2. MOE Key Lab of Micro-System and Micro-Structures Manufacturing, Harbin Institute of Technology, Harbin 150080, China;
3. State Key Laboratory of Robotics and System, Harbin Institute of Technology, Harbin 150080, China;
4. School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150080, China
In hot summer or during exercise, human need to maintain a stable body temperature by sweating. Sweat is an acidic fluid with a pH value about 4.0-6.8 which is secreted by eccrine and apocrine glands[1]. There is a lot of information substances in the sweat such as amino acids (AA), urea, glucose, lactate, and metal ions. This biomarkers information can be obtained in a non-invasive method instead of using a needle to penetrate the blood vessel, which makes it more suitable for designing wearable devices to detect diseases and monitor health data[2-4].
In systems biology, sweat belongs to skin metabolites, and the metabolomics aims to reveal the relationship between metabolites and some diseases mechanism[5]. There are many reports illustrating the relationship between skin metabolites and diseases such as cystic fibrosis, psoriatic skin lesions, atopic dermatitis (AD), and lung cancer[6]. Among them, the diagnosis of cystic fibrosis (CF) is currently mature and well-developed treatment method. In 1953, di Sant'Agnese and his colleagues[7]described elevated electrolyte concentrations in the sweat of CF patients. Since then, the sweat test became the gold standard for confirming the diagnosis of CF. After continuous improvement, the patients with positive family history whose sweat chloride is greater than 60 mmols/L were accepted as diagnostic of CF. However, the detection of skin metabolites is not routinely used in clinical diagnosis because of lacking standardized methods and clinical validation studies on large populations. At present, blood, urine, and feces are commonly used in clinical diagnosis for disease analysis, but the collection of these metabolites is always difficult and awkward. With the acquiring of wearable sweat sensors in the future, this situation will be improved gradually[8-9].
Another reason limiting the application of sweat detection is the difficulty of sweat collection. The traditional sampling techniques such as skin biopsy, suction blistering, microdialysis, and open-flow microperfusion are all in an invasive way, which may incur an uncomfortable experience[10-12]. With the development of non-invasive sampling techniques, the iontophoresis and sweat-inducing drug (pilocarpine) are proposed to get sweat without high environment temperature or exercise[13]. Because these perspiration-inducing methods may affect the composition of sweat samples, the better collection methods and standardized evaluation methods need to be developed. As the perspiration is difficult to collect, the absorbent materials (hydrogel, cotton pads, or filter paper) are used directly attached to the skin to extract sweat[14]. In recent years, microfluidic technology has been rapidly developed, and the use of microfluidics for sweat collection and analysis has gradually become widespread. The in-situ collection and analysis of sweat by microfluidic system can transmit the information to the mobile terminal through wireless device (Bluetooth and near-field communication (NFC))[8].
As early as in 1982, Shichiri et al.[15] proposed the concept of wearable devices for blood glucose monitoring. In 1997, the temperature wearable sensor was raised for fabrics systems[16], and the concept of electrocardiogram (ECG) wearable device was developed in 2000[17]. From the Web of Science, the number of research for wearable sensors is increasing year by year. Wearable sensors have developed rapidly in the past decade, mainly focusing on the physical information collection such as heartbeat[18], blood pressure[19], respiration[20], and body temperature[21]. In recent years, epidermal electronics technology has continued to progress, providing a basis for the development of wearable devices[22]. New materials and new technology were also applied in the wearable sensors for data process[23]. Qiu et al.[24] fabricated the skin thermal conductivity sensor by using a flexible thermosensor-based 3ω technology, which can detect the different skin status. Different from the common blood glucose monitoring, the physical processes do not involve chemical reactions, which makes it easier to convert into electrical signals for transmission. This real-time monitoring provides instant information for disease prevention and diagnosis. Sweat sensor is different from the physical information collection devices. It mainly focuses on the transmission of chemicals, especially electrochemical signals. Through the detection and analysis of biomarkers in sweat, the health status of the user can be explained, which can remind users to pay attention to their health and provide data support for disease prevention and diagnosis[25-26]. To advance the progress of sweat sensors, this article reviews the relationship between sweat composition and disease, as well as the newly emerging sweat sensors, and summarizes the main difficulties, and predicts the future direction of this field (Fig. 1).

|
Fig.1 Illustration of the researches in this article |
1 Sweat 1.1 Sweat Composition
Sweat is used to metabolize cells waste and ensure the stability of human body temperature in an overheated environment. Therefore, if a person does not sweat (anhidrotic patients) and the temperature is high or the person is doing exercise, the body temperature will rise continuously, which can lead to heat stroke. For the sweat section, sweat glands, as an excretion department of the body, can expel unnecessary substances (metabolic waste and toxicants) from the body[27]. There are three main types of sweat glands: eccrine, apocrine, and apoeccrine (Fig. 2). The eccrine sweat glands are the most widely distributed in the skin, so the researchers on the discharge of sweat mainly focus on the eccrine glands[2]. Therefore, sweat glands in this article refer to eccrine glands. Sweat is a complex aqueous solution, and the main components are water and NaCl. Its other ingredients include lactate, urea, glucose, etc., which can act as biomarkers for disease analysis. The current research mainly focuses on Na+, Cl-, K+, Ca2+, Cu2+, lactate, urea, ethanol, ammonia, glucose, heavy metals, antibodies, enzymes, peptides, and other proteins and AA[28].

|
Fig.2 Structure of different sweat glands (Reprinted with permission from Ref. [2]. Copyright 2019, Taylor & Francis Group) |
On the surface of our skin, there are also sebaceous glands widely distributed. Sebaceous glands are not sweat glands, but their secretions can affect the composition of sweat. The sebum contains more lipid-based triglycerides and fatty acids as well as cholesterol, wax esters, squalene, keratin, cellular debris, anti-microbial lipids, antioxidants, coenzyme Q10, vitamin E, and other various metabolites of fat-producing cells. So the sweat collected on the skin surface also contains sebaceous gland secretions[13, 29-30]. Since sebaceous glands and sweat glands are distributed together, their secretions are difficult to classify. Therefore, in this article, we will not distinguish between sweat gland components and sebaceous gland components in detail. The liquid that can be collected on the epidermis are collectively referred to as sweat.
In the stratum, the insoluble polyprotein profilaggrin produces monomeric filaggrin through dephosphorylation and degradation process. Subsequently, the proteolysis of filaggrin will turn protein into AA and their derivatives which are the natural moisturizing factors (NMF) on the skin. Although the excretory ability of sweat is far inferior to the kidneys, some special poisons are preferentially metabolized in sweat such as cadmium, lead, and mercury[31]. Some pharmaceutical drugs like amphetamines, cocaine, cannabis, opiates, and associated metabolites can be traced in the sweat[32]. Moreover, the surface of our skin still has some other compounds such as cosmetics or skin surface medication. The environmental pollution distributed on the skin surface also can affect the composition of sweat[33]. Therefore, the composition of sweat is complex and contains a variety of biomarkers which can reflect plenty of biological information.
1.2 Sweat CollectionThe perspiration phenomenon can be triggered by many factors such as exercise, high temperature, psychological state, relative humidity, drugs, diet, skin, and xenobiotics exposure. For sweat analysis, collection section means to get adequate amounts of sweat for analysis device, and the induction methods cannot pollute the sweat and the collection process is more patient friendly. The wildly applied method is the pilocarpine iontophoresis. The 0.5% pilocarpine nitrate solution with cholinergic parasympathomimetic activity which can stimulate the muscarinic receptors in the sweat glands is triggered by electrode for 5 min 1.5 mA electrical current and getting 50-60 μL sweat[34].
Other common forms for sweat collection are exercise, sauna, or hot weather. Different from the pilocarpine iontophoresis, the content of sweat exhibits obvious differences, and the airborne water droplets potentially containing bacteria, viruses, fungi, and/or xenobiotics can directly affect the sweat composition and concentration. Moreover, there are large variations between different individuals in different condition. Therefore, analyzing the sauna or exercise sweat always requires a control group[35].
Different sweat collection equipment display various analysis process. The current commercial sweat collection device including the Macroduct (ELITECH WescorⓇ Inc., Logan, UT, USA) device with a capillary-coil collection section and iontophoresis stimulation part can collect ~0.1 mL sweat, which is usually applied on the forearms[36]. Another device called MegaductⓇ increased the collection volume up to ~0.5 mL, which is suitable for long-time and profuse sweating. Apart from the catheter collection device, the sweat patches are also available for sweat gathering. The PharmCheckⓇ or PharmChekⓇ (PharmChem Inc., Fort Worth, TX, USA) with a medical-grade cellulose paper and a waterproof cover seal for sweat absorption. This collection method can allow subjects to have a bath and normal activates[37]. There are also other non-commercial sweat collection methods such as agarose hydrogel micropatch[38], glass rollers and glass pipettes sweat collection device[39], and skin surface components collection paper[40]. Moreover, the prosperity of the microfluidic device promotes the sweat collection and detection to a new field. The microfluidic device can directly combine injection, mixing, reaction, washing, separation, and detection into a centimeter-level chip[41]. By using the pressure of sweating itself, the detection part and collection part were integrated on the lab-on-a-chip device, which means the sweat can easily be collected on the sensing section, and the detection can be conducted on the chip. This integrated device opens up more possibilities for wearable sweat sensors.
In the present wearable sensor collection units, the microfluidic system, hydrogel[42], and superhydrophobic-superhydrophilic microarrays[43] are introduced for sweat collection. At present, various collection methods and collection equipment can be selected for different analytes, and some standardized collection models have been published[44]. But due to the large individual differences in sweat, the standardized analysis of collection is still very difficult.
1.3 Wearable Sweat Device Opportunities in Disease and HealthcareWearable devices generally refer to devices that can be worn daily to monitor human physiological activities. The non-invasive point-of-care testing methods have aroused the interest of many researchers because it can monitor activities of users all day and provide medical interpretation when necessary[45-46]. Nowadays, wearable devices have been widely used in sports for monitoring sports condition, in healthcare for real-time detection of heart rate, blood pressure and breathing and in environmental detection for detecting work state[47]. Major technology companies, such as Apple and Huawei, have launched their own smart watch for healthcare[48]. In December 2021, Huawei released a new products named HUAWEI WATCH D which can monitor blood pressure, ECG, blood oxygen, sleep, stress, and body temperature. The supporting APP can customize a personalized health management plan for users according to their health data, and manage their daily health with the reminder function of the watch. The current wearable devices are generally designed as watches, wristbands, or glasses, and connected to mobile terminals via Bluetooth technology or NFC.
Sweat sensors are different from other physical information sensors. It is more likely to detect chemical components. Therefore, the goal of the sweat sensor is to evaluate the health status through monitoring biomarkers in sweat, which can be used as evidence for clinical diagnosis when necessary. Moreover, sweat as a metabolite can monitor drug intake, and analysts can analyze the drug ingested through sweat metabolism. This method provides a rapid and real-time detection way of alcohol and drugs or doping abuse[49].
Apart from the components of sweat, the speed and time of sweating is also a health signal. For instance, the sweat retention syndrome, thyrotoxicosis, and Parkinson's disease (PD) are all reported with abnormal sweating rate. Besides, diabetes, as a common senile disease, is reported sweating at night. These changes of sweating often indicate the poor health condition and the wearable sweat sensor can remind users of these abnormal situations[50-51].
It is hoped in the future that wearable system will connect the sensors of the whole-body for personalized analysis according to everyone's living habits, and the rapid development of big data tools also brings sweat sensors more opportunity. The health data from the whole-body can be updated to the Cloud, and the system can summarize it and get a health model by analyzing the data of each user, which builds models to evaluate the health status and risk assessment for predictive analytics. It can not only provide data for clinical diagnosis, but also can be used to analyze the environmental conditions of a country or region.
2 Sweat-Related Disease DetectionFor detecting disease related biomarkers, the plasma[52] and urine[53] or tissues are the analysis materials in current clinical diagnosis. Therefore, there are more challenges and possibilities for sweat noninvasive diagnosis, and current studies about sweat biomarkers for human disease have been summarized in Table 1.
| Table 1 Sweat related diseases |
2.1 Sweat as Biomarkers of Skin Disease
The skin is the first barrier of human immunity, and sweat plays an important role in maintaining its stability. It transports water, moisturizing factors, and antimicrobial peptides to the skin[65]. Therefore, changes in sweat are closely related to many skin diseases. Mark and Harding[3] analyzed the contents of AA and its derivatives in sweat. The results show that the average sweat rate of the subjects was 1.03 mL/20 min, and the AA content in sweat was 3161.1 μmol/L. They found serine showed the most content which accounts for over one-fifth of the free AA. The next abundant composition is glycine followed by 5-carboxylic acid, alanine, citrulline, and threonine, respectively. The data of this article shows the content of AA in sweat is very similar to that of epidermal protein profilaggrin, and the free AAs are the main source of natural NMF for stratum corneum. It plays an important role in maintaining skin moisture, keeping skin stability, and resisting external disturbances. Therefore, if the amino acid composition in sweat changes, it will affect the NMF. AD can be reflected by detecting the composition of AAs in sweat[3].
Psoriasis is a chronic and immune-mediated inflammatory skin disease which 2%-3% of the global population suffer from[55]. It is considered the dysregulation of keratinocyte differentiation, disruption of the skin barrier, and immune dysfunction caused the skin problem[66]. Dutkiewicz et al.[67] used biocompatible hydrogel micro-patch probes for skin metabolome collection. By comparing 100 psoriatic patients and 100 healthy individuals, the sweat metabolites, including choline, glutamic acid, phenylalanine, lactic acid, urocanic acid, and citrulline, are associated with the plaque severity scores. Dutkiewicz et al.[67] also used hydrogel micro-patch sampling technique for psoriasis skin research. The choline (r=0.7847, p < 0.0001) showed positive correlation and citrulline (r=-0.6015, p < 0.0001) correlated negatively with the general disease severity.
Another skin surface disease related to sweat is AD. The perspiration blocked leads to the impairment of sweat glands, and the sweat leakage flows into tissues causing itch serious. It is found that the sweat of AD patients showed obvious variation in protein (F=52.67 (p < 0.0001)), sodium (F=1.316 (p=0.6942)), salt (F=1.612 (p=0.5029)), andanti-microbial peptides (LL37 (F =8294 (p < 0.0001))), and β-defensin, (F = 3709 (p < 0.0001)). Through NMR spectra, the glucose concentrations of AD patients (mean±SD: 33.54±45.57, n=21) was higher (p=0.0331) than healthy subjects (mean±SD: 0.999±0.2053, n=10), which displayed positive correlation with severity[54, 68].
Skin metabolites also include volatile organic compounds, and it can also act as biomarkers in wearable sensors. Abaffy et al.[56] compared melanoma and non-neoplastic skin with the headspace solid phase micro extraction method. The levels of lauric acid and palmitic acid increased which displayed a character of cancer. The metabolic profiling data of basal cell carcinoma has been investigated by using high-resolution magic angle spinning nuclear magnetic resonance, which have found 9 different metabolites compared with the normal skin tissue[69].
2.2 Sweat as Biomarkers of Systemic DiseaseIn addition to skin diseases that are directly related to sweat, some systemic diseases have also been proved to be related to the sweat disorder. CF is a hereditary exocrine gland disease which is caused by the disordered gene encoding CF transmembrane conductance regulator. The transmembrane transport of water and electrolytes in the mucosal epithelium of the respiratory tract is impaired, and the content of acid glycoprotein in the mucous gland secretion increases, which changes the rheological properties of the mucus[57, 70]. The patient's epithelial cell chloride channel regulation is defective, which means it can be diagnosed by applying the sweat chloride test[71-72]. In 1953, di Sant'Agnese and his colleagues put up a standard diagnosis method. If the patients' sweat concentration of Cl- is greater than 60 mEq/L, it can be highly suspected of CF[44].
PD is a common degenerative disease of the nervous system in middle-aged and elderly people. Trivedi et al.[58] used sebum as biofluid for detecting PD. They met a super smeller named Joy Milne who has an extremely sensitive nose. She can tell the difference in smell between patients with Parkinson's and others. Through cooperating with the super smeller, this group considered perillic aldehyde and eicosane were the biomarkers on the Parkinson patients' skin.
Lung cancer is the health killer with the highest mortality rate. Despite the progress made in diagnosing and therapy, the detection of lung cancer is still difficult in early stage. Therefore, there is an urgent need for rapid and convenient lung cancer detection methods. Calderon-Santiago et al.[60] obtained the scope of lung cancer detection by comparing metabolites in sweat of lung cancer patients, smokers, and non-smokers. This method can discriminate the smoker and lung cancer patients, which can avoid false positives, and 5 metabolites (p < 0.008) were considered as the biomarkers of lung cancer. Another lung-related disease is active tuberculosis. By conducting a global proteomic profile, Adewole et al.[61] found the 26 proteins exclusively found in active tuberculosis patients. The proteins related to auxiliary transport (1 vs. 0%), enzyme regulation (19 vs. 7%), and membrane proteins (39 vs. 27%) were richer than other lung disease patients, and ribosomal proteins were only detected in tuberculosis patients. The experimental results indicated that the sweat of active tuberculosis patients is significantly different from other lung diseases and healthy controls, and it is expected that active tuberculosis detection can be carried out by detecting sweat.
VKH disease generally caused eye disfunction and its pathogenic mechanism is not clear. Cui et al.[62] investigated sweat samples acquired from the patients suffered from VKH disease, and discovered 116 proteins and 21 expressed metabolites had abnormal changes. Their research is of great significance for the breakthrough of the pathogenic principles and diagnosis of diseases.
Malaria is a highly lethal disease in the world, so the screening of asymptomatic infections is very important. de Moraes et al.[73] reported that it can distinguish malaria patients before microscopy detection by analyzing skin metabolites.
2.3 Sweat as Biomarkers of Mental DiseaseSchizophrenia is a neurodevelopmental disorder of unknown etiology. The skin lipid was studied by Smesny et al.[59] It was found that the skin of schizophrenia exhibited alterations of specific peripheral sphingolipid. Through multi-group data analysis, the ceramide (NH/AS and AH) revealed a significant difference, which indicated the sphingolipids can be regarded as the biomarkers of schizophrenia. The proteomic analysis of the sweat has been investigated by Raiszadeh et al.[34] They utilized liquid chromatography-tandem mass spectrometry (LC-MS) and multiple reaction monitoring mass spectrometry (MRM-MS) to show the difference of schizophrenia, and they discovered 17 proteins with abnormal abundance for schizophrenia.
Major depressive disorder is a complex psychological and physical disease. The person who suffers from the mental disease always show social impairment and the mortality rate of this diseases is high[74]. Cizza et al.[63] studied the neuroimmune biomarkers of the patients with major depressive disorder, and found the concentration of proinflammatory cytokines, neuropeptide Y, substance P, and calcitonin-gene-related peptide was higher and vasoactive intestinal peptide was lower than the control group, and all the related components exhibited strong correlation in sweat (r=0.73-0.99, p < 0.004). Moreover, cortisol is considered a biomarker for mental stress, and the increasing of sweat cortisol concentration is related to high mental stress[75-76].
2.4 Monitoring Effects of Administered DrugsDrug abuse will cause serious damage to the human health and it is very addictive, which can lead to a great impact on society and the economy[77]. Thus, a rapid drug detection is essential for identifying addicts. The pharmacological metabolites, such as opioids[78], tetrahydrocannabinol[79], and methamphetamine[80] can be detected in the sweat. Pichini et al.[81] explored the concentration of 3, 4-methylenedioxymethamphetamine in sweat after a single 100 mg dose. Moreover, drunk driving is a very dangerous behavior, and it is vital for analyzing alcohol intake. Buono et al.[82] compared the alcohol concentration of sweat and blood, which displays a strong correlation, and the ethanol occurring in the sweat gland distributed evenly. It suggests there is a correlation between blood alcohol and sweat alcohol, and the distribution of alcohol in sweat is rapid and equalized. Fig. 3 shows the relationship between disease and biomarkers, and the abnormal signals are usually related to a lot of disease risk. Therefore, the wearable sweat devices for biomarkers sensing is needed for disease prevention.

|
Fig.3 Sweat metabolites that can be regarded as disease biomarkers |
3 Wearable Sweat Detection Sensors
Wearable sweat sensors are the personal health care devices for disease prevention and sports management. There are several detection methods for sensor construction which can divide the sensors into three types: electrochemical-based sensors, colorimetric-based sensors, and transistor-based sensors. This section summarized the design strategy of reported wearable sweat sensors and compared different detection ways and biomarkers, which is displayed in Table 2. A wearable sweat sensor generally includes 4 function parts: a flexible substance, a sweat collection part, analysis units, and supporting circuit or reference unit.
| Table 2 Comparison of different sweat sensors |
3.1 Substrate Material and Sweat Collection Unit
As a sweat sensor, it is generally required to be flexible and it can collect sweat close to the skin, which can reduce the pollution and evaporation. Therefore, different flexible polymers, such as polydimethylsiloxane (PDMS), polyethylene terephthalate (PET), polymide (PI), poly(methyl methacrylate) (PMMA), P(VDF/TrFE), and polyurethane (PU), show different properties and have been applied as the substrate.
PET is formed by esterification of terephthalic acid and ethylene glycol and followed by polycondensation. The PET has good mechanical properties and insulating ability and it is easy to form a film. Gao et al.[84] processed the PET substance by photolithography and the sensors located on the PET substance. PET has good flexibility which can be tied around the wrist or forehead. But PET has poor air permeability which may cause discomfort and itching on their skin. Roy et al.[102] fabricated a carbon nanotube-based ion sensor by facilitating PI as the substrate support material which can stick on the skin. This method improved the skin comfort, but it is difficult for this sensor to maintain the stability with the accumulation of sweat. Anastasova et al.[96] used PMMA as the sweat collection and packaging material. It can protect the paper microfluidic and the sweat can outflow the sensor which showed great potential for sweat continuous monitoring.
However, the thicker PMMA, PU, PET, or PEN substrate is hard, and the thin materials are easy to slit. Moreover, these polymers are difficult to form in the laboratory and generally require larger equipment. Although the PDMS shows mild swelling, it is often selected as substance for its simple synthetic method. For microfluidic devices, the PDMS is widely selected for it is easy to make channels, which means that PDMS can both act as the packaging and collection unit. As a sweat sensor, it is necessary for sweat rate monitoring to design channels of the sweat. Koh et al.[89] produced a serpentine sweat channel with PDMS to observe the flow rate of sweat. Parrilla et al.[103] synthesized a PU substrate with sweat collection channel for ions detection. The excellent flexibility, low elastic modulus, and great biocompatibility made this polymer very suitable for fitting skin and collecting sweat[104]. Seghir and Arscott[105] studied the hardening agent and the curing process of PDMS fabrication, and it showed the PDMS can be controlled from 800 kPa to 10 MPa with a rupture limit higher than 20%. Moreover, the surface of PDMS can be adjusted by various surface treatments, including oxygen plasma, ultraviolet exposure, and chemical functionalization, which made it strongly adhesive to the skin and other electronic components[106-107]. Through transfer printing, the PDMS can be equipped with microstructures which can improve the stretch ability and relieve the strain from the substrate[108]. Li et al.[109] synthesized a 2D wavy structure causing omnidirectional stretch ability up to 30%. For more applied examples, the utilization of PDMS is also illustrated in Section 3.2.
At present, flexible substrates have been widely used in sweat sensors and other wearable devices. At present, the more serious problem is aging, and it is a key problem for the flexible materials to keep good properties in the long-term high temperature environment.
3.2 Sensor UnitsThe sensing units are the core component of the sweat sensors, and there are a lot of biosignals that can be conducted. The most common analytes are the ions including Na+, Cl-, K+, Ca2+, H+, and NH4+[84, 94, 96], and the selective ionophores are used to sense it. Ionophores can induce ionic activity generating a specific electrical potential. Based on the Nernst equation, the logarithm of the ion concentration is linearly related to the voltage. By monitoring the potential of the electrode, the concentration of the target ions are recorded by the sensors. Gao et al.[84] employed Na+ and K+ selective membrane on the electrode to form the special ion electrode. Nyein et al.[93] fabricated a wearable sweat sensor for Ca2+ and pH monitoring. The Ca2+ selective ionophore and aniline, sensitive to pH, were obtained to synthesize selective electrode. Moreover, the sweat sensors can also detect some metabolites such as glucose[84], lactate[96], ethanol[99], uric acid[100], cortisol[64], and adrenaline[101]. The lactate, glucose, and ethanol have their own oxidases, and the oxidases can be fixed on the electrode to arouse the electrochemical signals changes.
The enzyme catalyzes the reduction-oxidation (redox) reaction to arouse the electron transfer process, and the current is linearly related to the concentration of target components, which is recorded and processed by the sensor. An electrochemical tattoo was fabricated by Windmiller et al.[100] They used carbon fiber skin-based ink to form a sweat sensor for uric acid.
Other structure sensor units are based on the transistor. The selective materials are located on the gate electrodes or channels and the signals are trigged by the change of gate voltage affected by the target components. The source-drain current of the sensor can be expressed by the following formula:
| $ I_{\mathrm{ds}}=\mu n \mathrm{e} \frac{W}{L} V_{\mathrm{ds}} $ |
where μ is the mobility of the semiconductor, n is the carrier density, e is the electronic charge (1.6 × 1019), Vds is the source-drain voltage, and W and L represent the width and length of the channel. If the semiconductor material is an intrinsic semiconductor and the carrier density is all supplied by the gate voltage, the formula can become
| $ I_{\mathrm{ds}}=\mu \frac{W}{L} C_{\mathrm{g}} V_{\mathrm{ds}}\left(V_{\mathrm{g}}-V_{\mathrm{th}}\right) $ |
where Cg is the effective gate capacitance and Vth is the semiconductor surface potential. The above formula also changes slightly depending on different semiconductors. With the stable Vg, the signal of the chemicals can be reflected by the change of Ids. Parlak et al.[64] constructed an organic electrochemical device with cortisol molecularly imprinted polymers (MIPs) for selectivity. Moreover, Coppede et al.[101] monitored human stress by detecting adrenaline in sweat. This sensor has been modified on the cotton, which provided a new strategy for wearable sensors. By classifying the structure of the sensing part, the sweat sensors can be divided into three categories: colorimetric-based sensors, electrochemical-based sensors, and transistor-based sensors.
3.2.1 Colorimetric-based sensorsThe colorimetric method means the sweat sensor detects biomarkers by showing the color change, and the mobile terminal analyzes the data by analyzing the color change, which makes the users feel the difference intuitively. Koh et al.[89] fabricated a colorimetric sensing platform which can analyze lactate, chloride, pH, water, glucose, and chloride. The lactate detection range is about 1.5-100 mM, the limit of detection of glucose is about 200 μM, and the pH range is about 5.0 to 7.0. In Fig. 4(a), the sensor section is formed by 3 layers, and the colorimetric dye is sealed in the PDMS microfluidic channel. The microfluidic part for sweat collection and the NFC electronics for data transfer are on the first layer. There are some image process markers on the top for assessing color change. The white and black dots were reference markers to balance the light, and the four black crosses allowed rotations/translations of the images to figure out the sweat rate. The colors were transformed into %RGB format, and the concentration of the analytes was concluded by calibration curves. This work displayed a good colorimetric method for detecting sweat components, but its detection range and material fit still need to be improved.

|
Fig.4 Wearable sweat sensors by colorimetric method (a) Schematic of the sweat sensor's design. (Reprinted with permission from Ref. [89]. Copyright 2016, American Association for the Advancement of Science.) (b) Schematic of the production process of the thread/fabric-based sweat sensor. (Reprinted with permission from Ref. [90]. Copyright 2021 Royal Society of Chemistry.) (c) Synthesis and detection process of self-healable hydrogel patch. (Reprinted with permission from Ref. [42]. Copyright 2021 Royal Society of Chemistry.) (d) Detection phenomenon of the sweat sensor for sweat loss, pH, glucose, and lactate detection. (Reprinted with permission from Ref. [111]. Copyright 2019 Royal Society of Chemistry.) |
In order to get better material stability, the researchers made improvements to the base material. Wang et al.[42] reported a flexible and self-healable hydrogel patch for colorimetric sweat detection (Fig. 4(c)). The solvent displacement method was used for colorimetric hydrogel synthesis, and it can stick to the skin spontaneously. This method incorporates dye into the hydrogel and the material has better sticking ability than PDMS. The sensor displayed a stable measurement for pH, glucose, Cl-, and Ca2+, which is associated with the microphone APP for health management. The limits of detection is 4.23 mM, 0.028 mM, 12.69 mM, and 0.56 mM for pH, glucose, Cl-, and Ca2+, respectively. He et al.[43] also constructed a patch for pH, glucose, Cl-, and Ca2+ detection. In order to increase the ability of collecting sweat, this skin-mounted band was equipped with superhydrophobic-superhydrophilic microarrays with nanodendritic colorimetric biosensors. The superhydrophobic silica repelled the sweat to the superhydrophilic section which enhanced the ability to absorb sweat.
In addition to the patch type sweat sensor, Zhao et al.[90] fabricated a thread/fabric-based band for sweat sensing. As is displayed by Fig. 4(b), this thread/fabric-based analytical device included three layers. The PDMS layers covered the top of device to protect the sensing part. The second layer in the middle was responsible for the detection, the threads were dyed by colorimetric indicators, and the fabrics were hydrophobic-treated. Through embroidery, the superhydrophobic-superhydrophilic structure layer formed a sweat collection and analysis part, and a piece of thread was drawn out of the back for absorbing sweat. There are reference dots with various color around the sensor point, and the black and white dots are reference for RGB value calculation. For chloride, the detection range was about 10 to 150 mM, pH range was about 5.0 to 7.5, and that for glucose was about 10 to 2000 μM. The bottom layer closed to the skin were also PDMS. It can protect the sensing layer with a piece of thread for absorbing. This sensor is more suitable for loading on fabrics or clothes for easy wearing.
Part of the roles for the sweat sensor is to monitor movement and sports, and part of it is to detect disease. At present, the more mature technology is to detect CF by analyzing chloride ion concentration[71]. Zhang et al.[110] constructed a microfluidic system for biomarkers relevant to kidney disorders. The concentrations of creatinine, urea and pH can be analyzed by the color calibration markers, and the sweat rate and sweat loss can be evaluated by the microscale channels with a paper-based chemical assay or enzymes for colorimetric comparison. The reference color point showed the pH range from 5.0 to 7.0, the range of creatinine is from 0 to 0.5 mM, and urea range was from 0 to 250 mM. By utilizing the reference dots, the users can recognize the concentration by themselves, which made it more convenient for disease prevention. This analytical method did not require sweating through exercise and clinical medicine, which made it easier for infants and the elder to collect sweat. Sweat can not only be monitored as health signals, but also sense diseases by early warning.
Sweat detection exhibits great potential for point-of-care diagnosing. Zhang et al.[111] fabricated a sweat sensor for pH, glucose, and lactate detection (Fig. 4(d)). Among them, glucose can be used as a potential biomarker for diabetes. The glucose content in sweat is low, which is difficult for colorimetric detection. By optimizing the intake, evaporation, and storage of sweat, they concentrated the indicator on a small zone to improve the colorimetric detection sensitivity. This device can detect glucose in the range of 50 to 300 μM, which is in the physiological glucose concentration. This colorimetric method can be intuitively felt, and can also be measured more accurately through a mobile phone, which made it suitable for personalized health care and disease prevention.
Through the color change of the sensor sections, the colorimetric-based sensors showed great detection ability. The users can intuitively get the sweat state with their eyes, which is convenient for disease warning. Moreover, the colorimetric-based sensors do not require circuit support, making the sensors more compact and portable. However, the obvious disadvantage of this sensor is that it is difficult to quantify and easily affected by skin color or environment light, and the color changing substance sealed in the packaging material may become aged and deteriorated.
3.2.2 Electrochemical-based sensorsThe colorimetric method has the embarrassment of visual identity, which is more subjective and easily affected by skin tone. The electrochemical method is suitable for conducting chemical signals into the circuit, which facilitates the integration of equipment. The electrochemical detection has fast response and high sensitivity, and can directly output electrical signals, which is convenient for data analysis. Parrilla et al.[103] constructed a wearable ion monitoring sweat sensor, and it contains H+, Cl-, K+, and Na+ sensor channels embedded in flexible PU substrate (Fig. 5(a)). The electrodes included five circular patterns and the electrode path was fabricated by screen-printing technique. Subsequently, the sensor parts were functionalized by multiwalled carbon nanotubes (MWCNTs) to improve conductivity and covered by selective membranes for ions detection. This sweat sensor showed good prospects in detecting physiological and personal data of body during sports.
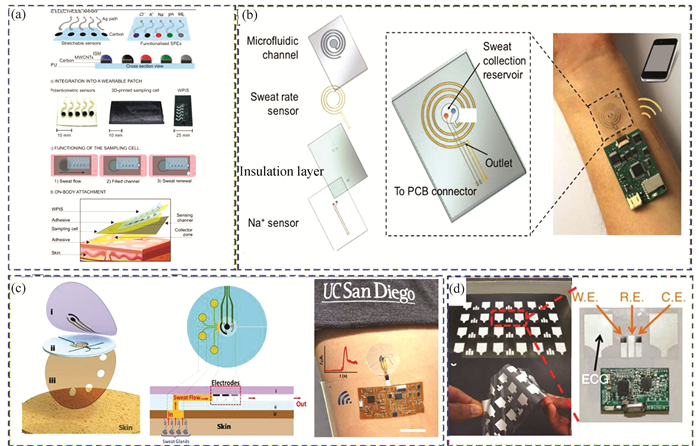
|
Fig.5 Wearable sweat sensors by electrochemical method (a) The design of the wearable sweater sensor for ions sensing. (Reprinted with permission from Ref. [103]. Copyright 2019 American Chemical Society.) (b) Structure schematic of sensing mode of sweating rate. (Reprinted with permission from Ref. [112]. Copyright 2018, American Chemical Society.) (c) Schematic of the structure for sweat collection. (Reprinted with permission from Ref. [97]. Copyright 2017, American Chemical Society.) (d) Structure of hybrid biosensor for lactate and electrophysiological signal detection[116] (Reprinted with permission from Ref. [116]. Copyright 2016, The Author(s)) |
Gao et al.[84] fabricated a fully integrated wearable sweat sensor for in site perspiration analysis. The sensor was based on the PET substrate with a stable skin contact. The sweat metabolites glucose and lactate, electrolytes Na+ and K+, and skin temperature were analyzed by selective electrodes and resistance-based temperature sensor, which divided the detection section of the sensor into seven parts. The glucose and lactate sensor electrode were modified by glucose oxidase and lactate oxidase. The sensitivities of glucose and lactate were 2.35 nA/mM and 220 nA/mM, respectively, and those of Na+ an K+ were 64.2 mV and 61.1 mV per decade of concentration. The ion-selective electrodes (ISEs) were fabricated by ion-selective members and temperature sensor parts were synthesized by Cr/Au metal microwires. The top layer was an insulating layer to prevent electrical contact. All signals were processed by an FPCB with Bluetooth which outputs the data to mobile handset containing sharing and uploading servers. This wearable device displayed good potential for profiling sweat details and monitoring human activities, and it was suitable for personalized health information management through data analysis. This device is very suitable for dynamic monitoring of changes in sweat composition during exercise, but it is not suitable for the non-exercise states. At the same time, the device has no sweat transport channel, which is not conducive to the device closing to the skin.
For individuals in sedentary, another work also was conducted by Emaminejad et al.[87] They built a sweat extraction system for CF diagnosing and glucose monitoring by adding iontophoresis interface in the analysis system. The sensor was constructed on the PET substrate with Cl- and Na+ selective electrodes with the iontophoresis electrodes around the detection zone. The hydrogels covered by thin stainless steel were located in the sensor-skin contact part which contained stimulating compounds such as pilocarpine for promoting sweat. The sensitivities of Cl- and Na+ were 63.2 mV and 55.1 mV per decade of concentration, and the Cl- and Na+ concentration performed as biomarkers for CF and the glucose level relating to blood served as diabetes warning. The sweat device exhibited two modes for both active and passive sweating which displayed wide application. But the device without sweat collection section was difficult to maintain a long-term close to the skin.
For long time monitoring, the microfluidic systems still manifest great observing advantages, and it can also be used in the electrochemical method. Nyein et al.[112] fabricated a spiral-patterned microfluidic platform for ions and sweat rate detection(Fig. 5(b)). Moreover, Martin et al.[97] combined lab-on-a-chip and electrochemical process in a flexible electronic board. It accomplished the real-time monitoring for glucose and lactate concentration (Fig. 4(c)). The sensitivities of glucose and lactate was 29.6 μM/μA and the limit detection was 50 μM. The small devices were easy to fix on the skin and had good elasticity. The electronic board can transmit data collected from sensor part to the mobile, which displayed potential for personalized sports training and diabetes disease detection. However, the portability of the battery is not conducive to the miniaturization of the device. Huang et al.[113] constructed an self-powered sweat sensor for glucose and lactate detection. The sensitivities of lactate and glucose were 2.48 mV/mM and 0.11 mV/μA, respectively. They employed enzymatic biofuel cell as the power supply section to minimize the device, which showed more energy efficiency for in situ detection.
For medicines intake and disease prevention, Bhide et al.[114] reported a sweat sensor for simultaneous detection of alcohol and glucose in ultra-low volumes, which was conducted by a gold-zinc oxide (ZnO) thin film electrode. The sensing region was immobilized by selective enzymes, and the electrochemical method impedance spectroscopy (EIS) and chronoamperometry (CA) was applied as biomarkers signal. Levodopa is the medication for PD. Monitoring levodopa level benefits the understanding of patients' physical and emotional condition. Tai et al.[115] fabricated a wearable sweat band for levodopa detection, which can track the real-time pharmacokinetic metabolism. The work electrode was modified by tyrosinase enzyme which can oxidize levodopa to dopaquinone and the sensitivity of levodopa is 15 nA/μM. The current signals can be processed and transmitted to the mobile phone through the circuit board facilitating the management of PD.
To improve the integration of sweat sensors, multiple sensors are attached together. Imani et al.[116] synthesized a chemical-electrophysiological hybrid biosensor for health care. As Fig. 5(d) shows, it combines lactate detection and ECG in one skin-worn device for simultaneous monitoring. The three-electrode lactate sensing part printed on the polyester sheet with bipolar ECG sensor and this system showed negligible cross-talk which meant the chemical sensor can be associated with physiological signal sensor. It is the trend of the future for different sensors on the body to jointly analyze various physiological signals to monitor the physiological state.
It is easy for the electrochemical-based sensors to convert the chemical signals into electrical signals, which makes the data easier to transmit, process, and record. But the companion circuit is bulky, and the electronics are not flexible enough to contact the skin. Multi-channel data integration and analysis and the establishment of individual models remain unstudied.
3.2.3 Transistor-based sensorsThe transistor-based sensors are based on the transistor principle for sensing target molecules. In general, the gate induced ion transfer of the analyte, and the semiconductor layer between source and drain electrode were changed, which can amplify the sensing signals. Sweat has a high content of ions, so many studies have reported the detection of ions[117-118]. For instance, Zhang et al.[91] fabricated an ion sensitive field effect transistors for sweat ions analyzing, which displayed high selectivity and ultralow power consumption. They used commercial CMOS to process and integrat the circuit communicating with the mobile phone by NFC. The analytes (pH, Na+, K+, Ca2+) sensitivities were 58 mV/pH, -57 mV/dec(Na+), -48 mV/dec(K+), and -26 mV/dec(Ca2+). Moreover, Keene et al.[92] also fabricated a sweat sensor for detecting calcium and ammonium ions. The ion selective members were covered by poly(ethylenedioxythiophene): poly(styrenesulfonate) (PEDOT: PSS) which formed the ion detection section. The constructed methods can form any cation of interest in general by changing the ionophores.
Cortisol, a well-known biomarker for human stress, is a hormone related to regulating metabolism and immune response. There are several reports about cortisol detection[119-121]. Parlak et al.[64] reported a sweat sensor for sensing cortisol by introducing the electrochemical transistor (Fig. 6(a)). The sensor were formed by three layers, the sample reservoir layer at the bottom, and the water-proof protection layer on the top. The molecularly selective organic electrochemical transistor (OECT) in the middle layer were constructed by MIPs and PEDOT: PSS which can selectively recognize cortisol. This device shows great detection ability with noninvasiveness, ease of operation, and user comfort for monitoring user's mental stress.
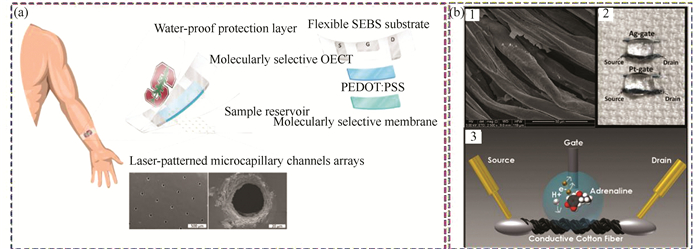
|
Fig.6 Transistor-based biosensors (a) Schematic of the wearable cortisol sensor. (Reprinted with permission from Ref. [64]. Copyright 2018 American Association for the Advancement of Science.) (b) Depiction of wearable adrenaline sensor. (Reprinted with permission from Ref. [101]. Copyright 2014 Royal Society of Chemistry.) (1) SEM image of the cotton wire modified by PEDOT: PSS. (2) Image of the cotton-OECT located on the cloth (3) Schematic of the detection process. |
Adrenaline is another hormone delivered by kidney which is secreted when a person is nervous or excited, and it is prohibited in the field of competitive sports. Therefore, it is urgent to monitor adrenaline in a fast, non-invasive, and portable way. Several analytical methods have been applied to sense it including electrochemical analysis[122-123] and fluorescence detection[124]. Mak et al.[125] and Coppede et al.[101] fabricated analogous OECTs by using Ag gate electrode and PEDOT: PSS. Mak et al.[125] synthesized OECT by deposited Ti/Pt electrode on the glass substrates and detected the adrenaline in the sample solution. Coppede et al.[101] constructed an adrenaline sensor by using cotton fiber. The cotton fibers were immersed by the PEDOT: PSS aqueous solution and followed by baking them at 150 ℃ for 3 hours, and the SEM image is exhibited in Fig. 6(b1). This process did not change the softness of cotton thread, and it can be fixed to the fabric (Fig. 6(b2)). The Ag and Pt wires were gate electrodes and the cotton fibers modified by PEDOT: PSS were source and drain electrodes, which shows selective detection performance (Fig. 6(b3)).
Because these sensors convert chemical signals to electrical signals, the transistor-based biosensors are similar to the electrochemical-based sensors. Sensors of this type can amplify the current signal and shows more flexible structure. However, the supporting circuit is still unclear and completely independent from usable devices which need to be developed. Same as the electrochemical-based sensors, the assembly of the supporting circuit, especially the battery, still affects the comfort of its skin fit. In the future, with the advancement of flexible electronic components and flexible batteries, the application of sweat sensors will be greatly improved.
3.3 Circuit Design UnitsAnother sensing component is the data transmission equipment. The electronics to perform monitoring schemes, process signals, and pass the data to mobile terminal are required to construct on a flexible substrate. The printed circuit board (PCB) has a good application prospect, as it can be industrially produced with existing industrial processes. As shown in Fig. 7, the whole PCB section is controlled by the microcontroller unit (MCU) including input and output units. The output part converts the digital signal into an analog signal through the DAC to control the external circuit, and the signal obtained by the sensor is processed by the ADC. The processing mainly includes amplifier, filter, ADC, other analog signal processing circuits, and MCU.
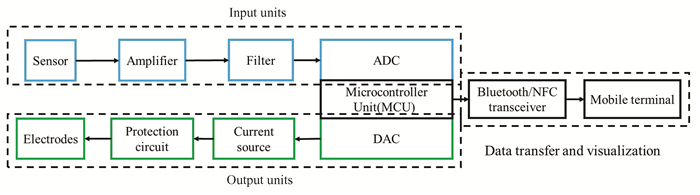
|
Fig.7 Schematic of the signal processing and transmission |
Gao et al.[84] integrated multiplexed sensing system consolidated on a flexible printed circuit board (FPCB) which solved the wearable problem. The received electric signal is current, voltage, or resistance information. Due to the weak current signal output by the sensor, the current signal was first to be processed by transimpedance amplifier, and then it is input to MCU through inverter and low-pass filter. For voltage signal, the voltage buffer, differential amplifier, and low-pass filter can be employed to process the voltage information. The resistance signal is processed by voltage divider, and all the signal can be processed by MCU. The MCU can connect to the Bluetooth module which can transmit information to the mobile terminal.
When doing exercise or the weather is hot, the sweat is easy to get. If the users are stationary, how do we get the sweat? The iontophoresis section was included to stimulate sweating. The hydrogels containing sweat-inducing drug were under the electrodes, and it is controlled by the current. Emaminejad et al.[87] fabricated an electrochemically enhanced iontophoresis interface with pilocarpine located in the hydrogels and used FPCB module to consolidate the whole sensor system. The DAC unit controlled the current source connecting to the hydrogel electrodes with the protection circuit. According to the sensor with different structure, its corresponding circuit design also needs to be adjusted. Zhang et al.[91] constructed ion sensitive filed effect transistors (ISFETs), and it can be integrated with complementary metal oxide semiconductor (CMOS).
Depending on the sensing unit, different sensing circuits can be configured in various formats. At present, the current circuit design is complete, but there is a lack of interpretation and advice of the data in the feedback.
4 Concluding Remarks and Future PerspectivesThe diseases related to sweat metabolites and the sweat sensors for sweat metabolites detection and monitoring are reviewed in this paper. There are many excellent experimental results that have been reported, and the wearable sensors for perspiration analysis show great potential for monitoring personal activities and sensing diseases. The integration of several chemical sensor parts can simultaneously transmit detection signal to the smart phones by wireless transmission module, which allows users to monitor their health in real time. With the advancement of medicine, there will be more and more biomarkers for disease monitoring, and sweat management will be closely related to the prevention of diseases and personal health care. For a specific disease, not only can the sweat sensor be used for prevention, but also its monitoring capabilities can be used to adjust the treatment plan in real time. For instance, the number of diabetic patients is increasing year by year, and it shows a trend of younger age. The glucose in sweat can be used as an biomarker of diabetes, and the sweat patch can monitor drug abuse[126]. According to the International Data Corporation (IDC), total global shipments of wearable devices reached 138.4 million units, with a year-on-year increase of 9.9%. Wearable devices have good application capabilities in disease prevention, detection, monitoring, and adjuvant therapy. Although wearable sweat sensors have achieved many results, there are still many problems that need to be solved.
(1) User experience
The sweat sensor needs to be close to the skin to maintain work condition, but the current supporting circuit equipment has poor flexibility and heavy weight, which affects the user experience. In the future, with the development of flexible electronic devices, screen printed circuits, and flexible batteries, the sweat sensor performance will be further improved. At the same time, improving the service life of flexible materials and delaying the aging of the flexible polymer will also improve the applicability of sweat sensors.
(2) Linking to proteomics
From the sweat related diseases summarization, there are a lot of disease metabolites which are proteins or peptides, but the wearable sweat sensor research about proteins is few. The analytes in sweat such as ions, lactate, and glucose are in high concentrations, which makes it easier to detect. However, the proteins and peptides in trace amounts, which shows extraordinary relation to many diseases, are hard to detect. These molecules are regulatory or signaling-function biomarkers which reflect changes in the body. Detecting the proteins and peptides allows us to better monitor the body's metabolism and accumulate data for clinical disease research.
(3) Analysis and processing of multiple signals
At present, some articles have reported on the simultaneous transmission of multiple detection signals, but these still cannot meet the demand of the health care. The physical quantities, including heart rate, acceleration, respiration, temperature, blood pressure, and chemical quantities such as optical and environmental conditions can be integrated in a whole analysis system. Through the analysis of the data around users, scientific health analysis can be carried out according to each person's living habits and environment.
(4) Personalized health analysis
Human have different lifestyles at different age stages, and different people's occupations will also present different life signals. Therefore, we can collect and process the signals of different people through big data to obtain different health models for health management of different people in different conditions. Especially for patients with special management and care, the automatic alarm for dangerous health signals can shorten the time for medical treatment.
With the development of the sweat device, more biocompatible materials, electronics, and sensing sections will be accepted, and the device will become more smart and portable. Patients can be monitored by the real-time signal, and the device can be designed in different models for different individuals, which is beneficial for users to manage health condition.
| [1] |
Bandodkar A J, Jeang W J, Ghaffari R, et al. Wearable sensors for biochemical sweat analysis. Annual Review of Analytical Chemistry, 2019, 12(1): 1-22. DOI:10.1146/annurev-anchem-061318-114910 (  0) 0) |
| [2] |
Baker L B. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature, 2019, 6(3): 211-259. DOI:10.1080/23328940.2019.1632145 (  0) 0) |
| [3] |
Mark H, Harding C R. Amino acid composition, including key derivatives of eccrine sweat: potential biomarkers of certain atopic skin conditions. International Journal of Cosmetic Science, 2013, 35(2): 163-168. DOI:10.1111/ics.12019 (  0) 0) |
| [4] |
Delgado-Povedano M M, Calderon-Santiago M, Priego-Capote F, et al. Study of sample preparation for quantitative analysis of amino acids in human sweat by liquid chromatography-tandem mass spectrometry. Talanta, 2016, 146: 310-317. DOI:10.1016/j.talanta.2015.07.066 (  0) 0) |
| [5] |
Elpa D P, Chiu H Y, Wu S P, et al. Urban, skin metabolomics. Trends in Endocrinology and Metabolism, 2021, 32(2): 66-75. DOI:10.1016/j.tem.2020.11.009 (  0) 0) |
| [6] |
Serag A, Shakkour Z, Halboup A M, et al. Sweat metabolome and proteome: recent trends in analytical advances and potential biological functions. Journal of Proteomics, 2021, 246: 104310. DOI:10.1016/j.jprot.2021.104310 (  0) 0) |
| [7] |
Taylor C J, Hardcastle J, Southern K W. Physiological measurements confirming the diagnosis of cystic fibrosis: the sweat test and measurements of transepithelial potential difference. Paediatric Respiratory Reviews, 2009, 10(4): 220-226. DOI:10.1016/j.prrv.2009.05.002 (  0) 0) |
| [8] |
Bariya M, Nyein H Y Y, Javey A. Wearable sweat sensors. Nature Electronics, 2018, 1(3): 160-171. DOI:10.1038/s41928-018-0043-y (  0) 0) |
| [9] |
Gao W, Ota H, Kiriya D, et al. Flexible electronics toward wearable sensing. Accounts of Chemical Research, 2019, 52(3): 523-533. DOI:10.1021/acs.accounts.8b00500 (  0) 0) |
| [10] |
Baumann K Y, Church M K, Clough G F, et al. Skin microdialysis: methods, applications and future opportunities-an EAACI position paper. Clinical and Translational Allergy, 2019, 9: 24. DOI:10.1186/s13601-019-0262-y (  0) 0) |
| [11] |
Lei B U W, Prow T W. A review of microsampling techniques and their social impact. Biomedical Microdevices, 2019, 21(4): 81. DOI:10.1007/s10544-019-0412-y (  0) 0) |
| [12] |
Kiistala U. Suction blister device for separation of viable epidermis from dermis. The Journal of Investigative Dermatology, 1968, 50(2): 129-137. DOI:10.1038/jid.1968.15 (  0) 0) |
| [13] |
Hussain J N, Mantri N, Cohen M M. Working up a good sweat-the challenges of standardising sweat collection for metabolomics analysis. The Clinical biochemist. Reviews, 2017, 38(1): 13-34. (  0) 0) |
| [14] |
Nalbant A A, Boyaci E. Advancements in non-invasive biological surface sampling and emerging applications. Separations, 2019, 6(4): 52. DOI:10.3390/separations6040052 (  0) 0) |
| [15] |
Shichiri M, Yamasaki Y, Kawamori R, et al. Wearable artificial endocrine pancrease with needle-type glucose sensor. The Lancet, 1982, 320(8308): 1129-1131. DOI:10.1016/S0140-6736(82)92788-X (  0) 0) |
| [16] |
de Rossi D, Santa A D, Mazzoldi A, et al. Dressware: wearable piezo- and thermoresistive fabrics for ergonomics and rehabilitation. Proceedings of the International Conference of the IEEE Engineering-in-Medicine-and-Biology-Society. Piscataway: IEEE, 1997, 6276193. DOI:10.1109/IEMBS.1997.758700 (  0) 0) |
| [17] |
Martin T, Jovanov E, Raskovic D, et al. Issues in wearable computing for medical monitoring applications: a case study of a wearable ECG monitoring device. Proceedings of the 4th International Symposium on Wearable Computers. Piscataway: IEEE, 2000. DOI:10.1109/ISWC.2000.888463 (  0) 0) |
| [18] |
Yin F X, Li X X, Peng H F, et al. A highly sensitive, multifunctional, and wearable mechanical sensor based on RGO/synergetic fiber bundles for monitoring human actions and physiological signals. Sensors and Actuators B-Chemical, 2019, 285: 179-185. DOI:10.1016/j.snb.2019.01.063 (  0) 0) |
| [19] |
Kim J, Chou E F, Le J, et al. Soft wearable pressure sensors for beat-to-beat blood pressure monitoring. Advanced Healthcare Materials, 2019, 8(13): 1900109. DOI:10.1002/adhm.201900109 (  0) 0) |
| [20] |
Al-Halhouli A, Al-Ghussain L, El Bouri S, et al. Clinical evaluation of stretchable and wearable inkjet-printed strain gauge sensor for respiratory rate monitoring at different measurements locations. Journal of Clinical Monitoring and Computing, 2021, 35(3): 453-462. DOI:10.1007/s10877-020-00481-3 (  0) 0) |
| [21] |
Park S J, Jeon J Y, Kang B C, et al. Wearable temperature sensors based on lanthanum-doped aluminum-oxide dielectrics operating at low-voltage and high-frequency for healthcare monitoring systems. Ceramics International, 2021, 47(4): 4579-4586. DOI:10.1016/j.ceramint.2020.10.023 (  0) 0) |
| [22] |
Kim D H, Lu N, Ma R, et al. Epidermal electronics. Science, 2011, 333(6044): 838-843. DOI:10.1126/science.1206157 (  0) 0) |
| [23] |
Qiu L, Zhu N, Feng Y H, et al. A review of recent advances in thermophysical properties at the nanoscale: from solid state to colloids. Physics Reports-Review Section of Physics Letters, 2020, 843: 1-81. DOI:10.1016/j.physrep.2019.12.001 (  0) 0) |
| [24] |
Qiu L, Ouyang Y X, Feng Y H, et al. In vivo skin thermophysical property testing technology using flexible thermosensor-based 3 omega method. International Journal of Heat and Mass Transfer, 2020, 163: 120550. DOI:10.1016/j.ijheatmasstransfer.2020.120550 (  0) 0) |
| [25] |
Bruen D, Delaney C, Florea L, et al. Diamond, glucose sensing for diabetes monitoring: recent developments. Sensors, 2017, 17(8): 1866. DOI:10.3390/s17081866 (  0) 0) |
| [26] |
van Hoovels K, Xuan X, Cuartero M, et al. Can wearable sweat lactate sensors contribute to sports physiology?. ACS Sensors, 2021, 6(10): 3496-3508. DOI:10.1021/acssensors.1c01403 (  0) 0) |
| [27] |
Dolores M, de Castro L. Sweat as a clinical sample: what is done and what should be done. Bioanalysis, 2016, 8(2): 85-88. DOI:10.4155/bio.15.229 (  0) 0) |
| [28] |
Hu Y, Converse C, Lyons M C, et al. Neural control of sweat secretion: a review. British Journal of Dermatology, 2018, 178(6): 1246-1256. DOI:10.1111/bjd.15808 (  0) 0) |
| [29] |
Niemann C. Differentiation of the sebaceous gland. Dermato-Endocrinology, 2009, 1(2): 64-67. DOI:10.4161/derm.1.2.8486 (  0) 0) |
| [30] |
Kumagai K, Yokoshiki S, Kobayashi K, et al. The structure of human sebaceous glands and its relation to skin viscoelasticity. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Piscataway: IEEE, 2018, 3460-3463. DOI:10.1109/embc.2018.8512966 (  0) 0) |
| [31] |
Sears M E, Kerr K J, Bray R I. Arsenic, cadmium, lead, and mercury in sweat: a systematic review. Journal of Environmental and Public Health, 2012, 2012: 184745-184745. DOI:10.1155/2012/184745 (  0) 0) |
| [32] |
Cone E J, Hillsgrove M J, Jenkins A J, et al. Sweat testing for heroin, cocaine, and metabolites. Journal of Analytical Toxicology, 1994, 18(6): 298-305. DOI:10.1093/jat/18.6.298 (  0) 0) |
| [33] |
Kazem S, Linssen E C, Gibbs S. Skin metabolism Phase Ⅰ and Phase Ⅱ enzymes in native and reconstructed human skin: a short review. Drug Discovery Today, 2019, 24(9): 1899-1910. DOI:10.1016/j.drudis.2019.06.002 (  0) 0) |
| [34] |
Raiszadeh M M, Ross M M, Russo P S, et al. Proteomic analysis of eccrine sweat: implications for the discovery of schizophrenia biomarker proteins. Journal of Proteome Research, 2012, 11(4): 2127-2139. DOI:10.1021/pr2007957 (  0) 0) |
| [35] |
Verde T, Shephard R J, Corey P, et al. Sweat composition in exercise and in heat. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology, 1982, 53(6): 1540-1545. DOI:10.1152/jappl.1982.53.6.1540 (  0) 0) |
| [36] |
Cole D E, Boucher M J. Use of a new sample-collection device (macroduct) in anion analysis of human sweat. Clinical Chemistry, 1986, 32(7): 1375-1378. DOI:10.1093/clinchem/32.7.1375 (  0) 0) |
| [37] |
Porucznik C A, Cox K J, Wilkins D G, et al. A preliminary study of biomonitoring for bisphenol-A in human sweat. Journal of Analytical Toxicology, 2015, 39(7): 562-566. DOI:10.1093/jat/bkv055 (  0) 0) |
| [38] |
Dutkiewicz E P, Lin J D, Tseng T W, et al. Hydrogel micropatches for sampling and profiling skin metabolites. Analytical Chemistry, 2014, 86(5): 2337-2344. DOI:10.1021/ac4039338 (  0) 0) |
| [39] |
Kutyshenko V P, Molchanov M, Beskaravayny P, et al. Analyzing and mapping sweat metabolomics by high-resolution NMR spectroscopy. PLoS One, 2011, 6(12): e28824. DOI:10.1371/journal.pone.0028824 (  0) 0) |
| [40] |
Shetage S S, Traynor M J, Brown M B, et al. Effect of ethnicity, gender and age on the amount and composition of residual skin surface components derived from sebum, sweat and epidermal lipids. Skin Research and Technology, 2014, 20(1): 97-107. DOI:10.1111/srt.12091 (  0) 0) |
| [41] |
Cai G Z, Xue L, Zhang H L, et al. A review on micromixers. Micromachines, 2017, 8(9): 274. DOI:10.3390/mi8090274 (  0) 0) |
| [42] |
Wang L R, Xu T L, He C, et al. Flexible, self-healable, adhesive and wearable hydrogel patch for colorimetric sweat detection. Journal of Materials Chemistry C, 2021, 9(41): 14938-14945. DOI:10.1039/d1tc03905a (  0) 0) |
| [43] |
He X C, Xu T L, Gu Z, et al. Flexible and superwettable bands as a platform toward sweat sampling and sensing. Analytical Chemistry, 2019, 91(7): 4296-4300. DOI:10.1021/acs.analchem.8b05875 (  0) 0) |
| [44] |
Rosenstein B J, Cutting G R. The diagnosis of cystic fibrosis: a consensus statement. Journal of Pediatrics, 1998, 132(4): 589-595. DOI:10.1016/s0022-3476(98)70344-0 (  0) 0) |
| [45] |
Dong M J, Fang B, Li J F, et al. Wearable sensing devices for upper limbs: a systematic review. Proceedings of the Institution of Mechanical Engineers Part H-Journal of Engineering in Medicine, 2021, 235(1): 117-130. DOI:10.1177/0954411920953031 (  0) 0) |
| [46] |
Sempionatto J R, Jeerapan I, Krishnan S, et al. Wearable chemical sensors: emerging systems for on-body analytical chemistry. Analytical Chemistry, 2020, 92(1): 378-396. DOI:10.1021/acs.analchem.9b04668 (  0) 0) |
| [47] |
Kim J, Kumar R, Bandodkar A J, et al. Advanced materials for printed wearable electrochemical devices: a review. Advanced Electronic Materials, 2017, 3(1): 1600260. DOI:10.1002/aelm.201600260 (  0) 0) |
| [48] |
Cheng Y M, Wang K, Xu H, et al. Recent developments in sensors for wearable device applications. Analytical and Bioanalytical Chemistry, 2021, 413(24): 6037-6057. DOI:10.1007/s00216-021-03602-2 (  0) 0) |
| [49] |
Chung M, Fortunato G, Radacsi N. Wearable flexible sweat sensors for healthcare monitoring: a review. Journal of the Royal Society Interface, 2019, 159(16): 1-15. DOI:10.1098/rsif.2019.0217 (  0) 0) |
| [50] |
Morris G C, Thomas T P L. Night sweats-Presentation of an often forgotten diagnosis. British Journal of Clinical Practice, 1991, 45(2): 145-145. (  0) 0) |
| [51] |
Viera A J, Bond M M, Yates S W. Diagnosing night sweats. American Family Physician, 2003, 67(5): 1019-1024. (  0) 0) |
| [52] |
Siddiqui M A, Pandey S, Azim A, et al. Metabolomics: an emerging potential approach to decipher critical illnesses. Biophysical Chemistry, 2020, 267: 106462. DOI:10.1016/j.bpc.2020.106462 (  0) 0) |
| [53] |
Bouatra S, Aziat F, Mandal R, et al. The human urine metabolome. PLoS One, 2013, 8(9): e73076. DOI:10.1371/journal.pone.0073076 (  0) 0) |
| [54] |
Murota H, Yamaga K, Ono E, et al. Sweat in the pathogenesis of atopic dermatitis. Allergology International, 2018, 67(4): 455-459. DOI:10.1016/j.alit.2018.06.003 (  0) 0) |
| [55] |
Dutkiewicz E P, Hsieh K T, Wang Y S, et al. Urban, hydrogel micropatch and mass spectrometry-assisted screening for psoriasis-related skin metabolites. Clinical Chemistry, 2016, 62(8): 1120-1128. DOI:10.1373/clinchem.2016.256396 (  0) 0) |
| [56] |
Abaffy T, Moeller M G, Riemer D D, et al. Comparative analysis of volatile metabolomics signals from melanoma and benign skin: a pilot study. Metabolomics, 2013, 9(5): 998-1008. DOI:10.1007/s11306-013-0523-z (  0) 0) |
| [57] |
Ratjen F, Bell S C, Rowe S M, et al. Cystic fibrosis. Nature Reviews Disease Primers, 2015, 1: 15010. DOI:10.1038/nrdp.2015.10 (  0) 0) |
| [58] |
Trivedi D K, Sinclair E, Xu Y, et al. Discovery of volatile biomarkers of Parkinson's disease from Sebum. ACS Central Science, 2019, 5(4): 599-606. DOI:10.1021/acscentsci.8b00879 (  0) 0) |
| [59] |
Smesny S, Schmelzer C E H, Hinder A, et al. Skin ceramide alterations in first-episode schizophrenia indicate abnormal sphingolipid metabolism. Schizophrenia Bulletin, 2013, 39(4): 933-941. DOI:10.1093/schbul/sbs058 (  0) 0) |
| [60] |
Calderon-Santiago M, Priego-Capote F, Turck N, et al. Human sweat metabolomics for lung cancer screening. Analytical and Bioanalytical Chemistry, 2015, 407(18): 5381-5392. DOI:10.1007/s00216-015-8700-8 (  0) 0) |
| [61] |
Adewole O O, Erhabor G E, Adewole T O, et al. Proteomic profiling of eccrine sweat reveals its potential as a diagnostic biofluid for active tuberculosis. Proteomics Clinical Applications, 2016, 10(5): 547-553. DOI:10.1002/prca.201500071 (  0) 0) |
| [62] |
Cui X, Su G, Zhang L, et al. Integrated omics analysis of sweat reveals an aberrant amino acid metabolism pathway in Vogt-Koyanagi-Harada disease. Clinical and Experimental Immunology, 2020, 200(3): 250-259. DOI:10.1111/cei.13435 (  0) 0) |
| [63] |
Cizza G, Marques A H, Eskandari F, et al. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study. Biological Psychiatry, 2008, 64(10): 907-911. DOI:10.1016/j.biopsych.2008.05.035 (  0) 0) |
| [64] |
Parlak O, Keene S T, Marais A, et al. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Science Advances, 2018, 4(7): eaar2904. DOI:10.1126/sciadv.aar2904 (  0) 0) |
| [65] |
Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. Journal of Dermatological Science, 2013, 72(2): 177-182. DOI:10.1016/j.jdermsci.2013.06.005 (  0) 0) |
| [66] |
Rendon A, Schaekel K. Psoriasis pathogenesis and treatment. International Journal of Molecular Sciences, 2019, 20(6): 1474. DOI:10.3390/ijms20061475 (  0) 0) |
| [67] |
Dutkiewicz E P, Hsieh K T, Urban P L, et al. Temporal correlations of skin and blood metabolites with clinical outcomes of biologic therapy in psoriasis. Journal of Applied Laboratory Medicine, 2020, 5(5): 877-888. DOI:10.1093/jalm/jfaa009 (  0) 0) |
| [68] |
Ono E, Murota H, Mori Y, et al. Sweat glucose and GLUT2 expression in atopic dermatitis: implication for clinical manifestation and treatment. PLoS One, 2018, 13(4): e0195960. DOI:10.1371/journal.pone.0195960 (  0) 0) |
| [69] |
Mun J H, Lee H, Yoon D, et al. Discrimination of basal cell carcinoma from normal skin tissue using high-resolution magic angle spinning H-1 NMR spectroscopy. PLoS One, 2016, 11(3): e015032. DOI:10.1371/journal.pone.0150328 (  0) 0) |
| [70] |
Wicks E. A patient's journey - Cystic fibrosis. British Medical Journal, 2007, 334(7606): 1270-1271. DOI:10.1136/bmj.39188.741944.47 (  0) 0) |
| [71] |
Hammond K B, Turcios N L, Gibson L E. Clinical-evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic-fibrosis. Journal of Pediatrics, 1994, 124(2): 255-260. DOI:10.1016/s0022-3476(94)70314-0 (  0) 0) |
| [72] |
Greaves R F, Jolly L, Massie J, et al. Laboratory performance of sweat conductivity for the screening of cystic fibrosis. Clinical Chemistry and Laboratory Medicine, 2018, 56(4): 559-564. DOI:10.1515/cclm-2017-0530 (  0) 0) |
| [73] |
de Moraes C M, Wanjiku C, Stanczyk N M, et al. Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(22): 5780-5785. DOI:10.1073/pnas.1801512115 (  0) 0) |
| [74] |
van Zoonen K, Buntrock C, Ebert D D, et al. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. International Journal of Epidemiology, 2014, 43(2): 318-329. DOI:10.1093/ije/dyt175 (  0) 0) |
| [75] |
Ouanes S, Popp J. High cortisol and the risk of dementia and Alzheimer's disease: a review of the literature. Frontiers in Aging Neuroscience, 2019, 11: 43. DOI:10.3389/fnagi.2019.00043 (  0) 0) |
| [76] |
Staufenbiel S M, Penninx B W J H, Spijker A T, et al. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology, 2013, 38(8): 1220-1235. DOI:10.1016/j.psyneuen.2012.11.015 (  0) 0) |
| [77] |
Xue W, Tan X T, Khaing Oo M K, et al. Rapid and sensitive detection of drugs of abuse in sweat by multiplexed capillary based immuno-biosensors. Analyst, 2020, 145(4): 1346-1354. DOI:10.1039/c9an02498k (  0) 0) |
| [78] |
Kintz P, Tracqui A, Mangin P, et al. Sweat testing in opioid users with a sweat patch. Journal of Analytical Toxicology, 1996, 20(6): 393-397. DOI:10.1093/jat/20.6.393 (  0) 0) |
| [79] |
de la Torre R, Pichini S. Usefulness of sweat testing for the detection of cannabis smoke. Clinical Chemistry, 2004, 50(11): 1961-1962. DOI:10.1373/clinchem.2004.040758 (  0) 0) |
| [80] |
Suzuki S, Inoue T, Hori H, et al. Analysis of methamphetamine in hair, nail, sweat, and saliva by mass fragmentography. Journal of Analytical Toxicology, 1989, 13(3): 176-178. DOI:10.1093/jat/13.3.176 (  0) 0) |
| [81] |
Pichini S, Navarro M, Pacifici R, et al. Usefulness of sweat testing for the detection of MDMA after a single-dose administration. Journal of Analytical Toxicology, 2003, 27(5): 294-303. DOI:10.1093/jat/27.5.294 (  0) 0) |
| [82] |
Buono M J. Sweat ethanol concentrations are highly correlated with co-existing blood values in humans. Experimental Physiology, 1999, 84(2): 401-404. DOI:10.1111/j.1469-445X.1999.01798.x (  0) 0) |
| [83] |
Schazmann B, Morris D, Slater C, et al. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Analytical Methods, 2010, 2(4): 342-348. DOI:10.1039/b9ay00184k (  0) 0) |
| [84] |
Gao W, Emaminejad S, Nyein H Y Y, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature, 2016, 529(7587): 509-514. DOI:10.1038/nature16521 (  0) 0) |
| [85] |
Li P, Zhang D Z, Wu Z L. Flexible MoS2 sensor arrays for high performance label-free ion sensing. Sensors and Actuators A-Physical, 2019, 286: 51-58. DOI:10.1016/j.sna.2018.12.026 (  0) 0) |
| [86] |
Patterson M J, Galloway S D R, Nimmo M A. Variations in regional sweat composition in normal human males. Experimental Physiology, 2000, 85(6): 869-875. DOI:10.1111/j.1469-445X.2000.02058.x (  0) 0) |
| [87] |
Emaminejad S, Gao W, Wu E, et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(18): 4625-4630. DOI:10.1073/pnas.1701740114 (  0) 0) |
| [88] |
Miller P R, Xiao X Y, Brener I, et al. Microneedle-based transdermal sensor for on-chip potentiometric determination of K+. Advanced Healthcare Materials, 2014, 3(6): 876-881. DOI:10.1002/adhm.201300541 (  0) 0) |
| [89] |
Koh A, Kang D, Xue Y G, et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Science Translational Medicine, 2016, 8(366): 366ra165. DOI:10.1126/scitranslmed.aaf2593 (  0) 0) |
| [90] |
Zhao Z Q, Li Q J, Chen L N, et al. A thread/fabric-based band as a flexible and wearable microfluidic device for sweat sensing and monitoring. Lab on a Chip, 2021, 21(5): 916-932. DOI:10.1039/d0lc01075h (  0) 0) |
| [91] |
Zhang J R, Rupakula M, Bellando F, et al. Sweat biomarker sensor incorporating picowatt, three-dimensional extended metal gate ion sensitive field effect transistors. ACS Sensors, 2019, 4(8): 2039-2047. DOI:10.1021/acssensors.9b00597 (  0) 0) |
| [92] |
Keene S T, Fogarty D, Cooke R, et al. Wearable organic electrochemical Transistor patch for multiplexed sensing of calcium and ammonium ions from human perspiration. Advanced Healthcare Materials, 2019, 8(24): 1901321. DOI:10.1002/adhm.201901321 (  0) 0) |
| [93] |
Nyein H Y Y, Gao W, Shahpar Z, et al. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano, 2016, 10(7): 7216-7224. DOI:10.1021/acsnano.6b04005 (  0) 0) |
| [94] |
Guinovart T, Bandodkar A J, Windmiller J R, et al. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst, 2013, 138(22): 7031-7038. DOI:10.1039/c3an01672b (  0) 0) |
| [95] |
Scheiblin G, Coppard R, Owens R M, et al. Referenceless pH sensor using organic electrochemical transistors. Advanced Materials Technologies, 2017, 2(2): 1600141. DOI:10.1002/admt.201600141 (  0) 0) |
| [96] |
Anastasova S, Crewther B, Bembnowicz P, et al. A wearable multisensing patch for continuous sweat monitoring. Biosensors and Bioelectronics, 2017, 93: 139-145. DOI:10.1016/j.bios.2016.09.038 (  0) 0) |
| [97] |
Martin A, Kim J, Kurniawan J F, et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sensors, 2017, 2(12): 1860-1868. DOI:10.1021/acssensors.7b00729 (  0) 0) |
| [98] |
Liu Q Z, Liu Y H, Wu F Q, et al. Highly sensitive and wearable In2O3 nanoribbon transistor biosensors with integrated on-chip gate for glucose monitoring in body fluids. ACS Nano, 2018, 12(2): 1170-1178. DOI:10.1021/acsnano.7b06823 (  0) 0) |
| [99] |
Kim J, Jeerapan I, Imani S, et al. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sensors, 2016, 1(8): 1011-1019. DOI:10.1021/acssensors.6b00356 (  0) 0) |
| [100] |
Windmiller J R, Bandodkar A J, Valdes-Ramirez G, et al. Electrochemical sensing based on printable temporary transfer tattoos. Chemical Communications, 2012, 48(54): 6794-6796. DOI:10.1039/c2cc32839a (  0) 0) |
| [101] |
Coppede N, Tarabella G, Villani M, et al. Human stress monitoring through an organic cotton-fiber biosensor. Journal of Materials Chemistry B, 2014, 2(34): 5620-5626. DOI:10.1039/c4tb00317a (  0) 0) |
| [102] |
Roy S, David-Pur M, Hanein Y. Carbon nanotube-based ion selective sensors for wearable applications. ACS Applied Materials and Interfaces, 2017, 9(40): 35169-35177. DOI:10.1021/acsami.7b07346 (  0) 0) |
| [103] |
Parrilla M, Ortiz-Gomez I, Canovas R, et al. Wearable potentiometric ion patch for on-body electrolyte monitoring in sweat: toward a validation strategy to ensure physiological relevance. Analytical Chemistry, 2019, 91(13): 8644-8651. DOI:10.1021/acs.analchem.9b02126 (  0) 0) |
| [104] |
Zhang S M, Hubis E, Tomasello G, et al. Patterning of stretchable organic electrochemical transistors. Chemistry of Materials, 2017, 29(7): 3126-3132. DOI:10.1021/acs.chemmater.7b00181 (  0) 0) |
| [105] |
Seghir R, Arscott S. Extended PDMS stiffness range for flexible systems. Sensors and Actuators A-Physical, 2015, 230: 33-39. DOI:10.1016/j.sna.2015.04.011 (  0) 0) |
| [106] |
Cai L H, Kodger T E, Guerra R E, et al. Soft poly(dimethylsiloxane) elastomers from architecture-driven entanglement free design. Advanced Materials, 2015, 27(35): 5132-5140. DOI:10.1002/adma.201502771 (  0) 0) |
| [107] |
Jeong S H, Zhang S, Hjort K, et al. PDMS-based elastomer tuned soft, stretchable, and sticky for epidermal electronics. Advanced Materials, 2016, 28(28): 5830-5836. DOI:10.1002/adma.201505372 (  0) 0) |
| [108] |
Su B, Gong S, Ma Z, et al. Mimosa-inspired design of a flexible pressure sensor with touch sensitivity. Small, 2015, 11(16): 1886-1891. DOI:10.1002/smll.201403036 (  0) 0) |
| [109] |
Li Y Z, Wang N X, Yang A N, et al. Biomimicking stretchable organic electrochemical transistor. Advanced Electronic Materials, 2019, 5(10): 1900566. DOI:10.1002/aelm.201900566 (  0) 0) |
| [110] |
Zhang Y, Guo H X, Kim S B, et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab on a Chip, 2019, 19(9): 1545-1555. DOI:10.1039/c9lc00103d (  0) 0) |
| [111] |
Zhang Z, Azizi M, Lee M, et al. A versatile, cost-effective, and flexible wearable biosensor for in situ and ex situ sweat analysis, and personalized nutrition assessment. Lab on a Chip, 2019, 19(20): 3448-3460. DOI:10.1039/c9lc00734b (  0) 0) |
| [112] |
Nyein H Y Y, Tai L C, Quynh Phuong N, et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sensors, 2018, 3(5): 944-952. DOI:10.1021/acssensors.7b00961 (  0) 0) |
| [113] |
Huang X C, Li J Y, Liu Y M, et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Design and Manufacturing, 2022, 5: 201-209. DOI:10.1007/s42242-021-00156-1 (  0) 0) |
| [114] |
Bhide A, Muthukumar S, Prasad S. CLASP (Continuous lifestyle awareness through sweat platform): a novel sensor for simultaneous detection of alcohol and glucose from passive perspired sweat. Biosensors and Bioelectronics, 2018, 117: 537-545. DOI:10.1016/j.bios.2018.06.065 (  0) 0) |
| [115] |
Tai L C, Liaw T S, Lin Y, et al. Wearable sweat band for noninvasive levodopa monitoring. Nano Letters, 2019, 19(9): 6346-6351. DOI:10.1021/acs.nanolett.9b02478 (  0) 0) |
| [116] |
Imani S, Bandodkar A J, Vinu Mohan A M, et al. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nature Communications, 2016, 7: 11650. DOI:10.1038/ncomms11650 (  0) 0) |
| [117] |
Nakata S, Arie T, Akita S, et al. Wearable, Flexible, and Multifunctional Healthcare Device with an ISFET Chemical Sensor for Simultaneous Sweat pH and Skin Temperature Monitoring. ACS Sensors, 2017, 2(3): 443-448. DOI:10.1021/acssensors.7b00047 (  0) 0) |
| [118] |
Cazale A, Sant W, Launay J, et al. Temple-boyer, study of field effect transistors for the sodium ion detection using fluoropolysiloxane-based sensitive layers. Sensors and Actuators B-Chemical, 2013, 177: 515-521. DOI:10.1016/j.snb.2012.11.054 (  0) 0) |
| [119] |
Upasham S, Tanak A, Jagannath B, et al. Development of ultra-low volume, multi-bio fluid, cortisol sensing platform. Scientific Reports, 2018, 8: 16745. DOI:10.1038/s41598-018-35199-5 (  0) 0) |
| [120] |
Kim Y H, Lee K, Jung H, et al. Direct immune-detection of cortisol by chemiresistor graphene oxide sensor. Biosensors and Bioelectronics, 2017, 98: 473-477. DOI:10.1016/j.bios.2017.07.017 (  0) 0) |
| [121] |
Murase N, Taniguchi Shin-ichi, Takano E, et al. A molecularly imprinted nanocavity-based fluorescence polarization assay platform for cortisol sensing. Journal of Materials Chemistry B, 2016, 4(10): 1770-1777. DOI:10.1039/c5tb02069g (  0) 0) |
| [122] |
Dhanjai, Yu N, Mugo S M. A flexible-imprinted capacitive sensor for rapid detection of adrenaline. Talanta, 2019, 204: 602-606. DOI:10.1016/j.talanta.2019.06.016 (  0) 0) |
| [123] |
Huynh T P, Bikram C K C, Lisowski W, et al. Molecularly imprinted polymer of bis(2, 2 '-bithienyl)methanes for selective determination of adrenaline. Bioelectrochemistry, 2013, 93: 37-45. DOI:10.1016/j.bioelechem.2012.07.003 (  0) 0) |
| [124] |
Wu W, Ding L Y, Lin H T, et al. A highly sensitive fluorescence sensor for adrenaline detection based on modified carbon quantum dots. Proceedings of the 10th International Conference on Information Optics and Photonics. Bellingham, WA, USA: SPIE, 2018. DOI: 10.1117/12.2506345.
(  0) 0) |
| [125] |
Mak C H, Liao C, Fu Y, et al. Highly-sensitive epinephrine sensors based on organic electrochemical transistors with carbon nanomaterial modified gate electrodes. Journal of Materials Chemistry C, 2015, 3(25): 6532-6538. DOI:10.1039/c5tc01100k (  0) 0) |
| [126] |
Quattrini C, Jeziorska M, Tavakoli M, et al. The neuropad test: a visual indicator test for human diabetic neuropathy. Diabetologia, 2008, 51(6): 1046-1050. DOI:10.1007/s00125-008-0987-y (  0) 0) |
 2023, Vol. 30
2023, Vol. 30


