Diabetes Mellitus (DM) is one of the most serious and widely known health issues over the globe that has risen substantially in recent decades. According to epidemiological research and studies, the number of diabetic patients each year has increased from roughly 30 million in 1985 to 177 million in 2000, 285 million in 2010, and more than 360 million by 2030 if the current trend continues[1-2]. Since it is classified as a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both, it leads to severe complications[3]. Around 15% of all diabetic patients suffer from DFU. DFU is an open sore that appears on the foot of a diabetic patient that is usually found on the bottom half of the foot[4]. Diabetic individuals at high risk for DFUs include those who have a history of DFUs, calluses, or Charcot's foot; such problems frequently lead to subsequent ulceration[5]. Although many ulcers may be healed with extra help, 14% to 24% of diabetic people require surgery. DFUs are responsible for 85% of all diabetes-related removals[4]. Apart from leading to consequences such as DFU, visual blindness, foot amputation, and kidney failure, diabetes also significantly raises the risk of coronary artery diseases, which leads to heart attack, strokes, and arterial narrowing, among other cardiovascular disorders. In 2020, 25% of India's 62 million diabetics developed DFUs, with 50% becoming infected and requiring hospitalization and 20% requiring amputation. According to published research, 100000 limb amputations are performed each year due to diabetes-related complications, costing $1960 for comprehensive DFU therapy. The lack of accurate DFU identification at an early stage is the key reason behind this.
Furthermore, patient outcomes and satisfaction with DFU and diabetes-related removal are unfavorable. At five years after a diabetes-related deduction, mortality for all patients exceeds 70%. Patients exposed to DFU had dismal outcomes; according to a new study, 1-, 2-, and 5-year endurance rates with DFU were 81%, 69%, and 29%, respectively[6]. In addition, 40% of all patients who had their limbs amputated had a survival expectancy of fewer than two years. Roughly 62% of persons living with DFU experience similar despair[7]. Only 25% to 50% of diabetic patients who experience sorrow are clinically evaluated and appropriately treated[8]. At the point where mental health concerns continue to go untreated, they can trigger a cycle that worsens both the diabetes condition and the depression of the individual[8]. Such complications may occur as a result of a person's poor mental health interfering with their ability to follow diabetes treatment recommendations[8].
Only hospitals and clinics have the equipment and resources necessary to identify and treat DFU. Treatment costs for neuropathic ulcers, infected neuropathic foot, advanced diabetic foot, and bypass were reported to be $56, $165, $1080, $960, $2650, and $1960, respectively, among Indian diabetics[9]. According to the American Diabetes Association, detecting and treating diabetic foot complications cost $43 billion in 2018. DFU treatment costs $2387 per month or $28644 per year per diabetes patient, based on a quiet premise[10-11]. Furthermore, half of DFU patients who had their limbs amputated have another amputation within two years. A DFU detection system that can be set up at home might save time and money by avoiding the need for frequent hospital visits.
Diabetes foot neuropathy is one of the key contributing factors for foot issues in diabetic patients, impairing the sense of sensation on the feet, and is one of the reasons for DFU. As a result, patients may struggle to notice any symptoms or damage as a result of DFU. Infection and ulceration in the foot cause the majority of these problems. Fissures, blisters, excessive callus development, redness, and elevated warmth are all early indications of DFU[12]. With proper treatment and early detection, it is possible to postpone or even prevent the development of DFUs. Typically, doctors evaluate the overall condition by looking at ankle-brachial pressure indices, plantar pressure profiles, and foot neuropathy tests. Advanced technologies such as corneal confocal microscopy, magnetic resonance tomography, and Doppler ultrasonography can also be used to determine the prevalence of peripheral neuropathy and angiopathy, as well as the risks associated with foot ulcers[13]. However, these approaches are considered invasive and expensive, and patient cooperation is low, especially with frequent doctor visits. Moreover, self-assessment and checkups for DFU have their own constraints, such as a lack of understanding of the ailment, problems with using specialized equipment and decreased physical mobility.
Many researchers have employed advanced models and interfaces to make home-based detection and treatment possible. The use of thermal scanning and image processing is one of the most frequent methods for the early diagnosis of a diabetic foot ulcer. Fraiwan et al.[14] developed a transportable thermal imaging device with an automated technique for detecting probable diabetic ulcers. To analyze and interpret the thermal scans and produce informative diagnoses, they used the Otsu thresholding approach and the Point-to-Point mean difference technique. Khandakar et al.[15] followed a similar approach, developing a traditional machine learning-based framework for early diabetes foot diagnosis using thermogram pictures obtained with smartphone Infrared (IR) cameras. Deep learning approaches for real-time DFU localization were developed by Goyal et al.[16]. They employed five-fold cross-validation, quicker R-CNN with InceptionV2 model employing two-tier transfer learning to obtain a precision of 91.8% on a database of 1775 DFU photos[16]. In December 2019, the US Veteran's Administration (VA)[17] devised SmartMat; A daily remote temperature monitoring foot mat that measures plantar foot temperatures using remote thermometry monitoring and transmits the data automatically to the respective podimetrics care team, who triage any concerning findings and help patients receive appropriate, preventive treatments under the direction of their clinician. Using sensing technologies to assess early symptoms in diabetes patients is another strategy many researchers have pursued in recent years. Compared to previous thermal scanning models, such systems were more accurate in recording factors such as callus of the foot and sensory loss to vibration. Sahana et al.[18] proposed a system that is developed and evaluated to identify the patients who are likely to develop diabetic foot ulcers at an early stage. They accomplished this by converting foot pressure readings into corresponding voltage output by the sensor. Using an amplification unit and a data acquisition device, they amplified the sensed data and determined the probability of developing DFU in diabetic patients[18]. Liu et al.[12] developed a plantar pressure measurement and analysis system based on a pressure-sensitive textile fabric sensor array that interfaces with the insole and wirelessly transmits the sensed data to a remote receiver over a Bluetooth network. In the seventh framework program European Union project SWAN-iCare. Salvo et al.[19] created a temperature and pH sensor capable of monitoring diabetic foot and venous leg ulcers. They built a pH sensor employing graphene oxide (GO) layers that vary their electrical potential when pH varies by leveraging differences in the electrical resistance of a nanocomposite composed of multi-walled carbon nanotubes and poly(styrene-b-(ethylene-co-butylene)-b-styrene) [19]. The involvement of IoT devices such as temperature sensors, pH sensors, insole sensors embedded in dressings, or bandaging for real-time monitoring of variables such as sensations and temperature in DF-ulcerated patients could aid clinicians in improving the quality of treatment for such complex pathology.
By considering the current literature, we investigated the leading techniques used for early DFU detection in diabetic patients, which includes various machine learning models, IoT architectures, and sensor technologies. Through this paper, we work on creating a machine learning model and an IoT ecosystem for efficient detection of diabetic foot ulcers. The major contributions of our proposed system are:
1) A machine learning model based on supervised learning using four different classification algorithms, namely XGBoost, Random Forest, K-SVM, Decision Tree.
2) An IoT device consisting of 8 motors that sense foot nodes via a shoe insole, and delivers sensory vibrations at each node to predict the areas of severity.
3) A mobile application that acts as an interface between the IoT device and the patients by collecting patient input and visualizing risk-prone zones on the patient's foot.
The organization of this paper is as follows: Our proposed methodology is discussed in Section 1, which consists of parameters and sub-parameters, dataset, architecture diagram, app interface, prediction technique, the consequent results are discussed in Section 2. The conclusion of the paper and its future scopes are discussed in Section 3.
1 Proposed Methodology 1.1 Parameters and Sub ParametersFor this model, we took 12 parameters that are highly responsible for predicting diabetic foot ulcers, as shown in Table 1.
| Table 1 Parameters and sub parameters used for our proposed model of DFU detection |
These parameters were considered with the help of a doctor who is a specialist in treating diabetic patients. We studied many patients' case reports who were suffering from diabetes type 2 and, in turn suffering from the diabetic foot. Each parameter is later divided into sub-parameters which patients select them using our mobile application. Our foot ulcer severity was predicted by our machine learning model using the sub-parameters chosen by the patient. The 12 parameters and sub-parameters are explained below.
1) Condition of Foot: This essential factor gives us a clear idea about patients' present foot problems. This parameter is divided into six sub-parameters: trauma, heel, bony prominence, sensory disturbances, foot ulcer, and skin atrophy. Here trauma is a condition where patients might be facing any ligament ruptures or ankle tears, any other muscular pain at the ankle caused by intense exercises or some other external factors like getting hit by a hard object, feeling tingling or weakness on the foot, or mild peroneal nerve injuries, etc. The heel is a condition where a patient faces pain at the heel of their foot due to Plantar fasciitis, Achilles tendinitis, Heel spur, Tenonitis, Flat feet, etc. Bony prominence is seen when a patient is suffering from Bunion, a bony bump at the joint of the big toe, or suffering from a navicular accessory syndrome where a bony bump is seen on the midfoot. A foot ulcer is a condition where a patient's skin layers have been ruptured due to surgery wounds, injuries caused by external procedures such as cuts, vehicular accidents, etc., and an ulcer is seen. Atrophy of the skin on foot is thinning of the epidermis and dermis, which may be caused by aging, steroid-induced skin atrophy, solar damage, radiodermatitis, chronic cutaneous lupus erythematosus, etc. These sub-parameters would help us predict the risk of occurrence of diabetic foot ulcers.
2) Level of Education: According to doctors and reports of diabetic patients who got their foot amputated due to diabetic foot ulcers, lack of awareness is one of the main reasons for not detecting foot ulcers at an early stage and preventing them from reaching a severe stage. Hence, if the patient's level of education is good enough to detect foot ulcers at an early stage or if they go to the regular diabetic checkups with the doctors, there are chances to prevent the occurrence of diabetic foot ulcers using simple preventive measures. Here level of education is further divided into sub-parameters like Illiterate, Primary, Secondary, Bachelor's degree, Master's degree, Ph.D.
3) Duration of Diabetic: According to Refs.[20-21], the prevalence of diabetic foot ulcers was 18.1%, and the risk of diabetic foot ulcers increased with the duration of diabetes more than ten years. Therefore we can conclude that people who have been suffering from diabetes type 2 for more than ten years have more risk of diabetic foot ulcer occurrence when compared to other diabetic patients.
4) Comorbidities: Comorbidity is another essential parameter that helps us know the patient's present underlying health conditions along with diabetes type 2, where those health conditions may increase the risk of diabetic foot ulcers. According to Refs.[22-24], diabetic patients have high chances of getting cardiovascular diseases like coronary artery disease, myocardial infarction, strokes, congestive heart failure peripheral vascular disease. These diseases may lead to diabetic neuropathy, which may lead to diabetic foot ulcers.
5) Occupation: Occupation is one of the most influencing factors that may increase the chances of diabetic foot ulcer formation in diabetic patients. People working as military officers, police officers, laborers, teachers, their work-life goes on mainly in standing positions where whole bodyweight load is bared by the foot, which causes nerve damage in diabetic patients. Furthermore, military officers, police officers, laborers' work-life is risky as they have many chances of getting their feet damaged. For example, military officers and police officers may be hit by criminals, and laborers may hurt their feet while carpentry, welding, etc. People who are unemployed, self-employed, and working in the IT (Information Technology) industry have fewer chances of developing foot ulcers.
6) Area of Residence: Area of residence is one factor that affects diabetic foot ulcer formation because the area of residence tells about the roads on which patients walk most of the time in their daily lives. Uneven roads may lead to an increase in foot pressure and may cause callus, high plantar pressures on the foot, which may, in turn, lead to ulcer formation on foot. According to the results in Ref.[25], there is a high prevalence of diabetic foot ulcers in people who live in rural, hilly, and forest areas when compared to urban areas.
7) Type of footwear: Type of footwear plays a vital role in preventing diabetic foot ulcer formation. If diabetic patients use footwear like open-toe shoes, laces shoes, high heels, etc., then the chances of getting foot ulcers are very high because these types of footwear increase the foot's pressure. As a result, doctors recommend diabetic patients use footwear with soft insoles and avoid footwear that increases pressure on the foot.
8) Foot deformity: We divided foot deformity into four sub-parameters skin ulcer, pain, infection, and cosmetic allergy. These sub-parameters help us understand the patient's foot conditions. The patient might be suffering from any skin ulcer anywhere on and around the foot; pain may be due to fracture, bony prominence, using high-heeled footwear, etc. Infection may be caused due to any reason, like when a patient exposes their foot to any bacteria, virus, etc. Cosmetic allergy may be caused due to high blood sugar levels; the patient might be allergic to some materials used in footwear or any other skincare products. Infection and skin ulcers highly contribute to foot ulceration when compared to other deformities.
9) Swelling: According to the doctors, swelling is one of the main symptoms of diabetic foot ulcer and the visual appearance of black tissue around some specific swollen area is due to the presence of unhealthy blood, which increases the chances of ulceration at that spot. Swelling may be caused by many reasons like fluid buildup, fractures, diabetic foot ulcers, etc. But if swelling is in one area, that may increase the chances of foot ulcer formation.
10) Movement of Ankle joint: 62 subjects were contemplated in three gatherings (controls, diabetic patients without foot issues, and diabetic patients with neuropathic ulceration) to decide if comparable changes happen in the joints of the foot and to analyze any conceivable relationship with neuropathic ulceration[26]. There was a significant disability of versatility in the scope of movement of the sub-talar joint in diabetic patients with ulcers when contrasted and controls (p=0.0001) or with the other diabetic patients (p=0.004). There was a critical connection between the sub-talar scope of movement and versatility in different foot joints, for example, at the hallux (r=0.59, p < 0.001). There was likewise a critical relationship between the clinical presentation of restricted joint portability in hand, Dupuytren's contracture, and versatility of the sub-talar joint (p < 0.05). Besides, the impedance of portability of the sub-talar joint was most noteworthy on the impacted side in those diabetic patients with neuropathic ulceration (p=0.029). They infer that the disorder of restricted joint portability additionally influences the joints of the feet of diabetic patients and may incline toward ulceration in defenseless neuropathic feet. Here we divided ankle movement into 4 different ranges 0-20, 0-35, 0-50, 0-75. If ankle movement is in the ranges 0-20 and 0-35, there is a high risk of diabetic foot ulcer formation.
11) Change of hormone level: The most important hormonal changes that increase the chances of diabetic foot ulcers are depression, menstruation, menopause. Depression and blood sugar are directly proportional to each other. Hence if depression increases, blood sugar levels increase too and vice versa. Women with diabetes have unpredictable or irregular menstrual cycles. Menstruation cycles sometimes cause insulin resistance due to an increase in progesterone levels. This leads to an increase in blood sugar levels in diabetic patients. During the transition to menopause, estrogen and progesterone hormone levels go up and down, leading to uncontrolled blood sugar levels. This slows down the metabolism and leads to an increase in weight, and may lead to nerve damage and vision loss. This is how diabetes and hormonal changes lead to a rise in foot ulceration.
12) Pain: With diabetic patients, foot pain helps us in the early detection of nerve damage that may, in turn, lead to foot ulceration. Hence, pain is an essential factor that helps predict foot ulcers.
1.2 DatasetThe baseline data collection involves a record of primary physical attributes, background medical information, metabolic and menstrual changes that act as relevant factors in this paper. Essentially, the data instantaneously from Dr. P. Srinivas helped get powerful insights regarding the parameters considered in the case of Diabetic Foot Ulcer (DFU). All patients are deemed to have type 2 diabetes. The most important obligation to be fulfilled by all factors is to directly influence the cause and the severity level of the DFU. Additionally, the recognition of background medical details and history of pain as factors in the dataset acknowledged the causes of the DFU. The attributes like gender, age, diabetes type, level of education, occupation, and area of residence supported the general census of the patients involved in the study that influenced the algorithm in regards to demographics. Basic factors of internal medicine like hormonal changes, positional pain, condition of the foot, the record of co-morbidities, foot deformity, swelling, movement of the ankle are taken into consideration. This amalgamation of factors aided the development of the algorithm that determined the severity level of DFU partly.
The gap in the data is met by follow-up data collection, which is the sensation of pressure perceived by the patient. The ulcer associated with diabetes does not heal, and nerve impairment further adds to the fact of leaving it untreated, gradually leading to sepsis and amputation. This focuses on the diabetic foot as the part affected by the ulcer. This paper proposes a wearable that is facilitated by eight sensors that send vibrations to eight points on the sole of the foot. The mobile application developed operates the sensors sequentially and collects responses from patients. The device proposed in this paper collects the response of the patients by sending vibrations to eight points on the sole of the foot through sensors. The patient's response is recorded through a mobile application that operates the sensors sequentially and collects the patient's feedback in real-time. The response collected from the patients is whether they can feel the vibrations at a certain point on the sole of the foot or not. The more severe the DFU, the higher the chances of the loss of sensation at a greater number of points, resulting in the patient being unable to feel the vibrations at certain points. The number of points of sensation loss is appended to the data collected, which influences the prediction percentile of severity. A cumulative score is calculated from the number of perceived vibrations by the user from sensors on an individual foot. Neuropathy is a condition where the person has a loss of sensation, especially in the peripherals like fingers and feet. Thus, when these peripherals undergo abrasion or extreme temperatures, the person does not feel pain stimuli leading to the part affected, untreated. Furthermore, high blood sugar levels narrow down the arteries at the peripherals, restricting the blood flow beginning to slow down the healing process. Thus, the collection of real-time responses of the patients, whether they can feel the vibrations at a certain point on the sole of the foot, proves to be a great asset to predict the severity of the foot ulcer. The more severe the ulcer is the loss of sensation at a greater number of points.
The number of points of sensation loss is appended to the data collected. This attribute is dynamic in nature, whose values are collected as real-time responses. The severity is calculated by giving sensation loss much priority as a deciding factor. The maintenance or worsening of the cumulative number of sensation loss points influences the sensation, which is reflected in the prediction percentile of severity.
1.3 Architecture DiagramAs shown in Fig. 1, the patient interacts with a mobile application through a questionnaire that takes input on their medical profile; these act as the parameters and the sub-parameters for our data. Additionally, the user undergoes a sensory test while using our application and IoT device. As soon as the user starts a sensory test, the IoT device gets switched on, and sensory information at all 8 points of the foot is taken. Additional sensory tests were conducted on the foot of each volunteer to collect more sensory information. These tests were performed using the same IoT device and involved stimulating additional points on the foot to collect sensory data. The collected sensory data was added to the dataset for further analysis and model training. Using all these questionnaire data and sensory data, a dataset is generated. That dataset is sent to our supervised machine learning module, where our prediction model gets trained. Dataset undergoes data pre-processing, clustering and that data is also used for visualization. After which, the data gets split into two parts one is training set, and the other one is test set. The training set is used to train the prediction model using classifier algorithms like XGBoost, K-SVM, Random forest, Decision tree. The prediction model is created and tested using a test set. The results are in the form of infographics shown on the application interface.
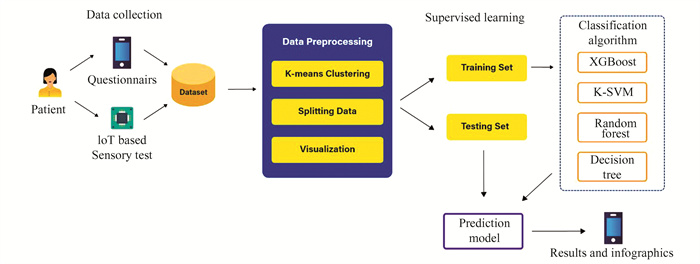
|
Fig.1 Architecture diagram |
1.4 Algorithm and Prediction Technique 1.4.1 IoT interface
We used an IoT device to do sensory tests on the patient's foot. The IoT device contains 8 vibrators, an Arduino board, and a Bluetooth module. 8 vibrators are placed at 8 different coordinates of the foot. Vibrators are attached to the insole of the shoe and are connected to Arduino. When the patient starts the mobile application triggers vibrators when the user starts sensory test via Bluetooth module which is connected to Arduino, then each vibrator runs sequentially for a specific duration of time, during which the patient would be given input for the sensory loss of vibration at each node. Similarly, we test this at all 8 points and represent the sensation at all the points of the user's foot using a heat map.
1.4.2 Data pre-processingFirst and foremost, the computation of the level of severity of DFU is made using a weightage allocation algorithm that prioritizes the attributes that contribute the most to the prognosis of DFU. Each value of the parameters in the dataset is allotted a weight based on its influence on DFU. The target parameter "Severity" is split up into low, mid, and high (-1, 0, and 1). The weights allotted to each value of most influential features are shown in Table 2.
| Table 2 Prioritized attributes based on weightage allocation algorithm categorized according to their severity measure |
Now, based on the information given by the patient involving both baseline and follow-up data collection, a cumulative(x) is calculated, taking Table 3 as reference. With the help of the below Table 3, the severity level of DFU is decided and appended to the patient information for prediction.
| Table 3 Cumulative range for x based on severity measure |
The categorical values of the parameters are converted to numerical data type, and also attributes similar to phrases were renamed to include underscores for easier visualizing of classification methods
1.4.3 ClusteringTo defend the decision of splitting up the parameter "Severity" into three classes; High, Mid, and Low(1, 0, -1), clustering is used. The optimal number of clusters(k) for K-means clustering is found with the help of the Elbow method. This method yielded three clusters regarding the dataset involved, backing the decision of the three classes.
1.4.4 VisualizationBasic visuals like histograms and correlation heatmap are realized, as shown in Fig. 2, which are powerful in discerning the relationship not only between target parameters and features but also the inter-dependency of features alike.

|
Fig.2 Correlation heatmap |
1.4.5 Splitting up of dataset into train and test set
An important step in this proposal sets the premise of machine learning classification. This starts with preparing independent features, which are gender, age, type of diabetes, condition of the foot, level of education, duration of diabetes, co-morbidities, occupation, area of residence, type of footwear, foot deformity, swelling, movement of the ankle joint, hormone changes, pain, and sensation loss. The parameter severity is the target or dependent variable, where the test cases get classified into or the feature that gets predicted by the classifiers. The dataset is split into 80∶20 for training and testing, respectively, using the scikit learn library. The trainset comprising components and target is known upon, whereas the test set with only features is classified for target parameter, as shown in Fig. 3.
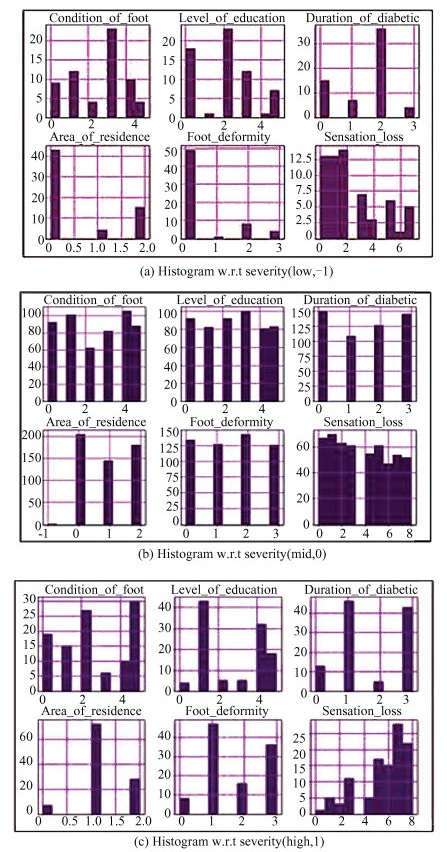
|
Fig.3 Pair plots and Histogram visualization with respect to severity measure |
1.4.6 Model selection and evaluation
Since the target parameter severity comprises classes, we used the most relevant classification algorithms of supervised learning.
a) XGBoost
This ensemble consisting of decision-tree and gradient boost proved to be most accurate for predicting the severity level based on the patient information. The maximum accuracy of 81.02% is attributed to individual evaluation by decision tree and minimization of errors by gradient boosting. The decision tree is visualized, clearly expressing the evaluating factor at each level where parameters like foot deformity, sensation loss, comorbidities, occupation, and area of residence are highlighted.
b) K-SVM
Kernel Support Vector Machine is a machine learning algorithm that extracts critical features and increases dimensionality in terms of correlation by an accommodating boundary. The kernel used in this proposal is the radial basis function. It achieved an accuracy of 75.18%. The inclusion of hyperplane while classifying the support vectors is the characteristic factor.
c) Random forest
This machine-learning classification algorithm depends on the majority deemed by multiple individual decision trees. Ten estimators with entropy as criteria are considered. Random forest accomplished an accuracy of 72.99%. Python visualization libraries helped in realizing the majority class in the random forest. The decrease in accuracy is seen due to a lack of better generalization of independent features.
d) Decision tree
This machine-learning classification algorithm evaluates considering a criterion, in this case, entropy which measures the disarranges in the given features and decides the class. It gave an accuracy of 67.15% due to its assumption of non-interdependence between the features, which leads to missing or ignoring important relations.
1.5 Mobile App InterfaceTo create a user-centered system, we developed a mobile application that acts as an interface between the IoT device and the patients. Through the app, the patients are prompted to answer a questionnaire that acts as an input for all our parameters and sub-parameters in our model. Based on the responses we create a foot profile of each patient to predict the risk-prone areas accurately. On activating the IoT device, sensory points on the foot profile are marked, and based on the responses for each vibration a heat map is generated. As shown in Fig. 4, the map depicts the risk-prone zones for diabetic foot ulcers and generates a medical report to be shared with a doctor or medical counsel.
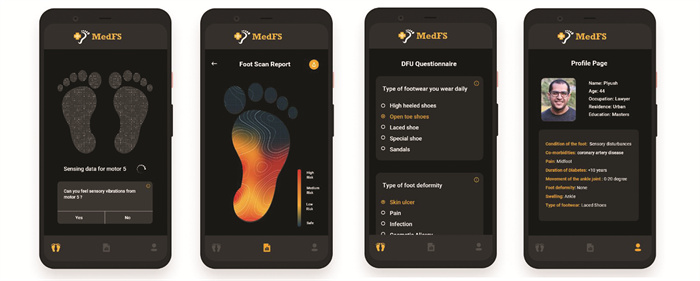
|
Fig.4 Mobile app interface used for early DFU detection and IoT interaction |
2 Results and Discussion
As seen in Table 4, the XG Boost classifier was 81% accurate in predicting the severity level of DFU, the highest among the rest of the classifiers chosen. The intelligent ensemble of gradient boosting and evaluators of XG Boost provided a desirable outcome. Whereas the Decision Tree classifier was least accurate with 67% due to the ignorance of interdependency between the features in the independent variables. The follow-up data collection was correctly carried out through the IoT setup of eight sensors and a sophisticated application that also collected the patient information and responses, yielding the prediction results and infographics for the user to interact with.
| Table 4 Comparative analysis of results obtained for each classifier |
3 Conclusions
If diagnosed early enough, foot ulcers are treatable and can be cured. Initial symptoms are hard to self-diagnose, and the danger of infection increases as time passes. This paper proposed a home-based system for the early detection and prediction of diabetic foot ulcers. For this, we developed a mobile application and an appropriate IoT setup of eight sensors to collect patient data. Through our collected data, we created a machine learning model based on supervised learning of 4 classification algorithms. As a result, the XG Boost classifier received an 81% accuracy in predicting the severity level of DFU, the highest among the rest of the classifiers. The prediction result is displayed in the form of infographics via the mobile application. Based on the severity measure and report of the patient, necessary treatment measures and suggestions are recommended via the application. Although the system successfully achieves preliminary results, there is a scope for further improvement. To produce faster results with large datasets, a classifier algorithm incorporated with deep learning and neural network can be developed. Enhanced automation features via the IoT interface can make the diagnosis more efficient and seamless.
AcknowledgmentsThe scholars Dr. J Jayashree, Dr. J.Vijayashree, Mr. Rishi Raghu, Ms. Kamasamudram Bhavya Sai, and Mr. Sai Surya Varshith Nukula, are thankful to the Doctors, Dr. P. Srinivas of Balaji Hospitals, Hanmakonda, Telangana, Dr. Nithin Kapoor CMC, Vellore for providing us the necessary help.
| [1] |
Shaw J E, Sicree R A, Zimmet P Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice, 2010, 87(1): 4-14. DOI:10.1016/j.diabres.2009.10.007 (  0) 0) |
| [2] |
Whiting D R, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice, 2011, 94(3): 311-321. DOI:10.1016/j.diabres.2011.10.029 (  0) 0) |
| [3] |
Kharroubi A T, Darwish H M. Diabetes mellitus: The epidemic of the century. World Journal of Diabetes, 2015, 6(6): 850-867. DOI:10.4239/wjd.v6.i6.850 (  0) 0) |
| [4] |
Leone S, Pascale R, Vitale M, et al. Epidemiologia del piede diabetico[Epidemiology of diabetic foot]. Infez Med., 2012, 20(Suppl 1): 8-13. (  0) 0) |
| [5] |
Boulton A J M, Whitehouse R W, Fiengold K R, et al. The diabetic foot. South Dartmouth (MA): MDText. com, Inc., 2020, PMID: 28121117.
(  0) 0) |
| [6] |
Brennan M B, Hess T M, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. Journal of Diabetes and Its Complications, 2017, 31(3): 556-561. DOI:10.1016/j.jdiacomp.2016.11.020 (  0) 0) |
| [7] |
Jiang F H, Liu X M, Yu H R, et al. The incidence of depression in patients with diabetic foot ulcers: a systematic review and meta-analysis. The International Journal of Lower Extremity Wounds, 2020, 21(2): 161-173. DOI:10.1177/1534734620929892 (  0) 0) |
| [8] |
Garrett C, Doherty A. Diabetes and mental health. Clinical Medicine, 2014, 14(6): 669-672. DOI:10.7861/clinmedicine.14-6-669 (  0) 0) |
| [9] |
Ghosh P, Valia R. Burden of diabetic foot ulcers in India: evidence landscape from published literature. Value in Health, 2017, 20(9): A485. DOI:10.1016/j.jval.2017.08.489 (  0) 0) |
| [10] |
Barshes N R, Saedi S, Wrobel J, et al. A model to estimate cost-savings in diabetic foot ulcer prevention efforts. Journal of Diabetes and Its Complications, 2017, 31(4): 700-707. DOI:10.1016/j.jdiacomp.2016.12.017 (  0) 0) |
| [11] |
Snyder R J, Kirsner R S, Warriner 3rd R A, et al. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage, 2010, 56(4 Suppl): S1-24. (  0) 0) |
| [12] |
Liu C, van der Heijden F, Klein M E, et al. Infrared dermal thermography on diabetic feet soles to predict ulcerations: a case study.. Proceedings of SPIE, Advanced Biomedical and Clinical Diagnostic Systems.San Francisco, California: SPIE, 2013, 85720N. DOI:10.1117/12.2001807 (  0) 0) |
| [13] |
Singh N, Armstrong D G, Lipsky B A. Preventing foot ulcers in patients with diabetes. Jama, 2005, 293(2): 217-228. DOI:10.1001/jama.293.2.217 (  0) 0) |
| [14] |
Fraiwan L, AlKhodari M, Ninan J, et al. Diabetic foot ulcer mobile detection system using smart phone thermal camera: a feasibility study. Biomedical Engineering Online, 2017, 16(1): 1-19. DOI:10.1186/s12938-017-0408-x (  0) 0) |
| [15] |
Khandakar A, Chowdhury M E H, Reaz M B I, et al. A machine learning model for early detection of diabetic foot using thermogram images. Computers in Biology and Medicine, 2021, 137: 104838. DOI:10.1016/j.compbiomed.2021.104838 (  0) 0) |
| [16] |
Goyal M, Reeves N D, Rajbhandari S, et al. Robust methods for real-time diabetic foot ulcer detection and localization on mobile devices. IEEE Journal of Biomedical and Health Informatics, 2019, 23(4): 1730-1741. DOI:10.1109/JBHI.2018.2868656 (  0) 0) |
| [17] |
Soban L. Tech Takes on Diabetic Foot Ulcers. DFCON2019* showcased new technologies coming online to aid in the treatment and prevention of diabetic foot ulcers. https://lermagazine.com/article/tech-takes-on-diabetic-foot-ulcers.
(  0) 0) |
| [18] |
Sahana H R, Shivani S U, Pallavi B, et al. Diabetic foot neuropathy monitoring system. International Journal of Engineering and Science, 2016, 5(12): 1-6. (  0) 0) |
| [19] |
Salvo P, Calisi N, Melai B, et al. Temperature-and pH-sensitive wearable materials for monitoring foot ulcers. International Journal of Nanomedicine, 2017, 12: 949-954. DOI:10.2147/IJN.S121726 (  0) 0) |
| [20] |
Orpyx Medical Tech Inc. Orpyx SI Sensory Insoles Help Prevent Diabetic Foot Ulcers 2020 Available from: https://www.orpyx.com/for-patients.
(  0) 0) |
| [21] |
Almobarak A O, Awadalla H, Osman M, et al. Prevalence of diabetic foot ulceration and associated risk factors: an old and still major public health problem in Khartoum, Sudan?. Annals of Translational Medicine, 2017, 5(17): 340. DOI:10.21037/atm.2017.07.01 (  0) 0) |
| [22] |
Naito R, Kasai T. Coronary artery disease in type 2 diabetes mellitus: Recent treatment strategies and future perspectives. World Journal of Cardiology, 2015, 7(3): 119-124. DOI:10.4330/wjc.v7.i3.119 (  0) 0) |
| [23] |
Jacoby R M, Nesto R W. Acute myocardial infarction in the diabetic patient: pathophysiology, clinical course and prognosis. Journal of the American College of Cardiology, 1992, 20(3): 736-744. DOI:10.1016/0735-1097(92)90033-j (  0) 0) |
| [24] |
Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome as a possible cardiovascular marker in diabetic patients. Journal of Diabetes Research, 2015, 2015: 268390. DOI:10.1155/2015/268390 (  0) 0) |
| [25] |
Mariam T G, Alemayehu A, Tesfaye E, et al. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow-up clinic at the University of Gondar Referral Hospital, North West Ethiopia, 2016: institutional-based cross-sectional study. Journal of Diabetes Research, 2017, 2017: 2879249. DOI:10.1155/2017/2879249 (  0) 0) |
| [26] |
Delbridge L, Perry P, Marr S, et al. Limited joint mobility in the diabetic foot: relationship to neuropathic ulceration. Journal of the British Diabetic Association, 1998, 5(4): 333-337. DOI:10.1111/j.1464-5491.1988.tb01000.x (  0) 0) |
 2024, Vol. 31
2024, Vol. 31


