general, PAN-based precursors would be changed into carbon materials through the oxidative stabilization and carbonization process. Therefore, it is a remarkable fact that the structure and morphology of PAN precursors would have a great effect on the properties of carbon materials. Consequently, a number of processing methods have been developed for producing PAN-based precursors with variable structures and compositions[20-22].
In this review, the polymerization methods of PAN is briefly introduced and the carbonization procedure of PAN is described. As shown in Scheme 1, the review mainly focuses on the discussion of different PAN processing methods. Additionally, diverse applications of PAN-derived carbon are reported. Finally, future prospects of PAN processing and the preparation of carbon materials are provided. The innovative aspects of this review includ but are not limited to: 1) advancement of various PAN processing methods; 2) specific processing mechanism of PAN and derived carbon; 3) linkage between the processing, structure and application; 4) recent advances in the applications of PAN derived carbon materials.
|
Scheme 1 Processing and applications of PAN derived carbon materials |
1 PAN and Derived Carbon
PAN is a commercially important polymer because of its unique properties including high physical, chemical resistance and stability[23-24]. The molecular structure of PAN displays that the macromolecular chain consists of acrylonitrile units in a head-to-tail connection manner. The strong dipole-dipole interaction between nitrile groups limits the bond rotation to form stiffer chains, which may endow PAN with higher crystalline, melting point and superior mechanical functions[25]. Meanwhile, PAN can only dissolve in polar organic solvents, such as N, N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and dimethylacetamide (DMAA).
Acrylonitrile (AN) is a reactive vinyl monomer and can be polymerized under different conditions. Free radical polymerization (FRP) is usually employed to synthesize PAN[26-28]. PAN with controlled molecular dimension and structure can be prepared by anionic polymerization using organometallic initiators[29-30]. For example, Siemeling et al. [31] synthesized PAN with high-molecular weight through the anionic polymerization using di(organoimido)- chromium(VI) complexes as catalysts. Reversible deactivation radical polymerization (RDRP) methods, such as atom transfer radical polymerization (ATRP), reversible addition fragmentation chain-transfer polymerization (RAFT) and nitroxide-mediated polymerization (NMP), have also been used to synthesize functional PAN[32-34]. These polymerization methods depend on a fast dynamic equilibrium between dormant chain ends and active radical species for controlling the reaction. Specifically, ATRP uses transition metal (Cu, Ru, Fe, etc.) complexes as catalysts to reverse transferring halogen atoms from growing chains, while RAFT uses dithioesters as chain transfer agents and NMP uses nitroxides as radical catchers. ATRP has the advantages of mild polymerization conditions, excellent molecular design ability, relative molecular weight controllability and low dispersion coefficient. RAFT can be used to prepare pure PAN without metal residues and NMP is commonly used for PAN block copolymer production. For instance, Hou et al. [35] prepared PAN with narrow polydispersity via reverse ATRP catalyzed by FeCl3/Isophthalic acid. Additionally, random or block copolymerization of acrylonitrile with other monomers, like styrene, ethyl methacrylate, maleic anhydride and acrylamide, have been successfully synthesized to enhance the chemical and mechanical properties[36-40]. The previous publication has reviewed the polymerization methods of PAN polymers in detail[41].
Two-step thermal treatment is employed to produce carbon materials from PAN-based precursors (as shown in Fig. 1): 1) oxidative stabilization process under moderate temperature (200-300 ℃); 2) following carbonization/graphitization process at high temperature and inert atmosphere[42]. During the oxidative stabilization, cyclization of nitrile groups and dehydrogenation of PAN occur to form ladder-like polymer structure. The existence of oxygen atmosphere promotes the dehydrogenation of PAN to maintain the highly oriented structures for subsequent carbonizations. The following carbonization treatment is then carried out under inert atmosphere to remove non-carbon components through forms of various gases. At the same time, further dehydrogenation and denitrogenation take place to form the final carbonized network structure. PAN-based precursors with different morphologies and compositions can be formed by using different processing methods. As a result, it may further influence the structures and properties of the resulting carbon materials. Moreover, the introduction of different substances during PAN processing may lead to the derived carbon nanomaterials with the development of porosity and the generation of surface functional groups.

|
Fig.1 Two-step thermal processing of PAN into carbon: oxidative stabilization and formation of a ladder polymer (left); carbonization/graphitization (right)[42]. (Reprinted with permission from Ref. [42]. Copyright 2012, John Wiley and Sons) |
2 PAN Processing and Derived Carbon Materials 2.1 Infiltration
The infiltration method is usually employed to prepare three-dimensional (3D) polymer/silica composites, in which polymer and silica opal are used as carbon source and hard template, respectively. Firstly, the polymer solution is filled into the silica opal template. Then, the assembly of polymer and silica takes place after solvent evaporation[43]. For example, Yang et al. [44] synthesized N-doped 3D porous carbon after the carbonization of PAN-silica gel assembly. It was found that the porous structure would be different when the carbonization temperature is changed. Sree Manu and the co-workers successfully prepared self-lubricating bidirectional PAN-based carbon fiber reinforced smart aluminum composites by squeezing infiltration[45].
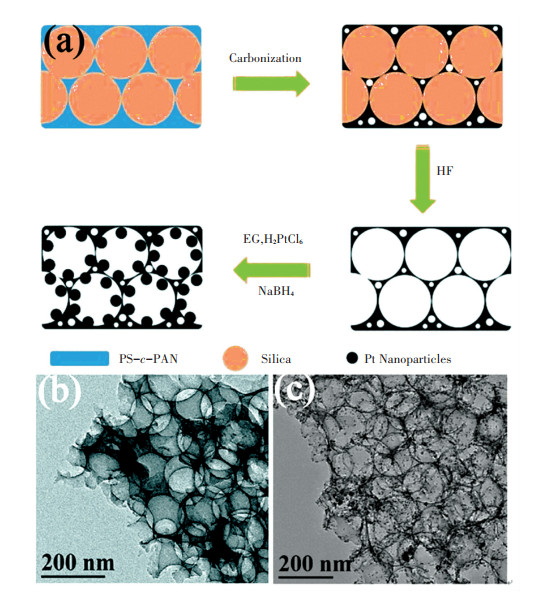
However, the low solubility and flexibility of PAN polymer may restrict the efficiency of PAN infiltration into the silica template. One efficient strategy is incorporating soft polymer blocks (e.g., styrene, butyl acrylate) into PAN rigid backbone[46-47]. For instance, Liu et al. [48] prepared PAN copolymer-silica assembly through the infiltration and converted it into 3D hierarchical porous carbon. As shown in Fig. 2(a), the solution dissolving polystyrene-co-polyacrylonitrile (PS-c -PAN) was dropped into the hard template silica, followed by the evaporation of solvent.The copolymer/silica assembly was finally acquired via multi-cycles of infiltration and evaporation. After carbonization, micropores and mesopores are obtained in carbon structure due to the decomposition of PS blocks. The TEM image in Fig. 2(b) shows that interconnected macropores were formed in the 3D carbon owing to the removal of silica template after HF etching. Pt NPs could be further uniformly deposited and distributed over the porous carbon (Fig. 2(c)).
|
Fig.2 (a) Formation process of 3D carbon and Pt/3D carbon through co-assembly of PAN copolymer and silica; (b)TEM images of 3D carbon; (c) Pt/3D carbon[48]. (Reprinted with permission from Ref. [48]. Copyright 2017, Royal Society of Chemistry) |
2.2 Flash Nano Precipitation (FNP)
FNP is a rapid self-assembly technique for producing polymeric nanoparticles (NPs) with adjustable size, narrow size distribution and stable morphological structure [49-51]. Typically, confined impinging jet (CIJ) or multi-inlet vortex mixer (MIVM) is used for rapidly mixing a good solvent stream dissolving polymer and an anti-solvent stream in the space-confined chamber during the FNP process[52-53]. The FNP technique features advantages of low energy consumption, simple device and high reproducibility[54]. During FNP, soft polymer blocks are usually incorporated into PAN backbone to enhance solubility and decrease stiffness[55]. Zhou et al. [56] synthesized pure polystyrene-co-polyacrylonitrile (PS-c-PAN) nanospheres through FNP. After amidoximation, the as-obtained NPs could be used for the adsorption of uranium.
Hydrophobic molecule can be encapsulated into PAN copolymer NPs via FNP. For example, Li et al.[57] employed FNP to prepare PS-c-PAN nanoparticles (NPs) entrapped hydrophobic organoferrous compound, (Ph3P)2Fe(CO)3, which was converted into Fe, P, N co-doped carbon materials. Fig. 3(a) represents the synthesis process of (Ph3P)2Fe(CO)3@PS-c-PAN NPs and Fe-P-N-doped carbon materials. In detail, one syringe containing THF solution of PS-c -PAN and (Ph3P)2Fe(CO)3 was placed at the inlet of stream 1, while the other syringe containing H2O was placed at the inlet of stream 2. The two fluids were subsequently injected into a CIJ mixing chamber and further diluted into a water reservoir to form a stable colloid solution. During the interdiffusion process, co-assembly of PS-c-PAN and hydrophobic active ingredient (Ph3P)2Fe(CO)3 successfully took place and small aggregates of (Ph3P)2Fe(CO)3 were encapsulated within the polymer shell. As shown in Figs. 3(b)-3(c), pure PS- c -PAN NPs was spherical in shape with a diameter of ~140 nm, while entrapment of (Ph3P)2Fe(CO)3 resulted in the increase of NPs size. After carbonization, Fe-based NPs were uniformly dispersed within carbon materials, in which the size of Fe-based NPs depended on the feed amount of (Ph3P)2Fe(CO)3 (as shown in Figs. 3(d)-3(e)). PS-c-PAN NPs entrapping hydrophobic Pt(acac)2 were produced by Liu and co-workers by FNP as well[58]. Also, the protection of the polymer shell could lead to the uniform distribution of Pt NPs in the carbon matrix after pyrolysis.

|
Fig.3 (a) Schematic illustration of FNP for (Ph3P)2Fe(CO)3@PS-c -PAN NPs and conversion into Fe-P-N-doped carbon materials; TEM images of (b) pure PS-c -PAN NPs; (c) (Ph3P)2Fe(CO)3@PS-c -PAN NPs; Fe/P/N carbons with different ((Ph3P)2Fe(CO)3 feeding amounts at (d) 5 mg and (e) 3 mg[57]. (Reprinted with permission from Ref. [57]. Copyright 2018, Springer Nature) |
In addition, metal-polyphenol complex could be entrapped within PSc-PAN NPs through FNP. For instance, Wang et al. [59] synthesized PS-c -PAN NP-entrapped hydrophobic tannic acid-iron (TA-Fe) complexes via FNP. During the mixing procedure, hydrophobic TA-Fe complexes were in-situ formed due to the fact that polyphenol ligand may connect with metal ions through its catechol group. At the same time, co-assembly of polymer and TA-Fe complexes happened, and small aggregates of TA-Fe complexes were encapsulated within the polymer matrix. The precursors were further converted into carbon nanomaterials with uniformly dispersed Fe-based NPs after carbonization.
2.3 ElectrospinningElectrospinning has emerged as a direct and continuous technique for preparing polymer nanofibers (NFs) and nanofibrous membranes with high porosity, large specific surface area and uniformity[60-63]. This method also features the advantages of low cost, simple equipment and high efficiency. The electrospinning device consists of three components: high-voltage supplier, syringe/capillary tube with needles and metal collector[64]. The requirements for the electrospinning process have been deeply investigated by: (a) selecting a suitable solvent necessary to dissolve the polymer precursors; (b) controlling the viscosity and surface tension of the precursor solutions to ensure the solutions fluidity; (c) the voltage of electrostatic field overcoming viscoelastic force and surface tension of precursor solutions to form continuous jets[65]. Electrospinning of PAN precursors followed by oxidative stabilization and carbonization has been widely employed as a straightforward and convenient method to produce carbon NFs[66-69].
In addition, porous structures can be formed via adding porogens into PAN precursors during electrospinning. For example, Tran et al. [70] produced PAN/Nafion composite NFs by electrospinning. After carbonization, porous carbon NFs were obtained after the removal of Nafion. Li et al.[71] obtained PAN@SiO2-Ag composite nanofibrous film via electrospinning, in which Ag NPS were deposited onto the surface of PAN@SiO2 nanofibers from wet electroless deposition. Liu et al.[72] prepared Co, N co-doped porous carbon NFs through electrospinning of PAN and Co-ZIF precursors followed by carbonization as well as acid leaching treatment. The electrospinning technique could also introduce various active ingredients into NFs[73]. For instance, Li et al. [74] synthesized Fe/Co-PAN/PVP composite NFs via electrospinning and the N-doped carbon NFs with distributed FeCo NPs were further obtained after pyrolysis(Fig. 4(a)). TEM image in Fig. 4(b) exhibits that Fe/Co-PAN/PVP NFs showed the nanofibrous structure with an average diameter of ~300 nm, while the obtained carbon NFs performed a little shrinkage in diameter after thermal treatment (Fig. 4(c)). As shown in Fig. 4(d), FeCo alloy NPs were homogeneously distributed in the resulting carbon NFs with mesoporous structure after the removal of PVP. Similarly, carbon NFs with dispersed FeCoNi alloy NPs were also produced by using FeCoNi-PAN/PVP NFs as carbonizable precursors[75].

|
Fig.4 (a) Schematic synthesis process of FeCo alloy nanoparticles decorated mesoporous Fe/Co-N-C nanofibers; (b) TEM images of FeCo-polymer nanofibers; (c) SEM and (d) TEM images of FeCo@MNC[74]. (Reprinted with permission from Ref. [74]. Copyright 2019, Elsevier) |
The incorporation of active ingredients and porogens into 3D carbon network can be achieved through the combination of electrospinning and other surface modification methods[76-79]. For example, Yang et al. [80] synthesized PAN/GO composite NFs via the electrospinning process. Then, the NFs were impregnated within the Fe3+ solution to realize the doping of Fe into the PAN/GO precursors. The connection between PAN and GO was formed via the active functional groups on the modified GO (e.g., epoxide, hydroxyl and carboxyl). Meanwhile, the NFs could adsorb more Feions owing to the addition of modified GO. After carbonization, N, Fe dual-doped carbon NFs were produced. Additionally, Gong et al. [81] produced N, F co-doped porous carbon NFs derived from PAN/PVDF/PVP tricomponent polymer NFs through successive electrospinning, hydrothermal and thermal treatment. The removal of PVP during the hydrothermal process resulted in the formation of pores on the fibers.
2.4 Solution Blow SpinningThe solution blow spinning technique is a simple, novel and efficient processing method for preparing polymer micro/nanofibers with large specific surface area and high porosity. Compared with electrospinning, solution blow spinning is free of high voltage and can blow the polymer precursor solution into fibrous structures by using a high-speed gas flow[82]. Additionally, this method is suitable for almost all kinds of polymers, particularly for PAN, which is unable to be molten[83]. de Gonzaga et al.[84] produced PAN and lignin derived carbon nanofibers by solution blow spinning, in which more lignin containing fibers showed lower thermal stability and smaller diameters. Rheological behavior in solution blow spinning was studied by Lu[85] and gel-like solution spinning has been applied in the fabrication of PAN and PMMA-b-PAN fibers[86].
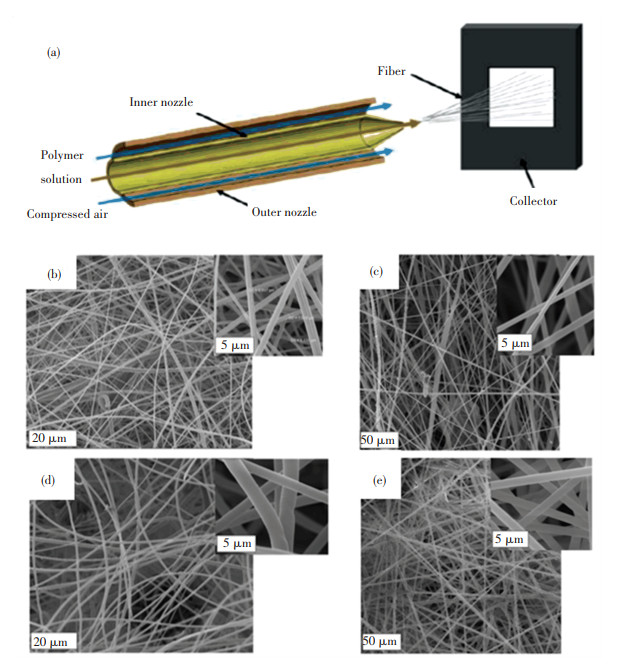
Moreover, core-shell fibrous PAN structure can be achieved through solution blow spinning. For example, Ergün et al. [87] synthesized metallophthalocyanine/PAN nanofibers via solution blow spinning (Fig. 5(a)). Figs. 5(c)-5(e) shows the SEM image of composite fibers, in which the smooth texture of fibers could be observed. As a result, spun PAN fibers coating with nanostructures of CoPc and ZnPc had been successfully produced. Chen et al. [88] prepared composite SiC@SiO2/carbon NFs through the solution blow spinning of SiO2/PAN precursors and calcination. The blow-spun SiO2/PAN hybrid NFs with uniformly dispersed SiO2 NPs possessed a uniform structure with an average diameter of ~368 nm. A core-shell structure was formed after calcination, in which the shell was mainly composed of amorphous SiO2.
|
Fig.5 (a) Schematic drawing of the setup for solution blow spinning and SEM images of composites, (b) pristine PAN, (c) ZnPc/PAN, (d) CoPc/PAN, (e) ZnPc-TiO2/PAN[87]. (Reprinted with permission from Ref. [87]. Copyright 2020, John Wiley and Sons) |
2.5 Centrifugal Spinning
In recent years, centrifugal spinning has been considered as an innovate and simple method to produce polymer micro/nanofibers. The centrifugal spinning system is composed of spinneret, rod collectors, DC motor, speed controller and fiber collectors[89]. During the process of centrifugal spinning, polymer NFs are extruded from polymer solutions via a high-rate rotary and perforated spinneret. With free of high-voltage electric field, less solvent and high rotational speed, the centrifugal spinning technique can improve the security and production rate as well as reduce the production cost[90].
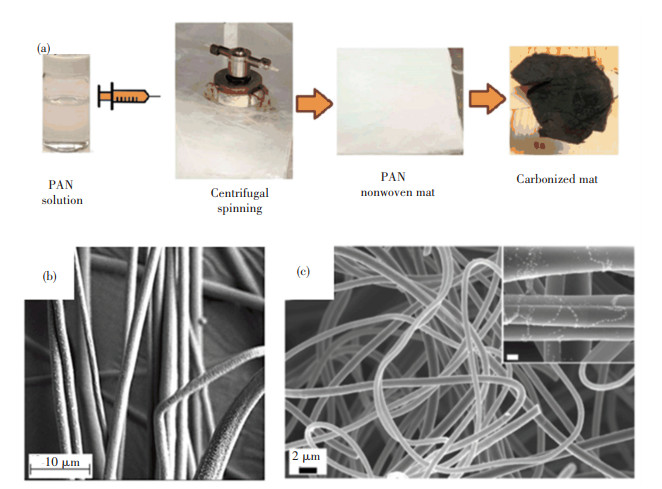
Carbon NFs with porous structure can be successfully obtained by incorporating additional substances into PAN precursors via the centrifugal spinning process and thermal treatment[91]. For example, Akia et al. [92] employed centrifugal spinning technique to prepare PAN-based carbon fibers with small diameter. As shown in Fig. 6(a), PAN solution containing sodium chloride was put into the spinning vessel and further injected from the holes on the vessel wall at a high rotational speed to form NFs after the evaporation of solvent. The SEM image in Fig. 6(b) obviously shows the existence of pores over the PAN fibers. Fig. 6(c) further proves the formation of porous structure in the resulting carbon fibers. Xu et al. [93] also fabricated hollow carbon microfibers via the oxidative stabilization and carbonization of PAN/PVP microfibers. The PAN/PVP microfibers were obtained through coaxial centrifugal spinning, in which PAN solution was placed in the outer spinneret while PVP solution was put into the inner spinneret. Then, the hollow structured fibers were received as a result of PVP elimination through ultrasonic treatment.
|
Fig.6 (a) Schematic of the preparation of PAN-based carbon fiber via centrifugal spinning; SEM micrographs of (b) PAN with 5 wt% of NaCl based fibers, (c) carbonized fibers[92]. (Reprinted with permission from Ref. [92]. Copyright 2018, John Wiley and Sons) |
2.6 Dry-wet Spinning
Dry-wet spinning combines the characteristics of dry spinning and wet spinning. During the process of dry-wet spinning, the dope is extruded through an air gap and further coagulation is carried out by a conventional method like wet spinning[94]. The viscosity of spinning precursors plays an important role in spinnability. Commonly, a relatively low viscosity may result in the dope fracture, while a large viscosity may cut down the spinning rate and coagulation speed. PAN fibers obtained from dry-wet spinning usually possess uniform structure, high elasticity, less surface wrinkles and optimized orientation, all of which result in the improvement of fiber's mechanical property. For example, Xu et al. [95] proposed a fabrication method that combines dry-jet-wet spinning and forced assembly for hierarchically structured composite fibers consisting of carbon nanotube (CNT)/PAN alternating layers. The mechanical properties of the fiber gradually increased with increasing layer numbers.
In addition, the using of PAN copolymers with comparatively high solubility endows the obtained fibers with more extensibility and makes it less prone to fibrillation[96]. For example, Sun et al. [97] synthesized hollow fibers via dry-wet spinning by using copolymers of acrylonitrile, methyl methacrylate and itaconic acid as spinning precursors (Fig. 7(a)). The obtained fibers were further oxidized, carbonized as well as CO2-activated to obtain hollow carbon fibers with high specific surface area. The surface area and pore size distribution of hollow carbon fibers could be varied by changing the carbonization temperature(Figs. 7(b)-7(d)). In addition, the dry-wet spinning technique can be used to obtain PAN-based fibrous membranes as well. For instance, Saufi et al. [98] prepared PAN hollow fiber membranes using dry-wet spinning by the simultaneous extrusion of spinning solution and bore fluids. The structures and properties of derived carbon membranes can be changed with the variation of pyrolysis temperature.

|
Fig.7 (a) The cross section of virgin PAN hollow fiber; SEM micrographs of the cross sections of carbon fibers carbonized at (b) 500 ℃; (c) 700 ℃; (d) 900 ℃[97]. (Reprinted with permission from Ref. [97]. Copyright 2005, John Wiley and Sons) |
3 Applications 3.1 Structural Materials
Due to the advantages of high strength-to-weight ratio and outstanding stiffness, carbon fibers have been widely employed as high performance structural materials in aerospace, defense, and other industries[99]. The principle to improve the elastic modulus and strength of carbon fibers is to use the characteristics that they can always shrink during the process of thermal treatment[100]. PAN is a favorable precursor because of its superior mechanical property and high carbon yield. The incorporation of PAN copolymer can result in additional beneficial effects: 1) partially obstructing the nitrile/nitrile interactions, 2) endowing the copolymer with more readily soluble in solvents, 3) allowing better macromolecular orientation in the precursor fibers, 4) making the carbon fibers more structurally homogeneous. Moreover, previous research has proved that the mechanical strength of carbon fibers would increase as the diameter of precursor fibers decreases, which means that a smaller diameter allows a higher fraction of the theoretical strength[101].
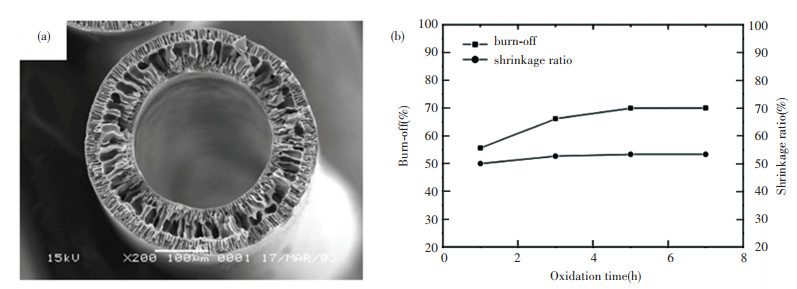
Liu et al.[102] investigated the structural conversion during the oxidative stabilization of electrospun PAN NFs and compared with those of conventional PAN fibers made from wet spinning process. The comparison shows that electrospun PAN NFs could be used to develop continuous carbon fibers with superior mechanical strength. Moreover, aligned fiber bundles outperform either single fiber or nonwoven fiber mat from the point of mechanical property[103-104]. Sun et al.[105] prepared PAN derived carbon hollow fibers (PAN-ACHF) through combination processing of dry-wet spinning, carbonization at 900 ℃ in nitrogen and activation by carbon dioxide. The SEM image in Fig. 8(a) shows the porous structure of the PAN hollow fiber. The burn-off and the shrinkage ratio of PAN-ACHF is shown in Fig. 8(b), in which the burn-off has slowly increased while shrinkage has not clear change with oxidation time increasing.
|
Fig.8 (a) SEM image for the cross section of virgin PAN hollow fiber; (b) Burn-off and shrinkage ratio of PAN-ACHF versus oxidation time[105]. (Reprinted with permission from Ref. [105]. Copyright 2007, John Wiley and Sons) |
3.2 Electrocatalysis
Oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) have been playing important roles in the charge and discharge processes for fuel cells and metal-air batteries[106-107]. It is urgent to develop efficient electrocatalysts to accelerate the slow kinetics taking place at the cathodes[108-109]. N-doped carbon materials have been widely employed as electrocatalysts because the introduction of N can increase the electronic conductivity of carbon materials to further improve the electrochemical performance[110-111]. PAN is a commonly used precursor for N-doped carbon materials. In addition, metals (e.g. Fe, Ni, Co) and heteroatoms (e.g. P, B, S) could be co-doped into the N-doped carbon materials to introduce more electrochemical active sites[112-113]. Li et al. [57] prepared Fe-P-N-doped carbon materials with large specific surface area through the combination of FNP and thermal treatment. The as-obtained carbon catalyst showed efficient electrocatalytic activity for ORR via a four-electron process. Importantly, the voids caused by the decomposition of PAN blends made the active sites more accessible to further enhance the ORR property. Moreover, PAN derived carbon has been also considered as a sustainable electrocatalyst for hydrogen evolution reaction (HER)[114-116].
It is worth noting that carbon NFs with large strength-to-weight ratio perform superior electrical conductivity, which may accelerate O2 and electrolyte diffusion for electrochemical processes[117]. For instance, Shang et al. [118] produced N-doped carbon NFs encapsulating Co NPs via the carbonization of electrospun PAN/Co (Ⅲ) precursors. As shown in Fig. 9(a), the as-obtained Co-carbon NFs maintained the weblike structure and were beneficial for the mass transport and access of reactant species. TEM image of Co-carbon NFs in Fig. 9(b) exhibits that Co NPs were uniformly distributed over the carbon NFs and pores were formed owing to the pyrolysis of organics, all of which resulted in the outstanding electrocatalytic performance (Figs. 9(c)-9(d)). Li et al. [119] fabricated N-doped hierarchical porous carbon NFs with distributed Fe3C/Fe NPs through the immersions and carbonization of electrospun PAN and ZIF-8 composite NFs. The co-doping of N and Fe could supply numerous active sites. In addition, the hierarchical porous structure derived from the removal of ZIF-8 NPs could endow the active sites more accessibility. Hence, the resulting Fe/N co-doped carbon NFs performed great catalytic activities for both ORR and OER.

|
Fig.9 (a) Typical FESEM image and (b) TEM image of Co-carbon NFs; (c) LSV curves and (d) K-L plots of Co-carbon NFs[118]. (Reprinted with permission from Ref. [118]. Copyright 2016, John Wiley and Sons) |
3.3 Supercapacitors
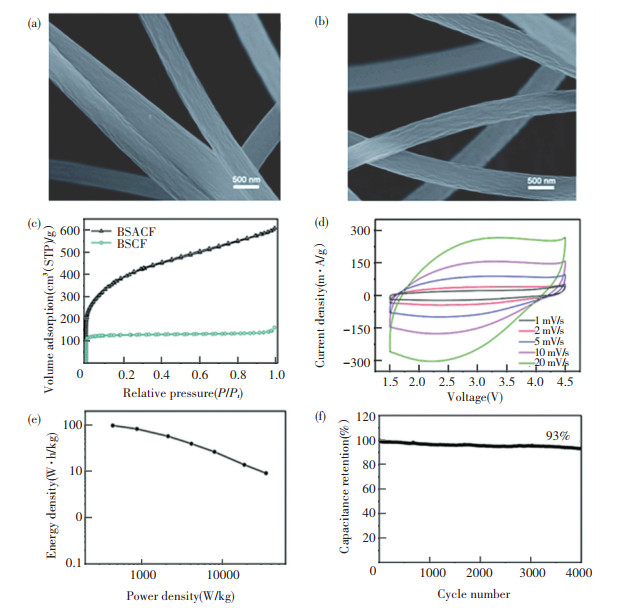
Generally, supercapacitors are composed of electrode materials, electrolyte, diaphragm and collector, in which electrode materials play the most important role[120-123]. N-doped porous carbon materials have been widely employed as electrode materials and the introduction of N heteroatom can dramatically improve the capacitance while retaining the great intrinsic properties of carbons[124-125]. PAN is a widely used precursor due to its great spinnability in solution, high nitrogen content and carbon yield[126-129]. For example, Zhan et al. [130] prepared carbon fibers (BSCFs) via the direct carbonization of solution blow spun PAN fibers and activated carbon fibers (BSACFs) through additional ammonia activation. The shrinkage in fiber diameter took place due to the evolution of gases during the process of carbonization and ammonia activation (Figs. 10(a)-10(b)). The N2 adsorption/desorption isotherms show that microporous structure was formed in BSCFs while both micropores and mesopores were observed in BSACFs (Fig. 10(c)). The flexible hybrid supercapacitors (FHSC), which was composed with BSACFs and BSCFs, showed superior energy density, power density and cycle stability due to the abundant N content and porous structure (Figs. 10(d)-10(f)). Lota et al. [131] investigated the effect of N content on the capacitance performance of carbon materials derived from PAN and its blends with coal-tar pitch. The result showed proportional correlation between N content and capacitance values in the acidic electrolytic solution. The reason may be that the introduction of N-doped pseudocapacitance can improve polarity and accessibility of carbon surface.
|
Fig.10 SEM images of (a) carbon fibers and (b) activated carbon fibers; (c) N2 adsorption/desorption isotherms of carbon fibers and activated carbon fibers; (d) CV curves of FHSC at different sweep rates from 1 mV/s to 20 mV/s; (e) Ragone plots of FHSC; (f) Cycle performance of FHSC[130]. (Reproduced from an open access article, Ref.[130]) |
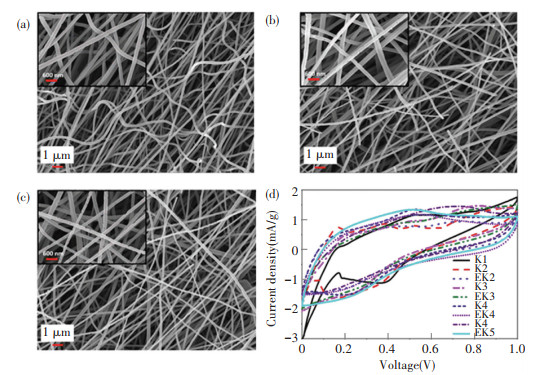
In addition, the morphology of N-doped carbon materials could be varied via incorporating sacrificial substances into the PAN precursor, such as Nafion, tetraethoxy orthosilicate (TEOS) and polymethylmethacrylate (PMMA)[132-134]. Altin et al.[135] prepared carbon NFs through the electrospinning of PAN and polyvinyl alcohol (PVA) followed by carbonization. The morphologies of porous carbon nanofibers varied from different contents of PVA (Figs. 11(a)-11(c)). Fig. 11(d) shows cyclic voltammograms measurements of the series of carbon NFs and exhibits that the addition of PVA could be beneficial for the specific capacitance of supercapacitors due to the interconnected structure.
|
Fig.11 SEM images of PAN/PVA carbonized nanofibers with different contents of PVA: (a) 0; (b) 5%; (c) 10%; (d) CV curves of different porous carbon nanofibers at 5 mV/s[135]. (Reprinted with permission from Ref. [135]. Copyright 2021, John Wiley and Sons) |
3.4 Batteries
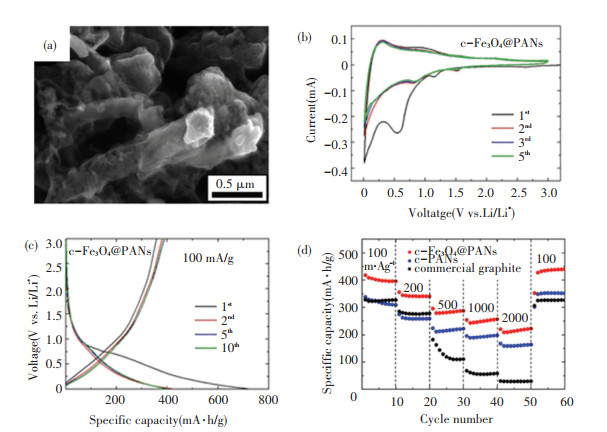
Among the energy storage and conversion devices, high performance lithium-ion batteries (LIBs) have been considered as one of the most promising technologies owing to their advantages of high energy density, long cycle performance and elastic design[136]. Generally, the performance of LIBs relies dominantly on the activities of their anode materials[137]. PAN derived N-doped carbon materials have emerged as efficient anode materials for LIBs due to the features of large specific surface area, high reversible capacity and stable cycle properties[138]. For example, Kwon et al. [139] produced magnetite (Fe3O4) -incorporated PAN derived carbon composites as LIBs anodes (Fig. 12(a)). The presence of Fe3O4 NPs improved the graphitization efficiency of PAN. As a result, both capacity and cycling stability of anode materials for LIBs have been enhanced (Figs. 12(b)-12(d)). In addition, incorporating other components into PAN precursors could obtain porous structures[140]. For instance, LIBs performances of porous carbon NFs derived from PAN/PLLA NFs and nonporous carbon NFs derived from pure PAN NFs were compared by Ji et al[141]. The contrast results certificated that the porous carbon NFs made the transport distances of electrons and lithium ions shorter and the electrode-electrolyte contact areas larger, thereby performing a greater cycling stability and a higher reversible capacity than nonporous carbon NFs.
|
Fig.12 (a) SEM image of c-Fe3O4@PANs; (b) CV profiles of c-Fe3O4@PANs at a scan rate of 0.1 mV/s; (c) Voltage profiles of c-Fe3O4@PANs at a current density of 100 mA/g; (d) Rate properties of carbon electrodes at various current densities[139]. (Reprinted with permission from Ref. [139]. Copyright 2022, American Chemical Society) |
Moreover, adding metal species into N-doped carbon materials can introduce more active sites to enhance the electronic conductivity[142]. For instance, Jiang et al. [143] employed SnCl2/PAN as a centrifugal spinning precursor to obtain tin-containing carbon NFs via a two-step thermal treatment. SnCl2/PAN NFs possessed smooth surface and their diameters increased with the increment of SnCl2 content. After carbonization, wrinkles and grooves could be observed on the surface of Sn/carbon composite NFs as well as the occurrence of diameter shrinkage. The fibrous structure was beneficial for the accommodation of Sn particles volume expansions during the process of lithiation and delithiation. The resulting Sn/carbon NFs performed superior electrochemical performance owing to the synergistic effect of carbon NFs and Sn NPs.
Furthermore, PAN derived carbon materials can also be used in other batteries, such as sodium-ion batteries(SIBs), solid-state lithium batteries (SSLBs) and lithium-sulfur batteries (LSBs)[144-147]. For example, Zhu et al. [148] prepared sulfur/carbonized PAN composite via the carbonization of PAN with sulfur, which was employed as a cathode in all-solid-state sodium-sulfur battery (Fig. 13(a)). CV curves in Fig. 13(b) exhibit two continuous steps of the S/CPAN reduction. The battery with S/CPAN composite cathode and PEO electrolyte showed the second discharge capacity of 252 mA·h/g (713 mA· h/gsulfur) and an appreciable capacity profile during the cycling process (Figs. 13(c)-13(d)).

|
Fig.13 (a) SEM images of S/CPAN composites; (b) CV profiles of all-solid-state Na-S battery; (c) The galvanostatic discharge-charge (GDC) voltage profiles of all-solid-state Na-S battery at 0.1 A/g; (d) Cycling performance of all-solid-state Na-S battery at a constant current density of 0.1 A/g[148]. (Reprinted with permission from Ref.[148]. Copyright 2019, American Chemical Society) |
3.5 Adsorption and Separation
Adsorption has been considered as one of the most efficient techniques for pollutant removal because of its advantages of low cost, simple operation, high efficiency and recyclability[149-152]. Porous carbon with large specific surface area and high nitrogen content can result in more adsorption sites, which are beneficial for adsorption capability[153]. For instance, Wang et al. [154] prepared a series of carbon NFs derived from the electrospinning ZIF-8 with PAN. The hierarchical porous structure was obtained by the decomposition of ZIF-8, leading to the efficient adsorption properties for both small-sized dye molecule MB (methylene blue) and large dye molecule CR (congo red) (Figs. 14(a)-14(b)). Besides, Ni ions were introduced into the carbonized precursors to obtain carbon NFs with dispersed Ni NPs, which could be removed easily via a magnet (Figs. 14(c)-14(d)). Tao et al. [155]produced activated carbon NFs with large specific surface area through the solution blow spinning of PAN precursor, followed by KOH activation and carbonization. It was found that the surface area and pore volume of activated carbon NFs could be adjusted by changing the activation conditions. The obtained activated carbon NFs performed a high adsorption capacity for phenol.

|
Fig.14 Adsorption isotherm curves of different solutions using PCNFs as absorbent: (a) MB; (b) CR; Illustration of different solutions color change using PCNFs-Ni as absorbent: (c) MB; (d) CR[154]. (Reprinted with permission from Ref. [154]. Copyright 2020, Elsevier) |
In addition, membrane separation has also been regarded as an efficient approach for environmental remediation. Specially, PAN derived carbon nanofibrous membranes are widely applied for separation due to high chemical resistance, thermal stability, large surface area and high inter-fiber porosity. Higher degree of porosity can lead to smaller driving force to make separation more energy efficient. For example, Tai et al. [156] prepared SiO2-carbon composite nanofibrous membranes via the electrospinning of PAN and TEOS precursors followed by the pyrolysis process. The quality test of oil-water separation exhibited that the obtained carbon membranes possessed outstanding separation performance of oil and water. Liu et al. [157] fabricated macroporous carbon membranes with superhydrophobicity and superlipophilicity by the carbonization of electrospun PAN/terephthalic acid NFs, which demonstrated excellent separation capacity in oil-water separation. Kim et al.[158] designed a carbon molecular sieve membrane derived from PAN to achieve efficient ethylene/ethane separation, which showed a high separation efficiency compared with other polymer derived membranes.
4 Conclusions and OutlookPAN is a well-known polymer with great stability, mechanical and chemical properties. Owing to its high carbon yield and flexibility, PAN has been widely used as an efficient carbonizable precursor. The properties of resulting carbon materials highly rely on the PAN-based precursors with diverse structures and compositions. By using specific processing methods, PAN-based materials with various morphologies can be formed. In addition, incorporating different substances into PAN during different processing technologies may endow derived carbon materials with additional functionality. As a result, the obtained carbon composite materials have been used in a variety of fields, such as structural materials, electrocatalysis, supercapacitors, batteries, adsorption and separation.
Although the advancements of PAN processing and derived carbon materials have been improved markedly in the past years, more challenges still need to be solved. Table 1 summarizes the PAN processing methods with their advantages, challenges and applications of derived carbon. The structures and properties of PAN and derived carbon materials are different dependent on the different processing methods. Currently, electrospinning is the most popular method to prepare high-performance PAN and derived fibers due to its simplicity and efficiency. The future development of PAN processing is mainly to combine multiple technologies and controlling factors, thereby eliminating the shortcomings of single method. On the other hand, the strong dipoles interaction of PAN restricts most of PAN precursors into fibrous structures. Self-assembly is a promising processing method to prepare PAN composite materials with particle morphology. For example, PAN polymeric NPs with uniform sizes have been prepared through FNP and functional cores could be simultaneously entrapped into the polymer shell. Consequently, the development of self-assembly is crucial for the preparation of PAN NPs beyond fibrous morphology to extend their applications.
| Table 1 Summary of PAN processing methods with their advantages, challenges and applications for PAN derived carbon |
Additionally, the selection of precursors also limits the development of PAN processing. It is noteworthy that the incorporation of soft polymer blocks (e.g., styrene, butyl acrylate) into PAN backbone could decrease its stiffness and improve its solubility. The soft polymer block may also be utilized as a sacrificial template during carbonization, which would result in the formation of porous structure. More PAN copolymers, blends and composites are expected to develop for different processing methods. Through selection of proper PAN precursor and processing method, functional carbon materials with variable structures and properties would be constructed. It is anticipated that the future will see more applications of PAN derived carbon materials, such as biological engineering and high-performance nanocomposite.
| [1] |
Thines R K, Mubarak N M, Nizamuddin S, et al. Application potential of carbon nanomaterials in water and wastewater treatment: A review. Journal of the Taiwan Institute of Chemical Engineers, 2017, 72: 116-133. DOI:10.1016/j.jtice.2017.01.018 (  0) 0) |
| [2] |
Liu S, Chevali V S, Xu Z G, et al. A review of extending performance of epoxy resins using carbon nanomaterials. Composites Part B: Engineering, 2018, 136: 197-214. DOI:10.1016/j.compositesb.2017.08.020 (  0) 0) |
| [3] |
DaRos T, Prato M. Medicinal chemistry with fullerenes and fullerene derivatives. Chemical Communications, 1999, 663-669. DOI:10.1039/A809495K (  0) 0) |
| [4] |
Popov V N. Carbon nanotubes: properties and application. Materials Science and Engineering R, 2004, 43: 61-102. DOI:10.1016/j.mser.2003.10.001 (  0) 0) |
| [5] |
Huang X, Qi X Y, Boey F, et al. Graphene-based composites. Chemical Society Reviews, 2012, 41: 666-686. DOI:10.1039/C1CS15078B (  0) 0) |
| [6] |
Llobet E. Gas sensors using carbon nanomaterials: A review. Sensors and Actuators B, 2013, 179: 32-45. DOI:10.1016/j.snb.2012.11.014 (  0) 0) |
| [7] |
Al-Hamadani Y A, Chu K H, Son A, et al. Stabilization and dispersion of carbon nanomaterials in aqueous solutions: A review. Separation and Purification Technology, 2015, 156: 861-874. DOI:10.1016/j.seppur.2015.11.002 (  0) 0) |
| [8] |
Pang J B, Ford C, Tan G, et al. Synthesis of mesoporous carbon using enzymatically polymerized polyphenolic precursor and simultaneously assembled silica template. Microporous and Mesoporous Materials, 2005, 85: 293-296. DOI:10.1016/j.micromeso.2005.06.030 (  0) 0) |
| [9] |
Zhang Z J, Chen X Y. Nitrogen-doped nanoporous carbon materials derived from folic acid: Simply introducing redox additive of p-phenylenediamine into KOH electrolyte for greatly improving the supercapacitor performance. Journal of Electroanalytical Chemistry, 2016, 764: 45-55. DOI:10.1016/j.jelechem.2016.01.017 (  0) 0) |
| [10] |
Wang J, Nie P, Ding B, et al. Biomass derived carbon for energy storage devices. Journal of Materials Chemistry A, 2017, 5: 2411. DOI:10.1039/C6TA08742F (  0) 0) |
| [11] |
Lee Y R, Kim J, Ahn W S. Synthesis of metal-organic frameworks: A mini review. Korean Journal of Chemical Engineering, 2013, 30(9): 1667-1680. DOI:10.1007/s11814-013-0140-6 (  0) 0) |
| [12] |
Sun X, Li Y F, Dou J, et al. Metal-organic frameworks derived carbon as a high-efficiency counter electrode for dye-sensitized solar cells. Journal of Power Sources, 2016, 322: 93-98. DOI:10.1016/j.jpowsour.2016.05.025 (  0) 0) |
| [13] |
Wei J, Liang Y, Hu Y X, et al. Hydrothermalsynthesis of metal-polyphenol coordination crystals and their derived metal/ n -doped carbon composites for oxygen electrocatalysis. Angewandte Chemie, 2016, 128: 12658-12662. DOI:10.1002/ange.201606327 (  0) 0) |
| [14] |
Wang X Y, Shi B B, Yang H, et al. Assembling covalent organic framework membranes with superior ion exchange capacity. Nature Communications, 2022, 13: 1020. DOI:10.1038/s41467-022-28643-8 (  0) 0) |
| [15] |
Teng H S, Wang S C. Preparation of porous carbons from phenol-formaldehyde resins with chemical and physical activation. Carbon, 2000, 38: 817-824. DOI:10.1016/S0008-6223(99)00160-8 (  0) 0) |
| [16] |
Kim C, Yang K S, Kojima M, et al. Fabrication of electrospinning-derived carbon nanofiber webs for the anode material of lithium-ion secondary batteries. Advanced Functional Materials, 2006, 16: 2393-2397. DOI:10.1002/adfm.200500911 (  0) 0) |
| [17] |
Prasetyo I, Rochmadi R, Wahyono E, et al. Controlling synthesis of polymer-derived carbon molecular sieve and its performance for CO2/CH4 separation. Engineering Journal, 2017, 21: 4. DOI:10.4186/ej.2017.21.4.83 (  0) 0) |
| [18] |
Wang J H, Liu R. Flash nano precipitation to prepare inorganic nanoparticles. Materials Reports, 2019, 33: 373-374. DOI:10.11896/j.issn.1005-023X.2019.3.h01.(inChinese) (  0) 0) |
| [19] |
Song C W, Wang T H, Qiu Y, et al. Effect of carbonization atmosphere on the structure changes of PAN carbon membranes. Journal of Porous Materials, 2009, 16: 197-203. DOI:10.1007/s10934-008-9185-z (  0) 0) |
| [20] |
Yang J H, Yang G Z, Yu D G, et al. Carbon foams from polyacrylonitrile-borneol films prepared using coaxial electrohydrodynamic atomization. Carbon, 2013, 53: 231-236. DOI:10.1016/j.carbon.2012.10.053 (  0) 0) |
| [21] |
Sullivan P, Moate J, Stone B, et al. Physical and chemical properties of PAN-derived electrospun activated carbon nanofibers and their potential for use as an adsorbent for toxic industrial chemicals. Adsorption, 2012, 18: 265-274. DOI:10.1007/s10450-012-9399-x (  0) 0) |
| [22] |
Hwang S, Kim S W, Cho G B, et al. Carbon nanotubes radially anchored on carbon fibers formed by polyacrylonitrile. Materials Research Bulletin, 2018, 97: 49-55. DOI:10.1016/j.materresbull.2017.08.039 (  0) 0) |
| [23] |
Nataraja S K, Yang K S, Aminabhavi T M. Polyacrylonitrile-based nanofibers: A state-of-the-art review. Progress in Polymer Science, 2012, 37: 487-513. DOI:10.1016/j.progpolymsci.2011.07.001 (  0) 0) |
| [24] |
Kausar A. Polyacrylonitrile nanocomposite with carbon nanostructures: A review. Polymer-Plastics Technology and Materials, 2019, 58: 707-731. DOI:10.1080/25740881.2018.1563113 (  0) 0) |
| [25] |
Hu X P. The molecular structure of polyacrylonitrile fibers. Journal of Applied Polymer Science, 1996, 62: 1925-1932. DOI:10.1002/(SICI)1097-4628(19961212)62:11<1925::AID-APP17>3.0.CO;2-X (  0) 0) |
| [26] |
Sui K Y, Gu L X. Preparation and characterization of amphiphilic block copolymer of polyacrylonitrile-block-Poly(ethylene oxide). Journal of Applied Polymer Science, 2003, 89: 1753-1759. DOI:10.1002/app.12036 (  0) 0) |
| [27] |
Boguslavsky L, Baruch S, Margel S. Synthesis and characterization of polyacrylonitrile nanoparticles by dispersion/emulsion polymerization process. Journal of Colloid and Interface Science, 2005, 289: 71-85. DOI:10.1016/j.jcis.2005.03.063 (  0) 0) |
| [28] |
Sui K Y, Liang H C, Zhao X, et al. Synthesis of amphiphilic poly(ethylene oxide-co-glycidol)-graft-polyacrylonitrile brush copolymers and their self-assembly in aqueous media. Macromolecular Chemistry and Physics, 2012, 213: 1717-1724. DOI:10.1002/macp.201200136 (  0) 0) |
| [29] |
Hirofusa S, Nagatomo Y, Inaki Y, et al. Vinyl polymerization by metal complexes. XV. polymerization of acrylonitrile initiated by imidazole-copper(Ⅱ) complex. Journal of Macromolecular Science-pure and Applied Chemistry, 1974, 8: 935-947. DOI:10.1080/00222337408066410 (  0) 0) |
| [30] |
Schimler S D, Ryan S J, Bland D C, et al. Anhydrous tetramethylammonium fluoride for room-temperature SNAr fluorination. The Journal of Organic Chemistry, 2015, 80: 12137-12145. DOI:10.1021/acs.joc.5b02075 (  0) 0) |
| [31] |
Siemeling U, Kölling L, Stammler A, et al. Polymerisation of acrylonitrile with di(organoimido)chromium(VI) complexes. Chemical Communications, 2000, 13: 1177-1178. DOI:10.1039/B002310H (  0) 0) |
| [32] |
Lazzari M, Chiantore O, Mendichi R, et al. Synthesis of polyacrylonitrile-block-polystyrene copolymers by atom transfer radical polymerization. Macromolecular Chemistry and Physics, 2005, 206: 1382-1388. DOI:10.1002/macp.200500159 (  0) 0) |
| [33] |
Niu S G, Zhang L F, Zhu J, et al. Synthesis of high molecular weight and narrow molecular weight distribution poly(acrylonitrile) via RAFT polymerization. Journal of Polymer Science, Part A: Polymer Chemistry, 2013, 51: 1197-1204. DOI:10.1002/pola.26488 (  0) 0) |
| [34] |
Kaim A, Megiel E. Transition states for deactivation reactions in the modeled 2, 2, 6, 6-tetramethyl-1-piperidinyloxy-mediated free-radical polymerization of acrylonitrile. Journal of Polymer Science: Part A: Polymer Chemistry, 2006, 44: 914-927. DOI:10.1002/pola.21203 (  0) 0) |
| [35] |
Hou C, Qu R J, Ji C N, et al. Synthesis of polyacrylonitrile via reverse atom transfer radical polymerization catalyzed by FeCl3/Isophthalic acid. Journal of Polymer Science: Part A: Polymer Chemistry, 2006, 44: 219-225. DOI:10.1002/pola.21174 (  0) 0) |
| [36] |
Brar A S, Saini T. Atom transfer radical copolymerization of acrylonitrile and ethyl methacrylate at ambient temperature. Journal of Polymer Science: Part A: Polymer Chemistry, 2006, 44: 1975-1984. DOI:10.1002/pola.21302 (  0) 0) |
| [37] |
Niu S G, Ding M Q, Chen M T, et al. Synthesis of well-defined copolymer of acrylonitrile and maleic anhydride via RAFT polymerization. Journal of Polymer Science, Part A: Polymer Chemistry, 2013, 51: 5263-5269. DOI:10.1002/pola.26956 (  0) 0) |
| [38] |
Nguyen-Thai N U, Hong S C. Controlled architectures of poly(acrylonitrileco-itaconic acid) for efficient structural transformation into carbon materials. Carbon, 2014, 69: 571-581. DOI:10.1016/j.carbon.2013.12.068 (  0) 0) |
| [39] |
Moskowitz J D, Wiggins J S. Semibatch RAFT copolymerization of acrylonitrile and nisopropylacrylamide: effect of comonomer distribution on cyclization and thermal stability. Polymer, 2016, 84: 311-318. DOI:10.1016/j.polymer.2015.12.035 (  0) 0) |
| [40] |
Zhou Z P, Liu T Y, Khan A U, et al. Block copolymer-based porous carbon fibers. Science Advances, 2019, 5(2): eaau6852. DOI:10.1126/sciadv.aau6852 (  0) 0) |
| [41] |
Kope c ' M, Lamson M, Yuan R, et al. Polyacrylonitrile-derived nanostructured carbon materials. Progress in Polymer Science, 2019, 92: 89-134. DOI:10.1016/j.progpolymsci.2019.02.003 (  0) 0) |
| [42] |
McGann J P, Zhong M J, Kim E K, et al. Block copolymer templating as a path to porous nanostructured carbons with highly accessible nitrogens for enhanced (electro) chemical performance. Macromolecular Chemistry and Physics, 2012, 213: 1078-1090. DOI:10.1002/macp.201100691 (  0) 0) |
| [43] |
Zhang S L, Chen L, Zhou S X, et al. Facile synthesis of hierarchically ordered porous carbon via in situ self-assembly of colloidal polymer and silica spheres and its use as a catalyst support. Chemistry of Materials, 2010, 22: 3433-3440. DOI:10.1021/cm1002274 (  0) 0) |
| [44] |
Yang X Q, Wu D C, Chen X M, et al. Nitrogen-enriched nanocarbons with a 3-d continuous mesopore structure from polyacrylonitrile for supercapacitor application. The Journal of Physical Chemistry C, 2010, 114: 8581-8586. DOI:10.1021/jp101255d (  0) 0) |
| [45] |
Sree Manu K M, Ajay Raag L, Rajan T P D, et al. Self-lubricating bidirectional carbon fiber reinforced smart aluminum composites by squeeze infiltration process. Journal of Materials Science & Technology, 2019, 35(11): 2559-2569. DOI:10.1016/j.jmst.2019.04.034 (  0) 0) |
| [46] |
Zhong M J, Kim E K, McGann J P, et al. Electrochemically active nitrogen-enriched nanocarbons with well-defined morphology synthesized by pyrolysis of self-assembled block copolymer. Journal of the American Chemical Society, 2012, 134: 14846-14857. DOI:10.1021/ja304352n (  0) 0) |
| [47] |
Tang C B, Tracz A, Kruk M, et al. Long-range ordered thin films of block copolymers prepared by zone-casting and their thermal conversion into ordered nanostructured carbon. Journal of the American Chemical Society, 2005, 127: 6918-6919. DOI:10.1021/ja0508929 (  0) 0) |
| [48] |
Liu M M, Li J, Cai C, et al. A polyacrylonitrile copolymer-silica template for three-dimensional hierarchical porous carbon as a Pt catalyst support for the oxygen reduction reaction. Dalton Transactions, 2017, 46: 9912-9917. DOI:10.1039/C7DT01081H (  0) 0) |
| [49] |
Zhang Z J, Sun L, Liu R. Flash nanoprecipitation of polymer supported Pt colloids with tunable catalytic chromium reduction property. Colloid and Polymer Science, 2018, 296: 327-333. DOI:10.1007/s00396-017-4231-5 (  0) 0) |
| [50] |
He Y Z, Priestley R D, Liu R. A one-step and scalable continuous-flow nanoprecipitation for catalytic reduction of organic pollutants in water. Industrial & Engineering Chemistry Research, 2016, 55: 9851-9856. DOI:10.1021/acs.iecr.6b02279 (  0) 0) |
| [51] |
Sosa C, Liu R, Tang C, et al. Soft multifaced and patchy colloids by constrained volume self-assembly. Macromolecules, 2016, 49: 3580-3585. DOI:10.1021/acs.macromol.6b00708 (  0) 0) |
| [52] |
Han J, Zhu Z X, Qian H T, et al. A simple confined impingement jets mixer for flash nanoprecipitation. Journal of Pharmaceutical Sciences, 2012, 101(10): 4018-4023. DOI:10.1002/jps.23259 (  0) 0) |
| [53] |
Russ B, Liu Y, Prud'homme R K. Optimized descriptive model for micromixing in a vortex mixer. Chemical Engineering Communications, 2010, 197: 1068-1075. DOI:10.1080/00986440903412985 (  0) 0) |
| [54] |
Liu R, Priestley R D. Rational design and fabrication of core-shell nanoparticles through a one-step/pot strategy. Journal of Materials Chemistry A, 2016, 4: 6680. DOI:10.1039/C5TA09607C (  0) 0) |
| [55] |
Nataraja S K, Yang K S, Aminabhavi T M. Polyacrylonitrile-based nanofibers: A state-of-the-art review. Progress in Polymer Science, 2012, 37: 487-513. DOI:10.1016/j.progpolymsci.2011.07.001 (  0) 0) |
| [56] |
Zhou Z W, Zhao J, Shen Y L, et al. Flash nanoprecipitation of poly(styrene-co-acrylonitrile) nanospheres and their amidoximation for uranium sorption. Journal of Radioanalytical and Nuclear Chemistry, 2017, 314: 2003-2007. DOI:10.1007/s10967-017-5596-0 (  0) 0) |
| [57] |
Li C L, Zhao J, Priestley R D, et al. Constrained-volume assembly oforganometal confined in polymer to fabricate multi-heteroatom doped carbon for oxygen reduction reaction. Science China Materials, 2018, 61(10): 1305-1313. DOI:10.1007/s40843-018-9269-x (  0) 0) |
| [58] |
Liu M M, Zhao J, Priestley R D, et al. Flash nanoprecipitation of poly(styrene-co-acrylonitrile) colloids in the presence of hydrophobic organoplatinum and their derived Pt-carbon nanocomposites for oxygen reduction reaction. Colloids and Surfaces A, 2018, 552: 118-123. DOI:10.1016/j.colsurfa.2018.05.032 (  0) 0) |
| [59] |
Wang J H, Zhang Z J, Zhao J, et al. Pyrolysis of a flash nanoprecipitated tannic acid-metal@polymer assembly to create an electrochemically active metal@nanocarbon catalyst. Polymer Journal, 2020, 52: 539-547. DOI:10.1038/s41428-020-0305-1 (  0) 0) |
| [60] |
Zhang Z J, Yang Y, Li C L, et al. Porous nanofibrous superhydrophobic membrane with embedded Au nanoparticles for the integration of oil/water separation and catalytic degradation. Journal of Membrane Science, 2019, 582: 350-357. DOI:10.1016/j.memsci.2019.04.024 (  0) 0) |
| [61] |
Yoon K, Kim K, Wang X F, et al. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer, 2006, 47: 2434-2441. DOI:10.1016/j.polymer.2006.01.042 (  0) 0) |
| [62] |
Wang R, Liu Y, Li B, et al. Electrospun nanofibrous membranes for high flux microfiltration. Journal of Membrane Science, 2012, 392: 167-174. DOI:10.1016/j.memsci.2011.12.019 (  0) 0) |
| [63] |
Zhang L F, Luo J, Menkhaus T J, et al. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. Journal of Membrane Science, 2011, 369: 499-505. DOI:10.1016/j.memsci.2010.12.032 (  0) 0) |
| [64] |
Ray S S, Chen S S, Li C W, et al. A comprehensive review: electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Advances, 2016, 6: 85495-85514. DOI:10.1039/C6RA14952A (  0) 0) |
| [65] |
Huang Z M, Zhang Y Z, Kotaki M, et al. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology, 2013, 63: 2223-2253. DOI:10.1016/S0266-3538(03)00178-7 (  0) 0) |
| [66] |
Jadhav S A, Dhavale S B, Patil A H, et al. Brief overview of electrospun polyacrylonitrile carbon nanofibers: preparation process with applications and recent trends. Material Design & Processing Communications, 2019, 1: e83. DOI:10.1002/mdp2.83 (  0) 0) |
| [67] |
Zhang L F, Aboagye A, Kelkar A, et al. A review: carbon nanofibers from electrospun polyacrylonitrile and their applications. Journal of Materials Science, 2014, 49: 463-480. DOI:10.1007/S10853-013-7705-y (  0) 0) |
| [68] |
Wang Y X, Zhang L J, Zhang Y F, et al. Study on preparation, structure and properties of polyacrylonitrile/boron nitride hybrid composite fibers via electrospinning. Materials Reports, 2020, 34(12): 12158-12162. DOI:10.11896/cldb.19060142 (  0) 0) |
| [69] |
Lee K J, Shiratori N, Lee G H, et al. Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent. Carbon, 2010, 48: 4248-4255. DOI:10.1016/j.carbon.2010.07.034 (  0) 0) |
| [70] |
Tran C, Kalra V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. Journal of Power Sources, 2013, 235: 289-296. DOI:10.1016/j.jpowsour.2013.01.080 (  0) 0) |
| [71] |
Li TT, Wang Y T, Peng H K, et al. Lightweight, flexible and superhydrophobic composite nanofiber films inspired by nacre for highly electromagnetic interference shielding. Composites Part A, 2020, 128: 105685. DOI:10.1016/j.compositesa.2019.105685 (  0) 0) |
| [72] |
Liu C, Wang J, Li J S, et al. Electrospun ZIF-based hierarchical carbon fiber as an efficient electrocatalyst for the oxygen reduction reaction. Journal of Materials Chemistry A, 2017, 5: 1211-1220. DOI:10.1039/C6TA09193H (  0) 0) |
| [73] |
Rajzer I, Kwiatkowski R, Piekarczyk W, et al. Carbon nanofibers produced from modified electrospun PAN/hydroxyapatite precursors as scaffolds for bone tissue engineering. Materials Science and Engineering C, 2012, 32: 2562-2569. DOI:10.1016/j.msec.2012.07.041 (  0) 0) |
| [74] |
Li C L, Wu M C, Liu R. High-performance bifunctional oxygen electrocatalysts for Zinc-air batteries over mesoporous Fe/Co-N-C nanofibers with embedding FeCo alloy nanoparticles. Applied Catalysis B: Environmental, 2019, 244: 150-158. DOI:10.1016/j.apcatb.2018.11.039 (  0) 0) |
| [75] |
Li C L, Zhang Z J, Wu M C, et al. FeCoNi ternary alloy embedded mesoporous carbon nanofiber: an efficient oxygen evolution catalyst for rechargeable Zinc-air battery. Materials Letters, 2019, 238: 138-142. DOI:10.1016/j.matlet.2018.11.160 (  0) 0) |
| [76] |
Moon S J, Park K, Seo E, et al. Mass production of functional amine-conjugated PAN nanofiber mat via syringeless electrospinning and CVD. Macromolecular Materials and Engineering, 2018, 303: 1700565. DOI:10.1002/mame.201700565 (  0) 0) |
| [77] |
Li R, Liu C J, Tang P F, et al. NiCoP nanowire arrays embedded in 3D integrated N-doped carbon network for enhanced electrochemical oxygen evolution. Vacuum, 2021, 192: 110395. DOI:10.1016/j.vacuum.2021.110395 (  0) 0) |
| [78] |
Liu Q, Cao S B, Qiu Y J. Effect of carbonization temperature on bimetallic FeCo-N/C nanofiber electrocatalysts for oxygen reduction reaction in sulfuric acid solution. International Journal of Hydrogen Energy, 2017, 4: 229274-229282. DOI:10.1016/j.ijhydene.2017.10.069 (  0) 0) |
| [79] |
Lei D Y, Li X D, Seo M K, et al. NiCo2O4 nanostructure-decorated PAN/lignin based carbon nanofiber electrodes with excellent cyclability for flexible hybrid supercapacitors. Polymer, 2017, 132: 31-40. DOI:10.1016/j.polymer.2017.10.051 (  0) 0) |
| [80] |
Yang W X, Zhang Y Q, Liu C Y, et al. Dual-doped carbon composite for efficient oxygen reduction via electrospinning and incipient impregnation. Journal of Power Sources, 2015, 274: 595-603. DOI:10.1016/j.jpowsour.2014.10.067 (  0) 0) |
| [81] |
Gong T, Qi R Y, Liu X D, et al. N, F-codoped microporous carbon nanofibers as efficient metal-free electrocatalysts for ORR. Nano-Micro Letters, 2019, 11: 9. DOI:10.1007/s40820-019-0240-x (  0) 0) |
| [82] |
Tang D Y, Zhuang X P, Zhang C, et al. Generation of nanofibers via electrostatic-induction-assisted solution blow spinning. Journal of Applied Polymer Science, 2015, 132: 42326. DOI:10.1002/app.42326 (  0) 0) |
| [83] |
Wang W L, Wang C G, Gao Q, et al. A new perspective on the internal structure of polyacrylonitrile-based preoxidized fibers through ultrathin sections. Polymer Degradation and Stability, 2019, 167: 269-276. DOI:10.1016/j.polymdegradstab.2019.07.012 (  0) 0) |
| [84] |
de Gonzaga L A C, Martins M C F, Correa A C, et al. Production of carbon nanofibers from PAN and lignin by solution blow spinning. Journal of Polymer Research, 2021, 28: 237. DOI:10.1007/s10965-021-02568-0 (  0) 0) |
| [85] |
Lu M X, Liao J S, Gulgunje P V, et al. Rheological behavior and fiber spinning of polyacrylonitrile (PAN)/Carbon nanotube (CNT) dispersions at high CNT loading. Polymer, 2021, 215: 123369. DOI:10.1016/j.polymer.2020.123369 (  0) 0) |
| [86] |
Ramachandran J, Serrano J M, Liu T Y, et al. Porous carbon fibers from gel-spun polyacrylonitrile and poly(methyl methacrylate)-block-poly(acrylonitrile). Carbon, 2022, 192: 332-346. DOI:10.1016/j.carbon.2022.02.044 (  0) 0) |
| [87] |
Ergün A B, Sevim A M, Kılıç A, et al. Metallophthalocyanine/polyacrylonitrile nanofibers by solution blow spinning technique for enhanced photocatalytic activity by visible light. Journal of Applied Polymer Science, 2021, 138: 50115. DOI:10.1002/app.50115 (  0) 0) |
| [88] |
Chen Y, Wang N, Jensen M, et al. Catalyst-free large-scale synthesis of composite SiC@SiO2/carbon nanofiber mats by blow-spinning. Journal of Materials Chemistry C, 2019, 7: 15233-15242. DOI:10.1039/C9TC05257G (  0) 0) |
| [89] |
Lu Y, Li Y, Zhang S, et al. Parameter study and characterization for polyacrylonitrile nanofibers fabricated via centrifugal spinning process. European Polymer Journal, 2013, 49: 3834-3845. DOI:10.1016/j.eurpolymj.2013.09.017 (  0) 0) |
| [90] |
Zhang X W, Lu Y. Centrifugal spinning: an alternative approach to fabricate nanofibers at high speed and low cost. Polymer Reviews, 2014, 54: 677-701. DOI:10.1080/15583724.2014.935858 (  0) 0) |
| [91] |
Jiang H, Ge Y Q, Fu K, et al. Centrifugally-spun tin-containing carbon nanofibers as anode material for lithium-ion batteries. Journal of Materials Science, 2015, 50: 1094-1102. DOI:10.1007/s10853-014-8666-5 (  0) 0) |
| [92] |
Akia M, Cremar L, Seas M, et al. High-throughput production with improved functionality and graphitization of carbon fine fibers developed from sodium chloride-polyacrylonitrile precursors. Polymer Engineering & Science, 2018, 58(11): 2047-2054. DOI:10.1002/pen.24816 (  0) 0) |
| [93] |
Xu W, Xia L, Zhou X H, et al. Hollow carbon microfibers fabricated using coaxial centrifugal spinning. Micro & Nano Letters, 2016, 11: 74-76. DOI:10.1049/mnl.2015.0346 (  0) 0) |
| [94] |
Xie W, Cheng H F, Chu Z Y, et al. Effect of carbonization time on the structure and electromagnetic parameters of porous-hollow carbon fibers. Ceramics International, 2009, 35: 2705-2710. DOI:10.1016/j.ceramint.2009.03.002 (  0) 0) |
| [95] |
Xu W H, Ravichandran D, Jambhulkar S, et al. Hierarchically structured composite fibers for real nanoscale manipulation of carbon nanotubes. Advanced Functional Materials, 2021, 31: 2009311. DOI:10.1002/adfm.202009311 (  0) 0) |
| [96] |
Bajaj P, Sreekumar T V, Sen K. Structure development during dry-jet-wet spinning of acrylonitrile/vinyl acids and acrylonitrile/methyl acrylate copolymers. Journal of Applied Polymer Science, 2002, 86: 773-787. DOI:10.1002/app.10973 (  0) 0) |
| [97] |
Sun J F, Wu G X, Wang Q R. The effects of carbonization temperature on the properties and structure of PAN-based activated carbon hollow fiber. Journal of Applied Polymer Science, 2005, 97: 2155-2160. DOI:10.1002/app.21955 (  0) 0) |
| [98] |
Saufi S M, d Ismail A F. Development and characterization of polyacrylonitrile (PAN) based carbon hollow fiber membrane. Songklanakarin Journal of Science and Technology, 2002, 24: 843-854. (  0) 0) |
| [99] |
Park S J. Carbon Fibers. Singapore: Springer Singapore, 2018. DOI:10.1007/978-981-13-0538-2
(  0) 0) |
| [100] |
Liu J, Yue Z R, Fong H. Continuous nanoscale carbon fibers with superior mechanical strength. Small, 2009, 5: 536-542. DOI:10.1002/smll.200801440 (  0) 0) |
| [101] |
Fitzer E. Pan-based carbon fibers—present state and trend of the technology from the viewpoint of possibilities and limits to influence and to control the fiber properties by the process parameters. Carbon, 1989, 27: 621-645. DOI:10.1016/0008-6223(89)90197-8 (  0) 0) |
| [102] |
Liua J, Zhou P X, Zhang L F, et al. Thermo-chemical reactions occurring during the oxidative stabilization of electrospun polyacrylonitrile precursor nanofibers and the resulting structural conversions. Carbon, 2009, 47: 1087-1095. DOI:10.1016/j.carbon.2008.12.033 (  0) 0) |
| [103] |
Shiratori H, Todoroki A, Ueda M, et al. Mechanism of folding a fiber bundle in the curved section of 3D printed carbon fiber reinforced plastics. Advanced Composite Materials, 2020, 29: 247-257. DOI:10.1080/09243046.2019.1682794 (  0) 0) |
| [104] |
Khan H, Kaur J, Naebe M, et al. Continuous, pilot-scale production of carbon fiber from a textile grade PAN polymer. Materials Today Communications, 2022, 31: 103231. DOI:10.1016/j.mtcomm.2022.103231 (  0) 0) |
| [105] |
Sun J F, He C J, Zhu S J, et al. Effects of oxidation time on the structure and properties of polyacrylonitrile-based activated carbon hollow fiber. Journal of Applied Polymer Science, 2007, 106: 470-474. DOI:10.1002/app.24508 (  0) 0) |
| [106] |
Zhao J, Qin R X, Liu R. Urea-bridging synthesis of nitrogen-doped carbon tube supported single metallic atoms as bifunctional oxygen electrocatalyst for Zinc-air battery. Applied Catalysis B: Environmental, 2019, 256: 117778. DOI:10.1016/j.apcatb.2019.117778 (  0) 0) |
| [107] |
Zhao J, Li C L, Liu R. Enhanced oxygen reduction of multi-Fe3O4@carbon core-shell electrocatalysts through a nanoparticle/polymer co-assembly strategy. Nanoscale, 2018, 10: 5747-6216. DOI:10.1039/C7NR09185K (  0) 0) |
| [108] |
Debe M K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature, 2012, 486: 43-51. DOI:10.1038/nature11115 (  0) 0) |
| [109] |
Lee Y M, Suntivich J, May K J, et al. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. The Journal of Physical Chemistry Letters, 2012, 3: 399-404. DOI:10.1021/jz2016507 (  0) 0) |
| [110] |
Su H Y, Gorlin Y, Man I C, et al. Identifying active surface phases for metal oxide electrocatalysts: a study of manganese oxide bi-functional catalysts for oxygen reduction and water oxidation catalysis. The Journal of Chemical Physics, 2012, 14: 14010-14022. DOI:10.1039/C2CP40841D (  0) 0) |
| [111] |
Esposito D V, Chen J G. Monolayer platinum supported on tungsten carbides as low-cost electrocatalysts: opportunities and limitations. Energy & Environmental Science Journal, 2011, 4: 3900. DOI:10.1039/C1EE01851E (  0) 0) |
| [112] |
Zhou R F, Qiao S Z. An Fe/N co-doped graphitic carbon bulb for high-performance oxygen reduction reaction. Chemical Communications, 2015, 51: 7516-7519. DOI:10.1039/C5CC00995B (  0) 0) |
| [113] |
Wang Y, Kong B, Zhao D Y, et al. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today, 2017, 15: 26-55. DOI:10.1016/j.nantod.2017.06.006 (  0) 0) |
| [114] |
Barhoum A, El-Maghrabi H H, Nada A A, et al. Simultaneous hydrogen and oxygen evolution reactions using free-standing nitrogen-doped-carbon-Co/CoOx nanofiber electrodes decorated with palladium nanoparticles. Journal of Materials Chemistry A, 2021, 9: 17724-17739. DOI:10.1039/d1ta03704h (  0) 0) |
| [115] |
Gong Y X, Xu L H, Li J J, et al. Confinement of transition metal phosphides in N, P-doped electrospun carbon fibers for enhanced electrocatalytic hydrogen evolution. Journal of Alloys and Compounds, 2021, 875: 159934. DOI:10.1016/j.jallcom.2021.159934 (  0) 0) |
| [116] |
Heckova M, Streckova M, Orinakova R, et al. Porous carbon fibers for effective hydrogen evolution. Applied Surface Science, 2020, 506: 144955. DOI:10.1016/j.apsusc.2019.144955 (  0) 0) |
| [117] |
Gómez-López P, Salatti-Dorado J Á, Rodríguez-Padrón D, et al. Mechanochemically synthetized PAN-based Co-N-doped carbon materials as electrocatalyst for oxygen evolution reaction. Nanomaterials, 2021, 11(2): 290. DOI:10.3390/nano11020290 (  0) 0) |
| [118] |
Shang C Q, Li M C, Wang Z Y, et al. Electrospun nitrogen-doped carbon nanofibers encapsulating cobalt nanoparticles as efficient oxygen reduction reaction catalysts. Chem Electro Chem, 2016, 3: 1437-1445. DOI:10.1002/celc.201600275 (  0) 0) |
| [119] |
Li Q, ZhaoJ, Wu M C, et al. Hierarchical porous n-doped carbon nanofibers supported Fe3C/Fe nanoparticles as efficient oxygen electrocatalysts for Zn-Air batteries. Chemistry Select, 2019, 4: 722-728. DOI:10.1002/slct.201803351 (  0) 0) |
| [120] |
Li Q, Li C L, Li Y L, et al. Fabrication of hollow n-doped carbon supported ultrathin NiO nanosheets for high-performance supercapacitor. Inorganic Chemistry Communications, 2017, 86: 140-144. DOI:10.1016/j.inoche.2017.10.005 (  0) 0) |
| [121] |
Zhao J, Li Q, Han L, et al. Spherical mesocrystals from self-assembly of folic acid and nickel(ii) ion for high-performance supercapacitors. Journal of Colloid and Interface Science, 2019, 538: 142-148. DOI:10.1016/j.jcis.2018.11.088 (  0) 0) |
| [122] |
Li M, Xue J M. Integrated synthesis of nitrogen-doped mesoporous carbon from melamine resins with superior performance in supercapacitors. The Journal of Physical Chemistry C, 2014, 118: 2507-2517. DOI:10.1021/jp410198r (  0) 0) |
| [123] |
Frackowiak E. Carbon materials for supercapacitor application. Physical Chemistry Chemical Physics, 2007, 9: 1774-1785. DOI:10.1039/B618139M (  0) 0) |
| [124] |
Jurewicz K, Vix-Guterl C, Frackowiak E, et al. Capacitance properties of ordered porous carbon materials prepared by a templating procedure. Journal of Physics and Chemistry of Solids, 2004, 65: 287-293. DOI:10.1016/j.jpcs.2003.10.024 (  0) 0) |
| [125] |
Zhong Y, Li Q, Liu R. Blueberry-peel-derived porous carbon for high-performance supercapacitors: the effect of N-doping and activation. Chemistry Select, 2020, 5: 1029-1036. DOI:10.1002/slct.201904820 (  0) 0) |
| [126] |
Kim C, Ngoc B T N, Yang K D, et al. Self-sustained thinwebs consisting of porous carbon nanofibers for supercapacitors via the electrospinning of polyacrylonitrile solutions containing Zinc chloride. Advanced Materials, 2007, 19: 2341-2346. DOI:10.1002/adma.200602184 (  0) 0) |
| [127] |
Yang K S, Joo Y E, Kim T C, et al. Performances of electrochemical hybrid supercapacitor of RuO2/activated carbon nanofibers from electrospinning. Proceedings of the 10th WSEAS International Conference on Computers. New York, NY: ACM, 2006: 888-892.
(  0) 0) |
| [128] |
Wang C H, Liu C, Li J S, et al. Electrospun metal-organic framework derived hierarchical carbon nanofibers with high performance for supercapacitors. Chemical Communications, 2017, 53: 1751-1754. DOI:10.1039/C6CC09832K (  0) 0) |
| [129] |
Wang H, Wang H J, Wang W Y, et al. Research progress in polyacrylonitrile (PAN) based carbon nanofibers electrode materials for supercapacitor. Materials Reports, 2018, 32(3): 730-734. DOI:10.11896/j.issn.1005-023X.2018.05.007 (  0) 0) |
| [130] |
Zhan C Z, Song J N, Ren X L, et al. Blow-spun N-doped carbon fiber based high performance flexible lithium ion capacitors. RSC Advances, 2020, 10: 9833-9839. DOI:10.1039/C9RA10348A (  0) 0) |
| [131] |
Lota G, Grzyb B, Machnikowska H, et al. Effect of nitrogen in carbon electrode on the supercapacitor performance. Chemical Physics Letters, 2005, 404: 53-58. DOI:10.1016/j.cplett.2005.01.074 (  0) 0) |
| [132] |
Lai C C, Lo C T. Preparation of nanostructural carbon nanofibers and their electrochemical performance for supercapacitors. Electrochimica Acta, 2015, 183: 85-93. DOI:10.1016/j.electacta.2015.02.143 (  0) 0) |
| [133] |
Tran C, Kalra V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. Journal of Power Sources, 2013, 235: 289-296. DOI:10.1016/j.jpowsour.2013.01.080 (  0) 0) |
| [134] |
Kim B H, Yang K S, Woo H G. Thin, bendable electrodes consisting of porous carbon nanofibers via the electrospinning of polyacrylonitrile containing tetraethoxy orthosilicate for supercapacitor. Electrochemistry Communications, 2011, 13: 1042-1046. DOI:10.1016/j.elecom.2011.06.024 (  0) 0) |
| [135] |
Altin Y, Bedeloglu A C. Polyacrylonitrile/polyvinyl alcohol-based porous carbon nanofiber electrodes for supercapacitor applications. International Journal of Energy Research, 2021, 45: 16497-16510. DOI:10.1002/er.6896 (  0) 0) |
| [136] |
Zhang W J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. Journal of Power Sources, 2011, 196: 13-24. DOI:10.1016/j.jpowsour.2010.07.020 (  0) 0) |
| [137] |
Datta M K, Kumta P N. Silicon and carbon based composite anodes for lithium ion batteries. Journal of Power Sources, 2006, 158: 557-563. DOI:10.1016/j.jpowsour.2005.09.016 (  0) 0) |
| [138] |
Lee J K, An K W, Ju J B, et al. Electrochemical properties of PAN-based carbon fibers as anodes for rechargeable lithium ion batteries. Carbon, 2001, 39: 1299-1305. DOI:10.1016/j.jpowsour.2005.09.016 (  0) 0) |
| [139] |
Kwon T G, Park H, Jo O H, et al. Facile Preparation of magnetite-incorporated polyacrylonitrile-derived carbons for Li-Ion battery anodes. ACS Applied Energy Materials, 2022, 5(1): 1262-1270. DOI:10.1021/acsaem.1c03679 (  0) 0) |
| [140] |
Ji L W, Yao Y F, Toprakci O, et al. Fabrication of carbon nanofiber-driven electrodes from electrospun polyacrylonitrile/polypyrrole bicomponents for high-performance rechargeable lithium-ion batteries. Journal of Power Sources, 2010, 195: 2050-2056. DOI:10.1016/j.jpowsour.2009.10.021 (  0) 0) |
| [141] |
Ji L W, Zhang X W. Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries. Nanotechnology, 2009, 20: 155705. DOI:10.1088/0957-4484/20/15/155705 (  0) 0) |
| [142] |
Li Y, Guo B K, Ji L W, et al. Structure control and performance improvement of carbon nanofibers containing a dispersion of silicon nanoparticles for energy storage. Carbon, 2013, 51: 185-194. DOI:10.1016/j.carbon.2012.08.027 (  0) 0) |
| [143] |
Jiang H, Ge Y Q, Fu K, et al. Centrifugally-spun tin-containing carbon nanofibers as anode material for lithium-ion batteries. Journal of Materials Science, 2015, 50: 1094-1102. DOI:10.1007/s10853-014-8666-5 (  0) 0) |
| [144] |
Zhang W L, Sun M L, Yin J, et al. A cyclized polyacrylonitrile anode for alkali metal ion batteries. Angewandte Chemie, 2021, 60: 1355-1363. DOI:10.1002/ange.202011484 (  0) 0) |
| [145] |
Liu W Y, Yi C J, Li L P, et al. Designing polymer-in-salt electrolyte and fully infiltrated 3D electrode for integrated solid-state lithium batteries. Angewandte Chemie, 2021, 60: 12931-12940. DOI:10.1002/ange.202101537 (  0) 0) |
| [146] |
Du K F, Zhang Q. Facile fabrication of sulfur entrapped carbonized material as cathode for high-performance lithium battery. RSC Advances, 2016, 6: 87690-87695. DOI:10.1039/C6RA22008H (  0) 0) |
| [147] |
Li Y, Zhu J D, Zhu P, et al. Glass fiber separator coated by porous carbon nanofiber derived from immiscible PAN/PMMA for high-performance lithium-sulfur batteries. Journal of Membrane Science, 2018, 552: 31-42. DOI:10.1016/j.memsci.2018.01.062 (  0) 0) |
| [148] |
Zhu T C, Dong X L, Liu Y, et al. An all-solid-state sodium-sulfur battery using a sulfur/carbonized polyacrylonitrile composite cathode. ACS Applied Energy Materials, 2019, 2(7): 5263-5271. DOI:10.1021/acsaem.9b00953 (  0) 0) |
| [149] |
Gong C, Zhou Z W, Li J, et al. Facile synthesis of ultra stable Fe3O4 @Carbon core-shell nanoparticles entrapped satellite au catalysts with enhanced 4-nitrophenol reduction property. Journal of the Taiwan Institute of Chemical Engineers, 2018, 84: 229-235. DOI:10.1016/j.jtice.2018.01.026 (  0) 0) |
| [150] |
Gong J L, Wang B, Zeng G M, et al. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. Journal of Hazardous Materials, 2009, 164: 1517-1522. DOI:10.1016/j.jhazmat.2008.09.072 (  0) 0) |
| [151] |
Kabita D, Mukhopadhyay S, Bhattacharjee S, et al. Chemical oxidation of methylene blue using a Fenton-like reaction. Journal of Hazardous Materials B, 2001, 84: 57-71. DOI:10.1016/S0304-3894(01)00202-3 (  0) 0) |
| [152] |
Li W H, Yue Q Y, Gao B Y, et al. Preparation and utilization of sludge-based activated carbon for the adsorption of dyes from aqueous solutions. Chemical Engineering Journal, 2011, 171: 320-327. DOI:10.1016/j.cej.2011.04.012 (  0) 0) |
| [153] |
DeLisi R, Lazzara G, Milioto S, et al. Adsorption of a dye on clay and sand. Use of cyclodextrins as solubility-enhancement agents. Chemospher, 2007, 69: 1703-1712. DOI:10.1016/j.chemosphere.2007.06.008 (  0) 0) |
| [154] |
Wang J H, Cai C, Zhang Z J, et al. Electrospun metal-organic frameworks with polyacrylonitrile as precursors to hierarchical porous carbon and composite nanofibers for adsorption and catalysis. Chemosphere, 2020, 239: 124833. DOI:10.1016/j.chemosphere.2019.124833 (  0) 0) |
| [155] |
Tao X X, Zhou G Q, Zhuang X P, et al. Solution blowing of activated carbon nanofibers for phenol adsorption. RSC Advances, 2015, 5: 5801-5808. DOI:10.1039/C4RA10897C (  0) 0) |
| [156] |
Tai M H, Gao P, Yong B Y L, et al. Highly efficient and flexible electrospun carbon-silica nanofibrous membrane for ultrafast gravity-driven oil-water separation. ACS Applied Materials & Interfaces, 2014, 6: 9393-9401. DOI:10.1021/am501758c (  0) 0) |
| [157] |
Liu H, Cao C Y, Wei F F, et al. Flexible macroporous carbon nanofiber film with high oil adsorption capacity. Journal of Materials Chemistry A, 2014, 2: 3557-3562. DOI:10.1039/C3TA14468B (  0) 0) |
| [158] |
Kim D H, Kwon Y S, Lee J H, et al. Tailoring the stabilization and pyrolysis processes of carbon molecular sieve membrane derived from polyacrylonitrile for ehylene/ethane separation. Membranes, 2022, 12(1): 93. DOI:10.3390/membranes12010093 (  0) 0) |
 2022, Vol. 29
2022, Vol. 29


