Compared with traditional energy sources, hydrogen energy has the advantages of high energy density, high energy conversion efficiency and zero carbon emissions. Green hydrogen produced from biomass has the advantages of low cost and zero carbon emission. The related technology is one of the potential ways to solve environmental pressure and energy demand. It is considered to have great economic and technological competitiveness by IEA[1-2]. The elements in biomass are rich in carbon, less in hydrogen, and more in oxygen[3]. The gasification of H2O to produce hydrogen mainly uses “carbon” in biomass to replace “oxygen” in H2O, that is C+H2O→CO+H2 and CO+H2O→CO2+H2 reaction to obtain hydrogen-rich synthesis gas[4-5]. Biomass-H2O gasification is a complex thermochemical reaction process, including volatile matter removal, homogeneous/heterogeneous reforming and biochar gasification etching[6-8]. Due to different reaction stages, chemical activity and mass transfer resistance, the gasification rate of biochar is far lower than the pyrolysis rate so the gasification of biochar is considered the rate control step of the whole biomass gasification reaction[9-10].
Although gasification is widely used, chemical reduction of H2O is usually difficult from the point of view of kinetics and thermokinetics[11] if high temperature and pressure are not applied[12]. However, some metal catalysts enable gasification to take place under significantly milder conditions. In particular, alkali metals show high catalytic activity, which can reduce the gasification temperature[13-14]. As a high-content element in biomass and a metal element with a high catalytic performance of AAEMs, K has a deeper impact on the thermochemical conversion of biomass than other AAEMs[15]. K has the highest catalytic activity in C-H2O gasification catalyzed by AAEMs, and the catalytic ability is K>Na>Mg from high to low[16].In a previous study,it was concluded that the function of K is mainly to form O-containing functional groups and to transform small ring systems to larger ones during biochar-H2O gasification[17]. Transition metal catalysts also play an obvious role in hydrogen production from biomass gasification and have excellent performance in hydrogen production from biomass gasification[18-20]. Nickel-based catalysts make the gas output of steam gasification of wood chips increase significantly, the H2 output increased from 30.1vol% to 50.6vol%[21]. It summarized and reported the kinetic process of methane catalytic steam reforming on the nickel-based catalyst, which is an oxygen transfer mechanism in essence. On this basis, the following mechanisms are proposed[22]:
| $ \begin{gathered} \mathrm{H}_2 \mathrm{O}(\mathrm{g})+* \rightarrow \mathrm{O} *(\mathrm{a})+\mathrm{H}_2(\mathrm{~g}) \\ \mathrm{CH}_4(\mathrm{~g})+2 * \rightarrow \mathrm{CH}_3(\mathrm{a})+\mathrm{H} *(\mathrm{a}) \\ \mathrm{CH}_3 *(\mathrm{a})+* \rightarrow \mathrm{CH}_2 *(\mathrm{a})+\mathrm{H} *(\mathrm{a}) \\ \mathrm{CH}_2 *(\mathrm{a})+* \rightarrow \mathrm{CH} *(\mathrm{a})+\mathrm{H} *(\mathrm{a}) \\ \mathrm{CH} *(\mathrm{a})+\mathrm{O} *(\mathrm{a}) \rightarrow \mathrm{CO} *(\mathrm{a})+\mathrm{H} *(\mathrm{a}) \\ \mathrm{CO} *(\mathrm{a}) \rightarrow \mathrm{CO}(\mathrm{g})+* \\ 2 \mathrm{H} *(\mathrm{a}) \rightarrow \mathrm{H}_2(\mathrm{~g})+2 * \end{gathered} $ |
where * represents the Ni atom on the catalyst surface.“a” and “g” represent free radicals and gas respectively.
Although these experiments put forward the structure and mechanism of catalytic active centers in gasification, no specific conclusion on catalysis has been drawn. Especially at high temperatures, it is difficult to conclude the catalytic mechanism through experiments.
Quantum chemistry and molecular dynamics calculation are important methods to study the reaction mechanism. Since 30 years ago, scholars[23-28] have studied the catalytic effect of metals on gasification by using this method, and have drawn many valuable conclusions. It is concluded that H2O dissociates from the active site at the edge of the graphite model, generating H2 and semiquinone[29]. Hydroxy groups are generated by water on the active sites of the graphite model edge and H of the hydroxy group transfers to the graphite model edge, converting the hydroxy group into a semiquinone[30]. It is believed that there are two forms of C-O-K: K2O-C and K2O2-C, they have a recycling process in the catalytic gasification reaction[31]. The mechanism of carbon dissolution[32] means that once the carbon enters the metal, it will get rid of the bondage of the graphite aromatic structure and get higher reactivity. Notably, these researchers just analyzed the reaction paths based on thermodynamics but did not calculate the transition states of the reaction paths.
Although the above research mentioned the influence of alkali metals and transition metals on the gasification reaction, it cannot explain the experimental phenomenon that the existence of alkali metals reduces the temperature and activation energy required for the reaction to occur, and there is still a lack of essential understanding of its specific mechanism. From this point of view, quantum chemical simulation is an effective method to analyze this problem. In this paper, density functional theory (DFT) was used to study the mechanism of K and Ni in the gasification and etching stage of biocarbon-H2O, and the specific mechanism of alkali metal and transition metal catalysts was discussed.
1 ComputationThe study on effect of ketones on coal indicated that coal will form a lamellar structure after gasification[33]. The properties of the carbon layer have little relationship with the number of benzene systems[34]. The previous density functional calculation of biochar often uses aromatic ring clusters as model compounds[35-37]. Comparing the single-layer graphite model with the multi-layer graphite model, it is found that the single-layer model is sufficient to characterize[38]. Among all sorts of models, it was finally found that the zigzag type and armchair type models were most suitable for the structures generated during coal gasification[39-40], as shown in Fig. 1. Also, electron microscope showed that the edge structure in the gasification environment is similar to the armchair type and zigzag type during the gasification process[41-42]. The reactivity of the zigzag edge is higher than that of the armchair edge[43] but their catalytic essence is the same[44]. Polycyclic hydrocarbons (PAH) are more reasonable for describing the structure of carbon materials[45], and consistent with Montoya's conclusion, the size of the benzene ring system in PAH almost does not affect the reaction path. Therefore, under the limited computing resources, the zigzag type with 6 rings was applied as the biochar model.
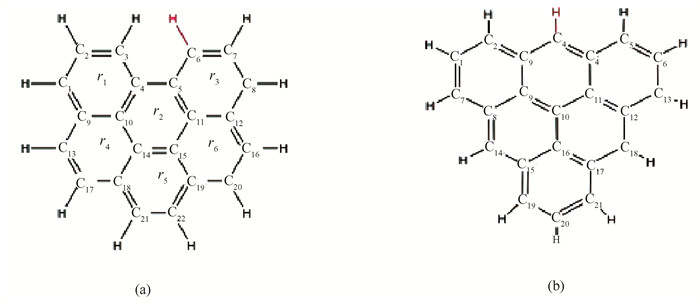
|
Fig.1 Two different biochar models (a) armchair model without active sites (Arm); (b) zigzag model without active sites (Zig) |
In addition, whether there are active sites is also a consideration for model selection. It was found that active sites were difficult to exist in practice in the coal structures[46], so H radical was used for closed-loop processing. And in the opinion of experimental researchers, the edge of such high-spin coke with multiple active carbon sites is difficult to exist. In high-temperature steam reactions, a large amount of free hydrogen can easily block these active carbon sites, so the biochar models in this paper do not contain active sites. The final biochar model is shown in Fig. 1(b).
The functional M06-2X can more effectively describe the reaction between metal and organic compounds than B3LYP[47-48]. And the def2svp basis set can well eliminate the error caused by dispersion[49], so this paper will calculate the single point energy with M062X/def2svp for non-catalytic and K-catalytic reaction. As for the Ni-catalytic reactions,a lower HF exchange function should be used. This paper applied PBE /def2-svp to calculate the single point energy for Ni-catalytic reaction because it's confirmed that PBE /def2-svp can well describe the role between the transition metal and coke models[50]. All reactants, products and intermediates are fully optimized to ensure that no virtual frequency occurs and all possible transition states are identified by the presence of a single imaginary frequency. In addition, all transition states are identified by IRC calculation, and their functional and basis sets are completely consistent with those of transition state search. All calculations are performed in Gaussian16. Gaussview is a visualization tool used with Gaussian to show the model. The electronic wave function information can be obtained when solving the Schrodinger equation. The wave function analysis can be used to study the distribution and behavior of electrons and the atomic bond-breaking process, describing the chemical reaction process from the perspective of electronic changes and analyzing the essence of the reaction. At present, the reduced density function (RDG)[51] is usually used to describe the interactions between molecules and atoms. RDG is defined as:
| $ \operatorname{RDG}(r)=\frac{1}{2\left(3 {\rm{ \mathsf{ π} }}^2\right)^{1 / 3}} \frac{|\nabla \rho(r)|}{\rho(r)^{4 / 3}} $ |
where (r) is the electron density, ▽ is the gradient operator, and |▽(r)| is the mode of the electron density gradient. RDG analysis in this paper is completed through Multiwfn[52]. The canonical molecular orbital generated by quantum chemical calculation is the eigenfunction of the KS operator. Regular molecular orbitals are highly delocalized and cannot be one-to-one corresponded with chemical bonds. If we want to directly link the orbitals and chemical bonds, we need to do the unitary transformation to occupy the regular molecular orbitals for orbital localization. The aromaticity of the system can be studied by localizing the π electrons in the molecular system through the localized orbital locator function[53](Localized orbital locator, LOL).
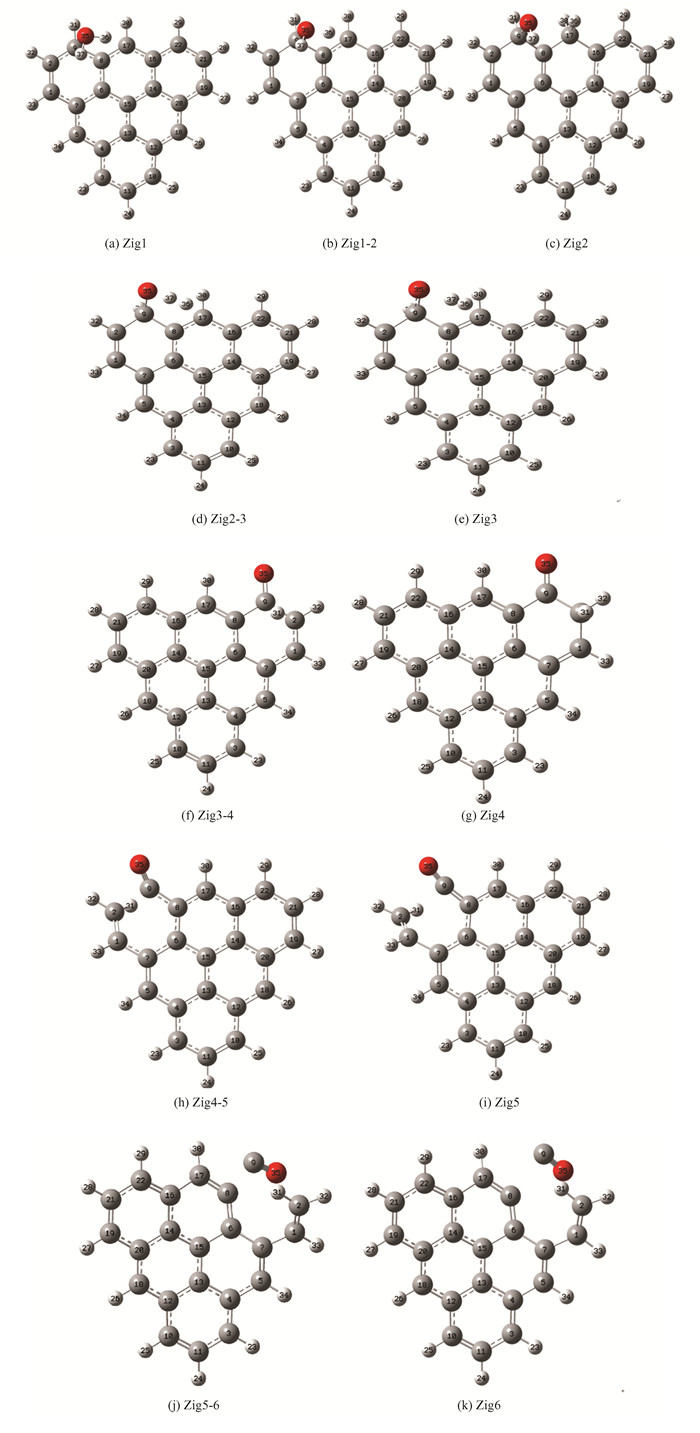
2 Results and Discussion 2.1 Zigzag Biochar Gasification without CatalysisThe reaction path of zigzag biochar and H2O-gasification process is shown in Fig. 2(a). Its transition state structure and corresponding reactants and products are all listed therein. The specific atomic number and virtual frequency size are given in the Supplementary Information. The reaction starts with the dissociation of H2O. The OH-H bond in H2O breaks to generate free H and OH. Then the O in the OH group will bind to a C atom on the biochar (O35 binds to C9). The H atom binds to C17, which is a hydrogen transfer process associated with the dissociation of water[54]. After the reaction is completed, C17 on the biochar matrix is occupied by two H atoms to form the H-C17-H structure, while C9 is occupied by OH and H to form the H-C9-OH structure, making C9 and C17 saturated. After that, H37 on the original hydroxyl starts to be far away from O35, H36 is far away from C17, and the two hydrogen atoms are far away from each other in the same direction. The bond length of the two hydrogen atoms gradually decreases. Finally, the bond length of H36-H37 is 0.74 angstrom, which is consistent with the H-H bond length in hydrogen[55], indicating that the process of Zig2 to Zig3 completes the transfer and desorption of hydrogen. After that, H31 connected to C9 will be transferred to adjacent C2. Under the oxidation of O35, the C2-C9 bond will break, which is a carbon dissolution process. Finally, the C9-C8 bond will break, generating CO, which is a CO desorption process.
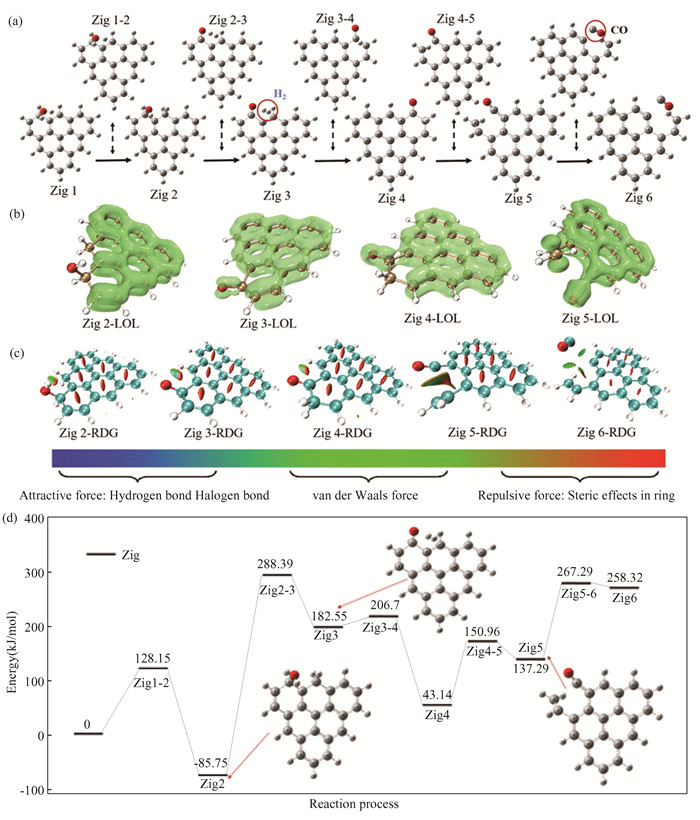
|
Fig.2 Calculation results (a)gasification process of zigzag biochar with water; (b)typical structure of LOL isosurface;(c)typical structure of RDG;(d)zigzag biochar water gasification reaction energy barrier |
In the model of biochar constructed, the electrons in the aromatic ring form conjugated π orbitals by sp2 hybridization, and the π orbitals are dispersed in the whole biochar plane. Conjugated large π bonds make it difficult for C-C in aromatic rings to break. Localization calculation of π electron orbit is carried out through LOL[53] to obtain LOL-π isosurface as shown in Fig. 2(b). The green area in the figure represents the π bond. It can be seen that the hydrolyzed OH and H break the conjugated π orbital, which makes its structure unstable and prone to hydrogen transfer and C-C bond breaking. The reduced density gradient function (RDG) can be used to analyze the weak interaction of the system. The RDG changes of typical structures in the reaction path are shown in Fig. 2(c). Blue, green and red represent attraction, van der Waals force and repulsion force respectively. In the whole reaction process, the relatively weak van der Waals force is dominant, and there is also some repulsive force in the process of hydrogen transfer and carbon dissolution, which is also the reason why the reaction can occur. After the dissociation of H2O molecules, there are two types of interactions between OH and O: the van der Waals force of O-H and repulsive force between H-H. This explains why hydrogen transfer is difficult to occur: the need of overcoming the repulsive force between H-H while preventing the binding of O-H. During carbon dissolution, CO and the carbon matrix exhibit a force between Van Der Waals force and repulsive force, which appears dark red in the RDG diagram. This is why this stage is more prone to occur than that of hydrogen transfer.
The energy barrier diagram of the reaction path is shown in Fig. 2(d). The highest energy barrier step is the dehydrogenation step of “hydrogen transfer”, and the energy barrier reaches 374.14 kJ/mol. The energy barrier of the carbon dissolution step is 107.82 kJ/mol and the energy barrier of CO desorption is 130 kJ/mol. The energy barrier of hydrolysis desorption is 128.15 kJ/mol, and the energy barrier of the second “hydrogen transfer” is not high(24.15 kJ/mol). It can be seen that the decisive step of the reaction is the first transfer of hydrogen[56-57], that is, the dehydrogenation step and the energy barrier is much higher than in other steps. In contrast, the energy barrier of water dissociation and carbon dissolution is not high, and the reaction is relatively easy to occur.
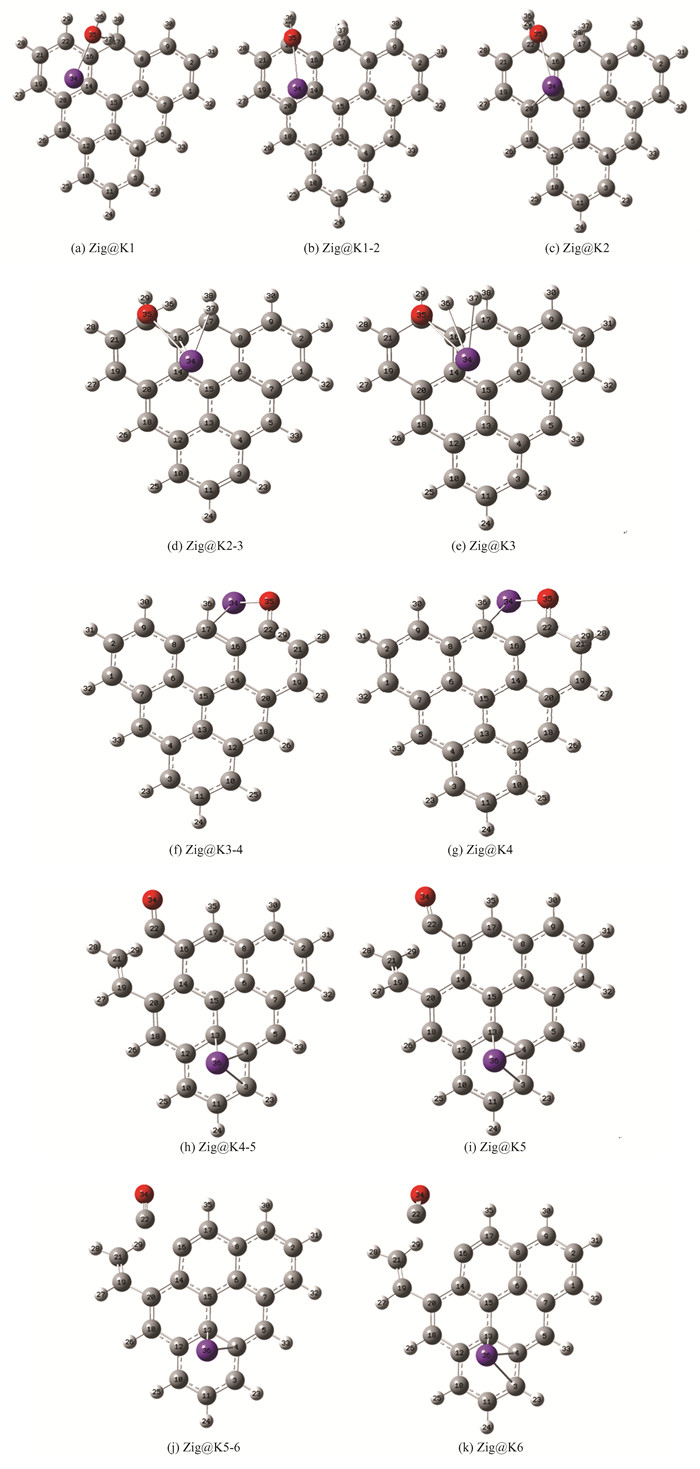
2.2 Zigzag Biochar Gasification with Catalytic KThe reaction path of zigzag biochar gasification with water under the catalysis of K is shown in Fig. 3(a). K first bonds with H2O to form the K-O structure, but not with the C atom. K is suspended on the carbon ring at this time, and there is only weak interaction traction, which is consistent with previous experimental results[58-59]. Later, O35 is close to C22, H37 is close to C17 and K is far away from the carbon matrix plane. About Zig@K2, H2O has been completely dissociated. When the dissociated hydroxyl group attacks the edge coke C22, K leaves the benzene ring and connects with O35 to form a C-O-K structure dominated by oxygen transfer. About Zig@K3, it can be seen that K directly participates in the hydrogen transfer process, which directly acts on H37, while the C-O-K structure makes K indirectly act on H36. K and C17 form the C-K structure, and No. 35 O form the C-O-K structure. This structure has no obvious effect on the second hydrogen transfer. The structure of C-O-K also indirectly affects the transfer of H29 from Zig@K3-4 to Zig@K4. Apart from K, Zig@K4-5 is consistent with Zig4-5. In fact, at this time, because the K atom has restored the C-K structure, in the reaction without the participation of the H, the connection between K and O is broken, and it is adsorbed on the coke structure by the weak interaction, which has little effect on the carbon dissolution process. In the CO desorption stage, K and O are still unconnected and adsorbed on the coke structure by the weak interaction. In this reaction, the mutual transformation of CK structure and C-O-K structure is consistent with the conclusion that C-O-K existing in oxygen transfer in the reaction process is the precursor of CK structure proposed by predecessors[60-61].

|
Fig.3 Calculation results (a)gasification process of zigzag biochar with catalytic K; (b)typical structure of LOL isosurface;(c)typical structure of RDG;(d)zigzag biochar water gasification reaction energy barrier comparison |
The obtained K-catalytic energy barrier diagram is compared with the catalyst-free energy barrier diagram, as shown in Fig. 3(d). Under the catalysis of K, the energy barrier of hydrolysis desorption is 141.82 kJ/mol, the hydrogen transfer barrier of hydrogen desorption is 152.41 kJ/mol, the carbon dissolution barrier is 175.09 kJ/mol, and the CO desorption barrier is 111.91 kJ/mol.Compared with the process without a catalyst, the most significant step for K to reduce the activation energy is the hydrogen transfer step, which decreases from 374.14 kJ/mol to 152.41 kJ/mol, while steps such as carbon dissolution have no obvious catalytic effect. At the beginning of the reaction, similar to the process without a catalyst, the whole coke molecular plane is scattered with π bonds. From the LOL-π isosurface graph in Fig. 3(b), it can be seen that K does not destroy the π bonds of biochar molecules. The destruction of π bonds is mainly due to the role of H free radicals, while the destruction of π bonds will lead to easier C-C bond rupture. The existence of K inhibits the destruction of π bonds by H free radicals, so in the CO desorption phase dominated by carbon dissolution, the reaction energy barrier of Zig@K(175.09 kJ/mol)is higher than that of catalytic free gasification(107.82 kJ/mol).The reduced density function of K catalysis is shown in Fig. 3(c).In the hydrogen transfer reaction, the existence of K causes the edge carbon to have a strong attraction to H, while the edge without K does not have a strong attraction to H. Therefore, K in the form of CK directly promotes the attraction of C to H, making the H transfer reaction easier to occur, reducing the reaction energy barrier, and achieving a significant catalytic gasification reaction. However, K also shows strong attraction to O and C (blue disk between K and O shown in Fig. 3(c)) after the desorption of H2 molecules generated by H transfer. In the structure of Zig@K5, although K is far away from the reaction area of marginal carbon, compared with the Zig5 structure, marginal C and adjacent C have a strong van der Waals and hydrogen bond attraction, which leads to the difficulty of breaking the C-C bond. That is why the decisive steps of K catalytic Char-H2O gasification reaction are carbon dissolution and CO desorption. It is obtained that K will inhibit the breaking of the C-C bond.
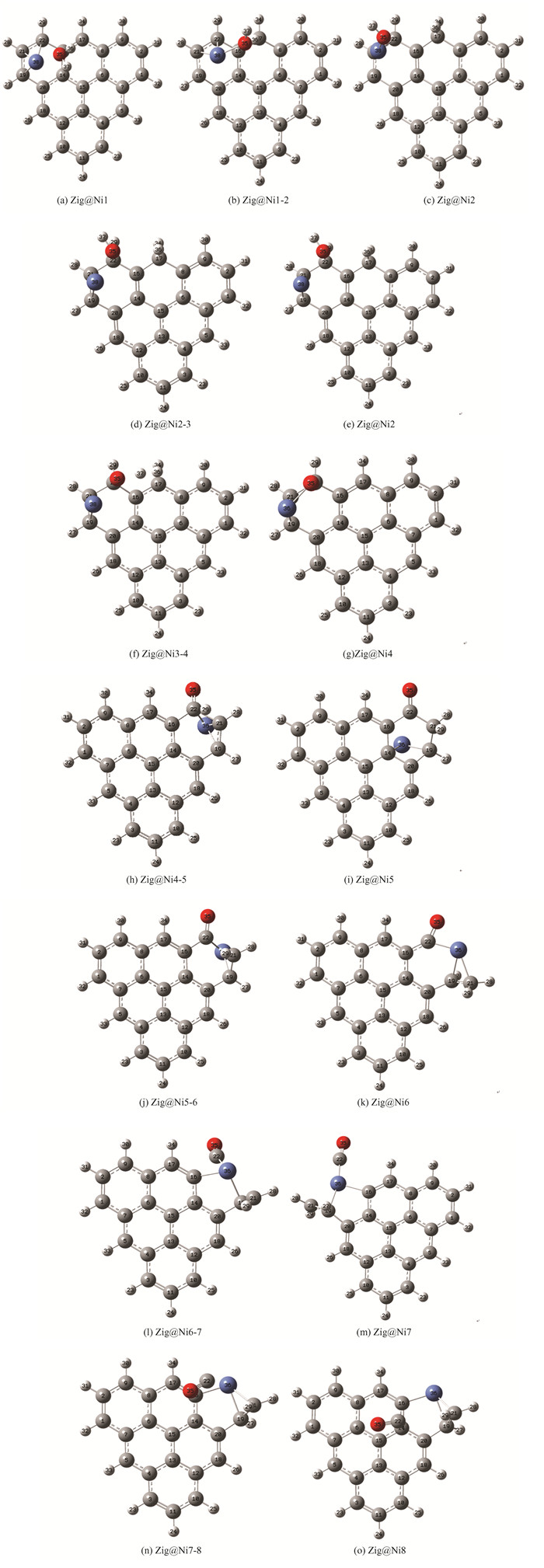
2.3 Zigzag Biochar Gasification with Catalytic NiThe reaction path of zigzag biochar gasification with water under the catalysis of Ni is shown in Fig. 4(a). It is reported that in the process of the gasification of char-CO2, Fe is firstly adsorbed on the carbon ring then dissociates the O in CO2 and transfers it to the coke molecules, forming a carbon-oxygen complex C(O) after absorbing CO2[44].Based on this conclusion and geometric optimization, the initial structure was gained. Firstly, it can be seen that the water molecule is now in a free state, far from the carbon matrix plane, which is not enough to form a C-O bond. At this time, Ni has formed a strong interaction with the carbon ring, which is also consistent with previous experiments on catalytic gasification of transition metals: Asami[62] conducted gasification experiments on FeCl3 treated coal, and found Fe3C substances in the gasification process, which indicates that transition metals can form stable bonds with carbon directly. Different from the dissociation step of catalyst-free water, O35 does not directly act on the carbon atom, but first acts with Ni, which may be conducive to the dissociation and adsorption of water. In Zig@Ni2, H36 and C17 have formed a stable C-H bond, while the dissociated hydroxyl group has not yet acted on the carbon matrix, but temporarily bonds with Ni. This process belongs to the proprietary step of Ni catalysis, which is not shown in K catalysis. Then O35 and Ni38 are far away from each other; O35 and C22 are close to each other. Zig@Ni3 structure is similar to its transition state, the difference is that O35 and C22 have been bonded, and the bond length is 1.48 angstrom, which is the stable bond length of the C-O bond. Secondly, Ni has shifted a certain distance to the left, increasing the bond length with O35, which indicates that water molecules have completed the dissociation adsorption process. It is not difficult to find that Ni did not participate in the hydrogen transfer process. In Zig@Ni4, Ni, O, and C form the C-Ni-O-C structure, which weakens the aromatic structure of the carbon ring and is conducive to subsequent carbon dissolution and CO desorption. In Zig@Ni5, Ni is adsorbed on C14 and C19. It can be seen that the carbon ring is deformed due to the role of transition metal Ni, and C19 deviates from the carbon matrix plane. Apart from the effect of Ni on the carbon ring, it has little effect on the hydrogen transfer step. In the process of carbon dissolution, Ni is bonded with C22, C21 and C19. The effect of Ni on the carbon ring reflects the etching effect of transition metal on the carbon matrix, and the effects of Ni, C22, and C21 prevent the re-establishment of the C-C bond, further strengthening its etching effect. In Zig@Ni6, it can be found that C21 has been completely extruded from the carbon plane at this time, and O35 has also deviated from a certain angle around C22, farther away from C16, showing a trend of CO desorption. In this structure, Ni still interacts with C22, C21 and C16, indicating that Ni participates in the whole process of carbon dissolution. Ni and O can achieve the function of synergistic etching in the carbon dissolution stage. Based on Zig@Ni6 searched for the basic structure, a transition state structure is obtained. The bond length between C16 and C22 is 1.81 angstrom, which is longer than the bonding distance of C-C. At this time, C22 has been removed from the carbon matrix plane, but C22 still interacts with Ni, and the bond length is 1.72 angstrom. This process is also different from the K catalytic process, which shows that in Char-H2O gasification with Ni participation, CO desorption also needs to overcome the effect of Ni on C.
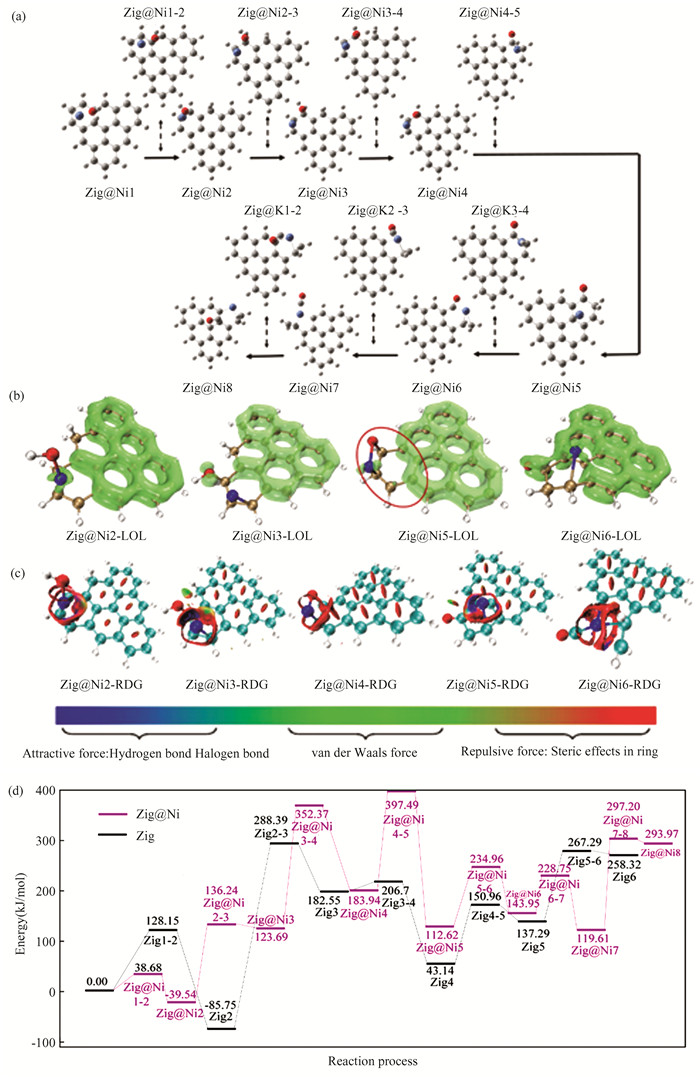
|
Fig.4 Calculation results (a) gasification process of zigzag biochar with catalytic Ni; (b) typical structure of LOL;(c) typical structure of RDG;(d) zigzag biochar water gasification reaction energy barrier comparison |
Under the catalysis of Ni, the hydrolysis barrier is 38.68 kJ/mol, the fully adsorbed barrier is 175.78 kJ/mol, the hydrogen transfer barrier of hydrogen desorption is 228.68 kJ/mol, the second hydrogen transfer barrier is 213.55 kJ/mol, the carbon dissolution barrier is 84.8 kJ/mol, and the second desorption barrier is 177.59 kJ/mol.Compared with the process without a catalyst, the activation energy of Ni reduction is not reflected in the hydrogen transfer step, while the energy barrier of the carbon dissolution step is significantly reduced. However, due to the second CO desorption, the overall desorption energy barrier increased from 111.91 kJ/mol to 177.59 kJ/mol.The addition of Ni also affects the dissociation process of water. It can be seen that the dissociation energy barrier of water becomes very low, only 38.68 kJ/mol, but at this time, the dissociated group acts with Ni and does not adsorb carbon. Therefore, second adsorption is required. At this time, the energy barrier is 175.78 kJ/mol, higher than the original 128.15 kJ/mol. From this point of view, the addition of Ni may have a side effect, that is, it increases the energy barrier of hydrolysis desorption and CO desorption. Whether the side effect is generated depends on whether the energy barrier brought by Ni is higher than the previous one.Fig. 4(b) shows the LOL-π isosurface map of typical structure in Char-Ni-H2O gasification reaction. It can be seen that the whole gasification reaction takes place on the two aromatic rings at the edge, and the π orbital of the aromatic ring is mainly destroyed by hydrolyzed H, OH, and catalytic metal Ni. In the hydrogen transfer stage, due to the combined action of H, and OH groups, the π orbitals of the two aromatic rings at the edge of the biochar are destroyed. In the carbon dissolution stage, it can be found that Ni can destroy the π orbitals of the benzene ring, making it easier to break, which corresponds to the previous energy barrier analysis.The contour map of the reduced density function of Ni catalysis is shown in Fig. 4(c). After the dissociation of water molecules to form OH and its binding with Ni, it exerts a repulsive effect on O. As the distance between OH and Ni increases, the repulsive force gradually becomes stronger hydrogen-bond attractive force. Therefore, the subsequent hydrogen transfer process needs to overcome this attractive force to happen. In the following reaction process, the type of force between Ni and O varies as the distance between the two changes. Throughout the entire process, there are obvious red discs around Ni, which means it exerts a strong repulsive force on the surrounding atoms. The specific mechanism of Ni weakening the aromaticity of biochar is reflected in the repulsive force of Ni on surrounding carbon atoms, which makes the structure of benzene ring more fragile.
2.4 K-Ni Synergistic Catalysis of Biochar-H2O GasificationIn the above study, it can be found that alkali metal K and transition metal Ni have different catalytic effects on the gasification of biocarbon-H2O. The decisive step of the gasification reaction of biocarbon-H2O is the H transfer process, which hinders the occurrence of the gasification reaction. In the reaction path of Char-H2O, where K catalyzes gasification alone, K will inhibit the destruction of π orbitals by OH and H and enhance the strong attraction of C-C, making the H transfer process easier to occur. The breaking process of the C-C bond is the key to hindering K catalytic gasification. In the reaction path of catalytic gasification of C-H2O by Ni alone, Ni mainly destroys the π orbital in the aromatic ring, thus reducing the energy barrier of carbon dissolution. The predecessors have done some research on the synergistic effect of multi-metals, It is found that the K-Co catalyst has the best catalytic activity for the gasification of carbon, while the Fe in the K-Fe catalyst is gradually oxidized to higher valence Fe, thereby reducing the carbon gasification rate[63]. Therefore, the overall energy barrier of the whole gasification process may become lower and the reaction rate may be faster due to the synergistic effect of the two. However, it is not ruled out that there is such a competitive relationship between the two (such as the competition between Ni and OH strong force and K against OH van der Waals force), which makes the reaction process more difficult to occur. The potential K/Ni interaction relationship is shown in Fig. 5.
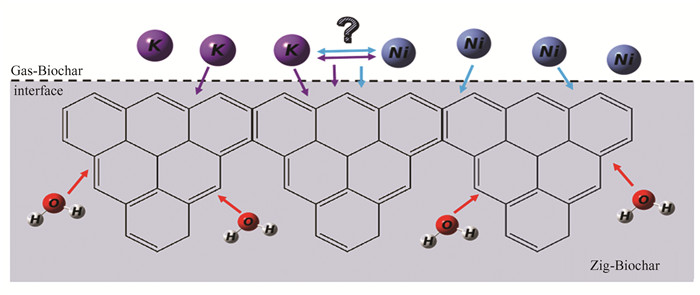
|
Fig.5 K-Ni synergistic mechanism |
3 Conclusions
1) The addition of a catalyst does not change the basic reaction, that is, no matter whether there is a catalyst or what kind of catalyst is, all reaction path reaction processes are composed of four basic reactions: dissociation adsorption of water, hydrogen transfer, carbon dissolution, and CO desorption.
2) In the Zig model, the highest energy barrier step in the gasification reaction of biochar is the dehydrogenation step of “hydrogen transfer”, and the energy barrier reaches 374.14 kJ/mol. The energy barrier of the “carbon dissolution” step is 107.82 kJ/mol, and the energy barrier of CO desorption is 130 kJ/mol.
3) The energy barrier of hydrolysis desorption is 128.15 kJ/mol, and the energy barrier of the second “hydrogen transfer” is not high (24.15 kJ/mol). The decisive step of the reaction is the first transfer of hydrogen and dehydrogenation reaction, and the energy barrier is much higher than the other steps. In contrast, the energy barrier of water dissociation and “carbon dissolution” is not high, and the reaction is relatively easy to occur.
4) The most significant step for K to decrease the activation energy was the hydrogen transfer step, which decreased from 374.14 kJ/mol to 152.41 kJ/mol. K in the form of CK directly promotes the attraction of C to H, making the H transfer reaction easier to occur, reducing the reaction energy barrier.
5) The catalytic effect of Ni is mainly embodied in the carbon dissolution step because Ni can destroy the aromatic structure directly, making the gasification easier to happen. The energy barrier is reduced from 122.34 kJ/mol to 84.8 kJ/mol, but the addition of Ni may cause a higher energy barrier for the hydrogen transfer due to the attractive force between Ni and OH.
6) There may be a synergistic effect between K-Ni and a complementary and enhanced effect between them, but there may also be a competitive relationship between them.
Supplementary Information
|
S1 Gasification process of zigzag biochar with water |
| Table S1 E and Erel of each structure and the frequency of their transition states |
|
S2 Gasification process of zigzag biochar with catalytic K |
| Table S2 E and Erel of each structure and the frequency of their transition states |
|
S3 Gasification process of zigzag biochar with catalytic Ni |
| Table S3 E and Erel of each structure and the frequency of their transition states |
| [1] |
Shahabuddin M, Krishna Bhavya B, Bhaskar T, et al. Advances in the thermo-chemical production of hydrogen from biomass and residual wastes: Summary of recent techno-economic analyses. Bioresource Technology, 2019, 299: 122557. DOI:10.1016/j.biortech.2019.122557 (  0) 0) |
| [2] |
Alazemi J, Andrews J. Automotive hydrogen fuelling stations: An international review. Renewable and Sustainable Energy Reviews, 2015, 48: 483-499. DOI:10.1016/j.rser.2015.03.085 (  0) 0) |
| [3] |
Li B, Mbeugang C F M, Huang Y, et al. A review of CaO based catalysts for tar removal during biomass gasification. Energy, 2022, 244(Part B): 123172. DOI:10.1016/j.energy.2022.123172 (  0) 0) |
| [4] |
Soni T, Salvi B L. A computational fluid dynamics study of condenser for condensation of bio-oil vapour from fast pyrolysis of biomass. International Journal of Renewable Energy Technology, 2021, 11(4): 335-345. DOI:10.1504/IJRET.2020.113770 (  0) 0) |
| [5] |
Feng D D, Yu Z, Zhao Y J, et al. Mechanism of in-situ dynamic catalysis and selective deactivation of H2O-activated biochar for biomass tar reforming. Fuel, 2020, 279(10): 118450. DOI:10.1016/j.fuel.2020.118450 (  0) 0) |
| [6] |
Zhao Y J, Feng D D, Zhang Y, et al. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Processing Technology, 2016, 141(Part 1): 54-60. DOI:10.1016/j.fuproc.2015.06.029 (  0) 0) |
| [7] |
Zhang C T, Chao L, Zhang Z M, et al. Pyrolysis of cellulose: Evolution of functionalities and structure of bio-char versus temperature. Renewable & Sustainable Energy Reviews, 2021, 135: 110416. DOI:10.1016/j.rser.2020.110416 (  0) 0) |
| [8] |
Guo F, Dong Y C, Tian B L, et al. Applications of microwave energy in gas production and tar removal during biomass gasification. Sustainable Energy & Fuels, 2020, 4(12): 5927-5946. DOI:10.1039/d0se01024c (  0) 0) |
| [9] |
Mallick D, Mahanta P, Moholkar V S. Synergistic Effects in Gasification of Coal/Biomass Blends: Analysis and Review. Coal and Biomass Gasification. Energy, Environment, and Sustainability. De S, Agarwal A, Moholkar V, Thallada B.(eds). Singapore: Springer, 2018.DOI: 10.1007/978-981-10-7335-9_19.
(  0) 0) |
| [10] |
Zhang Y, Wang S Z, Feng D D, et al. Functional biochar synergistic solid/liquid-phase CO2 capture: A review. Energy & Fuels, 2022, 36(6): 2945-2970. DOI:10.1021/acs.energyfuels.1c04372 (  0) 0) |
| [11] |
Calo J M, Perkins M T. A heterogeneous surface model for the “steady-state” kinetics of the boudouard reaction. Carbon, 1987, 25(3): 395-407. DOI:10.1016/0008-6223(87)90011-X (  0) 0) |
| [12] |
Zhao Y J, Feng D D, Zhang Y, et al. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Processing Technology, 2016, 141(Part 1): 54-60. DOI:10.1016/j.fuproc.2015.06.029 (  0) 0) |
| [13] |
Iwasa N, Yamane T, Arai M. Influence of alkali metal modification and reaction conditions on the catalytic activity and stability of Ni containing smectite-type material for steam reforming of acetic acid. International Journal of Hydrogen Energy, 2011, 36(10): 5904-5911. DOI:10.1016/j.ijhydene.2011.01.155 (  0) 0) |
| [14] |
Nzihou A, Stanmore B, Lyczko N, et al. The catalytic effect of inherent and adsorbed metals on the fast/flash pyrolysis of biomass: A review. Energy, 2019, 170: 326-337. DOI:10.1016/j.energy.2018.12.174 (  0) 0) |
| [15] |
Shen Y F, Zhang N Y, Zhang S. Catalytic pyrolysis of biomass with potassium compounds for Co-production of high-quality biofuels and porous carbons. Energy, 2020, 190: 116431. DOI:10.1016/j.energy.2019.116431 (  0) 0) |
| [16] |
Walker P L, Matsumoto S, Hanzawa T, et al. Catalysis of gasification of coal-derived cokes and chars. Fuel, 1983, 62(2): 140-149. DOI:10.1016/0016-2361(83)90186-2 (  0) 0) |
| [17] |
Feng D D, Zhao Y J, Zhang Y, et al. Catalytic mechanism of ion-exchanging alkali and alkaline earth metallic species on biochar reactivity during CO2/H2O gasification. Fuel, 2018, 212: 523-532. DOI:10.1016/j.fuel.2017.10.045 (  0) 0) |
| [18] |
Stonor M R, Chen J G, Park A. Bio-Energy with Carbon Capture and Storage (BECCS) potential: Production of high purity H2 from cellulose via Alkaline Thermal Treatment with gas phase reforming of hydrocarbons over various metal catalysts. International Journal of Hydrogen Energy, 2017, 42(41): 25903-25913. DOI:10.1016/j.ijhydene.2017.08.059 (  0) 0) |
| [19] |
Quan C, Wang H H, Gao N B. Development of activated biochar supported Ni catalyst for enhancing toluene steam reforming. International Journal of Energy Research, 2020, 44(7): 5749-5764. DOI:10.1002/er.5335 (  0) 0) |
| [20] |
Zhang C T, Zhang L J, Li Q Y, et al. Catalytic pyrolysis of poplar wood over transition metal oxides: Correlation of catalytic behaviors with physiochemical properties of the oxides. Biomass & Bioenergy, 2019, 124: 125-141. DOI:10.1016/j.biombioe.2019.03.017 (  0) 0) |
| [21] |
Wu C, Wang L, Williams P T, et al. Hydrogen production from biomass gasification with Ni/MCM-41 catalysts: Influence of Ni content. Applied Catalysis B Environmental, 2011, 108: 6-13. DOI:10.1016/j.apcatb.2011.07.023 (  0) 0) |
| [22] |
Wei J, Iglesia E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. Journal of Catalysis, 2004, 224(2): 370-383. DOI:10.1016/j.jcat.2004.02.032 (  0) 0) |
| [23] |
Chen S G, Yang R T. Unified mechanism of alkali and alkaline earth catalyzed gasification reactions of carbon by CO2 and H2O. Fuel & Energy Abstracts, 1997, 38(4): 230. (  0) 0) |
| [24] |
Calderón L A, Garza J, Espinal J F. Theoretical study of sodium effect on the gasification of carbonaceous materials with carbon dioxide. The Journal of Physical Chemistry A, 2015, 119(51): 12756-12766. DOI:10.1021/acs.jpca.5b07020 (  0) 0) |
| [25] |
Frankcombe T J, Smith S C. On the microscopic mechanism of carbon gasification: A theoretical study. Carbon, 2004, 42(14): 2921-2928. DOI:10.1016/j.carbon.2004.07.002 (  0) 0) |
| [26] |
Zhu Z, Lu G Q, Finnerty J, et al. Electronic structure methods applied to gas-carbon reactions. Carbon, 2003, 41(4): 635-658. DOI:10.1016/S0008-6223(02)00380-9 (  0) 0) |
| [27] |
Roberts M J, Everson R C, Domazetis G, et al. Density functional theory molecular modelling and experimental particle kinetics for CO2-char gasification. Carbon, 2015, 93: 295-314. DOI:10.1016/j.carbon.2015.05.053 (  0) 0) |
| [28] |
Feng D D, Shang Q, Dong H M, et al. Catalytic mechanism of Na on coal pyrolysis-derived carbon black formation: Experiment and DFT simulation. Fuel Processing Technology, 2021, 224: 107011. DOI:10.1016/j.fuproc.2021.107011 (  0) 0) |
| [29] |
Zhu Z H, Finnerty J, Lu G Q, et al. Molecular orbital theory calculations of the H2O Carbon reaction. Energy & Fuels, 2002, 16(4): 847-854. DOI:10.1021/ef010267z (  0) 0) |
| [30] |
Espinal J F, Mondragón F, Truong T N. Density functional theory study of Carbon-H2O reactions during gasification with steam American Chemical Society, Division of Fuel Chemistry, Preprints, 2004, 49(2): 49.
(  0) 0) |
| [31] |
Wang J, Jiang M, Yao Y, et al. Steam gasification of coal char catalysed b K2CO3 for enhanced production of hydrogen without formation of methane. Fuel, 2009, 88(9): 1572-1579. DOI:10.1016/j.fuel.2008.12.017 (  0) 0) |
| [32] |
Qiu Z, Song L, Zhao J, et al. The nanoparticle size effect in graphene cutting: a “Pac-Man” mechanism. Angewandte Chemie, 2016, 55(34): 9918-9921. DOI:10.1002/anie.201602541 (  0) 0) |
| [33] |
Sendt K, Haynes B S. Density functional study of the reaction of carbon surface oxides: the behavior of ketones. Journal of Physical Chemistry A, 2005, 109(15): 3438-3447. DOI:10.1021/jp045111p (  0) 0) |
| [34] |
Montoya A, Truong T, Mondragón F, et al. CO desorption from oxygen species on carbonaceous surface: 1. effects of the local structure of the active site and the surface coverage. The Journal of Physical Chemistry A, 2001, 105(27): 6757-6764. DOI:10.1021/jp010572l (  0) 0) |
| [35] |
Lin X, Zhang Y, Wang R, et al. Influence of the structural and surface characteristics of activated carbon on the catalytic decomposition of hydrogen iodide in the sulfur-iodine cycle for hydrogen production. International Journal of Hydrogen Energy, 2013, 38(35): 15003-15011. DOI:10.1016/j.ijhydene.2013.09.117 (  0) 0) |
| [36] |
Pi X, Sun F, Gao J, et al. A new insight into the SO2 adsorption behavior of oxidized carbon materials using model adsorbents and DFT calculations. Physical Chemistry Chemical Physics, 2019, 21: 9181-9188. DOI:10.1039/C8CP07782G (  0) 0) |
| [37] |
Zhao S, Xu W, Gu H, et al. Density functional theory and experimental study on the chemisorption and catalytic decomposition of benzene over exposed bio-char surface: The influence of unsaturated carbon atoms and potassium. Fuel, 2022, 326: 125032. DOI:10.1016/j.fuel.2022.125032 (  0) 0) |
| [38] |
Turn S, Kinoshita C, Zhang Z. An experimental investigation of hydrogen production from biomass gasification. International Journal of Hydrogen Energy, 1998, 23(8): 641-648. DOI:10.1016/S0360-3199(97)00118-3 (  0) 0) |
| [39] |
Chen N, Yang R T. Ab initio molecular orbital study of the unified mechanism and pathways for gas-carbon reactions. Journal of Physical Chemistry A, 1998, 102(31): 6348-6356. DOI:10.1021/jp981518g (  0) 0) |
| [40] |
Montoya A, Mondragón F, Truong T N. First-principles kinetics of CO desorption from oxygen species on carbonaceous surface. Journal of Physical Chemistry A, 2002, 106(16): 4236-4239. DOI:10.1021/jp0144294 (  0) 0) |
| [41] |
Cazorla-Amorós D, Linares-Solano A, Dekker F H M, et al. Isotopic steady-state and step-response study on carbon gasification catalyzed by calcium. Carbon, 1995, 33(8): 1147-1154. DOI:10.1016/0008-6223(95)00068-O (  0) 0) |
| [42] |
Lobo L S, Carabineiro Sónia A C. Kinetics and mechanism of catalytic carbon gasification. Fuel, 2016, 183: 457-469. DOI:10.1016/j.fuel.2016.06.115 (  0) 0) |
| [43] |
Zhu Z H, Lu G Q, Finnerty J, et al. Electronic structure methods applied to gas-carbon reactions. Carbon, 2003, 41(4): 635-658. DOI:10.1016/S0008-6223(02)00380-9 (  0) 0) |
| [44] |
Zhao D, Liu H, Lu P C, et al. DFT study of the catalytic effect of Fe on the gasification of char-CO2. Fuel, 2021, 292: 120203. DOI:10.1016/j.fuel.2021.120203 (  0) 0) |
| [45] |
Zhao D, Liu H, Sun C, et al. DFT study of the catalytic effect of Na on the gasification of carbon-CO2. Combustion and Flame, 2018, 197: 471-486. DOI:10.1016/j.combustflame.2018.09.002 (  0) 0) |
| [46] |
Radovic L, Bockrath B. On the chemical nature of graphene edges: Origin of stability and potential for magnetism in carbon materials. Journal of the American Chemical Society, 2005, 127(16): 5917-5927. DOI:10.1021/ja050124h (  0) 0) |
| [47] |
Hohenstein E G, Chill S T, Sherrill C D. Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules. Journal of Chemical Theory and Computation, 2008, 4(12): 1996-2000. DOI:10.1021/ct800308k (  0) 0) |
| [48] |
Umadevi D, Sastry G N. Molecular and ionic interaction with graphene nanoflakes: A computational investigation of CO2, H2O, Li, Mg, Li+, and Mg2+ interaction with polycyclic aromatic hydrocarbons. The Journal of Physical Chemistry C, 2011, 115(19): 9656-9667. DOI:10.1021/jp201578p (  0) 0) |
| [49] |
Fakheri H, Tayyari S F, Heravi M M, et al. Low frequency vibrational spectra and the nature of metal-oxygen bond of alkaline earth metal acetylacetonates. Journal of Molecular Structure, 2017, 1150: 340-348. DOI:10.1016/j.molstruc.2017.08.041 (  0) 0) |
| [50] |
Wu C, Gates I D. Methane activation by a single iron atom supported on graphene: Impact of substrates. Molecular Catalysis, 2019, 469: 40-47. DOI:10.1016/j.mcat.2019.03.002 (  0) 0) |
| [51] |
Yang J, Waller M P. Revealing noncovalent interactions in quantum crystallography: Taurine revisited. Journal of Computational Chemistry, 2013, 34(6): 466-470. DOI:10.1002/jcc.23155 (  0) 0) |
| [52] |
Tian L, Chen F. Multiwfn: A multifunctional wavefunction analyzer. Journal of Computational Chemistry, 2012, 33(5): 580-592. DOI:10.1002/jcc.22885 (  0) 0) |
| [53] |
Becke Axel D. Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. Journal of Chemical Physics, 1997, 107(20): 8554-8560. DOI:10.1063/1.475007 (  0) 0) |
| [54] |
Li M R, Lu Z, Wang G C. The effect of potassium on steam-methane reforming on the Ni-4/Al2O3 surface: A DFT study. Catalysis Science & Technology, 2017, 7(16): 3613-3625. DOI:10.1039/C7CY00986K (  0) 0) |
| [55] |
Chen C, Li W-Z, Song Y-C, et al. Structure and kinetics of hydrogen bonds in aqueous glucose solutions. Acta Physico-Chimica Sinica, 2011, 27(6): 1372-1378. DOI:10.3866/PKU.WHXB20110626 (  0) 0) |
| [56] |
Kozuch S, Shaik S. How to conceptualize catalytic cycles? The energetic span model. Accounts of Chemical Research, 2011, 44(2): 101-110. DOI:10.1021/ar1000956 (  0) 0) |
| [57] |
Murdoch J R. What is the rate-limiting step of a multistep reaction?. Journal of Chemical Education, 1981, 58(1): 32. DOI:10.1021/ED058P32 (  0) 0) |
| [58] |
Feng D, Zhao Y, Zhang Y, et al. Roles and fates of K and Ca species on biochar structure during in-situ tar H2O reforming over nascent biochar. International Journal of Hydrogen Energy, 2017, 42(34): 21686-21696. DOI:10.1016/j.ijhydene.2017.07.096 (  0) 0) |
| [59] |
Feng D, Zhao Y, Zhang Y, et al. Catalytic mechanism of ion-exchanging alkali and alkaline earth metallic species on biochar reactivity during CO2/H2O gasification. Fuel, 2018, 212: 523-532. DOI:10.1016/j.fuel.2017.10.045 (  0) 0) |
| [60] |
Zhao D, Liu H, Jiang L, et al. Investigation into the relationship between oxygen-containing groups and the release of Na and Cl during preoxidation and pyrolysis of Na-enriched Zhundong Coal. Energy & Fuels, 2017, 31(11): 11939-11946. DOI:10.1021/acs.energyfuels.7b02321 (  0) 0) |
| [61] |
Gea G, Sánchez JoséL, Murillo María B, et al. Kinetics of CO2 gasification of alkaline black liquor from wheat straw. Influence of CO and CO2 concentrations on the gasification rate. Industrial & Engineering Chemistry Research, 2004, 43(13): 3233-3241. DOI:10.1021/ie034338o (  0) 0) |
| [62] |
Asami K, Sears P, Furimsky E, et al. Gasification of brown coal and char with carbon dioxide in the presence of finely dispersed iron catalysts. Fuel Processing Technology, 1996, 47(2): 139-151. DOI:10.1016/0378-3820(96)01000-4 (  0) 0) |
| [63] |
Jiao W, Wang Z, Jiao W, et al. Influencing factors and reaction mechanism for catalytic CO2 gasification of sawdust char using K-modified transition metal composite catalysts: Experimental and DFT studies. Energy Conversion and Management, 2020, 208: 112522. DOI:10.1016/j.enconman.2020.112522 (  0) 0) |
 2024, Vol. 31
2024, Vol. 31


