2. College of Mechanical and Electrical Engineering, Harbin Engineering University, Harbin 150001, China
In the process of productivity development and technological innovation, traditional mechanical methods can no longer meet the current development trend of miniaturization, portability, intelligence and precision on account of their low efficiency and high energy consumption[1-4]. Because of the advantages of light weight, low manufacturing cost, low actuating voltage, good flexibility and fast response speed, ion actuator had extremely important application potential in aerospace, underwater equipment, biomedicine, micro-robotics and other fields[5-7]. In recent years, the researchers found that the actuate membrane materials, including sodium alginate[8], cellulose, chitosan[9-12], had exhibited certain properties. Due to their unique three-dimensional network structure and ability to retain a large amount of water, ion actuator had a wide range of applications in hydrogel artificial muscles[13] including biomedical applications, drug carriers[14-15], biosensors[16], medical devices[17], tissue engineering[18], separation systems[19-20], microfluidic systems[21], microvalves[22] and actuator[23-24]. Chitosan (CS) was a product of natural chitin. Owing to its rich hydroxyl and amino groups, the chemical properties of CS were more active. This was able to improve its strength, heat resistance, elastic modulus, electrical conductivity and other abilities through a variety of chemical modification reactions, while having good biocompatibility and biodegradable characteristics[25].
According to the conductive mechanism, ion actuator was divided into inorganic salt ion conductive actuator and ionic liquid conductive actuator. The preparation methods of ion actuator were generally classified into two types. One of them was to put inorganic salts into an ionic solution formed by the formed hydrogel material, after that the ions penetrated into the hydrogel network to obtain ion actuator. And the other one was to use the one-pot method, which involves adding all raw materials, including inorganic salt ions, into the reaction system to form an ionic conductive hydrogel. Compared with traditional doping reagents, NaCl had the more reliable purity and raw material price advantages, and the process flow was relatively simple without complex side reaction impurities. However, ion actuator still had the problems of small mechanical properties and narrow doping improvement paths. At the meantime, the preparation of chitosan-based ion actuator (CSIA) by NaCl dissolved in gel solution had not been tested, and the effect of CSIA materials on the output performance and electrochemical performance of gel material actuator needed to be further studied.
Therefore, this work proposes to immerse ionic conductive hydrogels in inorganic salt ion solutions through physical doping process to achieve the proportion of inorganic salt Na+ ions. Then, the effect of sodium chloride on CSIA was studied by testing its mechanical properties, porosity, electrochemical properties, as well as surface morphology and functional group measurements.
1 Experiments 1.1 Experimental and ReagentForce measurement software (FA1004) was produced by Shanghai Shangping Instrument Co., Ltd. (Shanghai, China). Vacuum drying oven (DZF-6050) was produced by Shanghai Sead Instrument Co., Ltd. (Shanghai, China). Electrochemical workstation (CHI760E) was produced by Shanghai Chenhua Instrument Co., Ltd. Infrared spectroscopy (FT-IR200) was produced by Tianjin Gangdong Technology Development Co., Ltd. Scanning electron microscope (S-240) was produced by Cambridge Instrument Company. Digital micrometer was produced by Guilin Haoli Measuring Instrument Technology Co., Ltd. Analytical balance (JJ224BC) was produced by Changshu Shuangjie Testing Instrument Factory.
Chitosan (CS), sodium chloride (NaCl), acetic acid (HAC), multi-walled carbon nanotubes (MCNT), Glycerol were provided by Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). All chemicals were of analytic grade and used without treating further for purification and the deionized water was self-restraint.
1.2 Preparation of Chitotan-Based Ion ActuatorChitosan-based ionic actuator was a new type of responsive electronic material, which mainly consisted of electrode membranes and actuate membranes prepared by sol-gel method[26] and its detailed preparation is shown in Fig. S1 in Supporting Information. The detailed preparation of NaCl-doped CSIA in this experiment is shown in Table S1 in Supporting Information. The CSIA actuator has a length and width of 30 mm℅3 mm and an average thickness of 0.4 mm. The MCNT electrode film thickness is 0.1 mm (micrometer measurement) and the electrode film mass percentage is 41%.
1.3 Experimental Setup 1.3.1 Output force and displacement test of CSIAThe force test platform is shown in Fig. S2(a) in Supporting Information, under the experimental test conditions of 300 s, voltage of 3 V and baud rate of 9600, the output force test of CSIA samples from S0-S5 was carried out, and the output force experimental test data of 6 groups were averaged. The deflection of CSIA on electrical stimulation is shown in Fig. S2(b) in Supporting Information.
Since the ion channel could improve the transmission ratio of charged ions inside the actuate membrane of CSIA, its mechanochemical characteristics and the porosity of the actuate membrane[27]. The formula for calculating porosity is shown below.
| $ P=\frac{m_1-m_0}{\rho \times V} \times 100 \% $ | (1) |
where P is the porosity of the drive membrane. m0(g) is the constant mass of the actuate membrane. m1(g) is the mass weight. ρ(g·cm-3) is the density of absolute ethanol and V(cm3) is the volume of the samples.
1.3.2 Electrochemical testing of CSIAThe electrochemical tests were conducted using the electrochemical workstation, as shown in Fig. S3(a). The three-electrode system (Fig. S3(b)) was formed using saturated calomel electrode as reference electrode and platinum electrode as auxiliary electrode and working electrode. The CHI760E was used for Cyclic Voltammetry (CV) test and Electrochemical Impedence Spectroscopy (EIS) test. The volt-ampere characteristic curve was obtained by the actuate membranes at 20 mV·s-1, 50 mV·s-1, 100 mV·s-1 scanning speeds. Because the clamping part of the electrochemical analyzer and the actuate membrane might have had certain poor contact, and there was voltage instability when the instrument started, so the method of cyclic scanning was used to obtain three sets of voltammetry characteristic curve data at scanning speed. Calculating the area enclosed by the plotted figure, which was approximately the value of the integral part, and then bringing it into the specific capacitance formula[28], the specific capacitance (Cp) of the material was caloulated. The specific capacitance was calculated as follows:
| $ C_p=\frac{A}{2\left(V_2-V_1\right) \times m \times k} $ | (2) |
where Cp was the specific capacitance. A was the scanning area. V1 and V2 were low and high potential, respectively. m was the sample quality and k was the scanning speed.
The equivalent circuit model is indicated in Fig. S3(c). It was used to analyze the electrochemical impedance data[29]. This analog circuit used Rs to represent the resistance of the electrolyte solution, Rct to denote the charge transfer resistance, Cd to show the electric double-layer capacitance, and Wo to represent the open potential. Data fitting was performed using Z-view software. The reverse extension of the data in the figure was connected to the x-axis, and its intercept was the measured resistance value.
The electrochemical performance of a CSIA sample was measured by its conductivity, which was calculated as follows:
| $ \sigma=\frac{L}{R_s \times S} $ | (3) |
where σ representes conductivity(S·m-1), RS is resistance(Ω), S is area(m2), and L is length(m).
1.3.3 Morphology and functional group testing of CSIAThe surface morphology of S0-S5 of the CSIA sample was observed microscopically by high-resolution scanning electron microscope (SEM). By Fourier transform, the infrared spectrum in the range of 4000-400 cm-1 was marked with potassium bromide (KBr) particles to study the chemical molecular structure and physical doping reaction of the actuating membrane.
2 Results and Discussion 2.1 Effect of NaCl on Mechanical Properties of CSIAThe output force and time curves of different CSIA samples are shown in Fig. 1(a).From the results it can be concluded that from S0 to S4, with the increase of the doping ratio of NaCl, the maximum output force of the CSIA sample S4 reached 2.939 mN, which was 3.4 times higher than that of the control group S0. With the doping ratio continuously increasing, the output force decreased to 0.3020 mN for S5 sample, and it reached 54.72% of S0. This showed at an appropriate amount NaCl doping, there was a certain optimization of the network structure of the actuate membranes of CSIA, which could improve the ion transfer rate inside the actuate membranes, thereby improving the output force of the CSIA. However, an excessive doping would change the arrangement frame of the internal structure of the actuate of CSIA, weakening its performance and leading to serious deformation bubbling, and energy loss in the sample during the electrical stimulation response.

|
Fig.1 Mechonical properties and deflection mechanism |
As shown in Fig. 1(b), the CSIA sample S5 increased the doping amount compared to S4, and then the reduction in the maximum displacement offset of S5 relative to S4 had accounted for 76.7% of that of S4, indicating that the doping ratio of NaCl had begun to exceed the limit.
The relationship between the maximum output force of the sample CSIA and the porosity of the actuate membranes is shown in Fig. 1(c). The trends of the two data sets are similar.With the increase of NaCl doping ratios, the change trend of porosity and output force increased first, and the porosity of actuate membranes CSIA of NaCl-doped reached the maximum at S4, and the maximum attained 12.98%. The lowest porosity reached its minimum at S5. This showed that NaCl had a certain optimization effect on the internal three-dimensional network structure of CS, which could improve the ion transfer rate inside the actuate membranes of CSIA, but excessive NaCl cross-linking doping would exacerbate ion blockage inside the actuating membranes, resulting in a decrease in their water content, and the CSIA to lose water and harden, resulting in the greater internal stress during deflection, thereby weakening the output force. The specific performance parameters of CSIA are detailed in Table S2 in Supporting Information.
The displacement deflection versus time is shown in Fig. 1(b). It can be concluded that the largest displacement deflection of the samples is S4, and the deflection change reaches its maximum value of 4.025 mm around 700 s, and then stabilizes at 3.9 mm after 1000 s. The other samples were first subjected to a slow fluctuating rise and then leveled off after 1000 s.
Among them, the curves of sample S3 in Fig. 1(a) and S4 in Fig. 1(b) first increased and then decreased, and the reason for the curve fluctuation was mainly due to the occurrence of tremor. After the CSIA sample underwent work and dehydration, the material's toughness decreased and it became brittle. This resulted in the dynamic equilibrium of the CSIA's mechanical properties being slowly disrupted, and ultimately led to tremors.
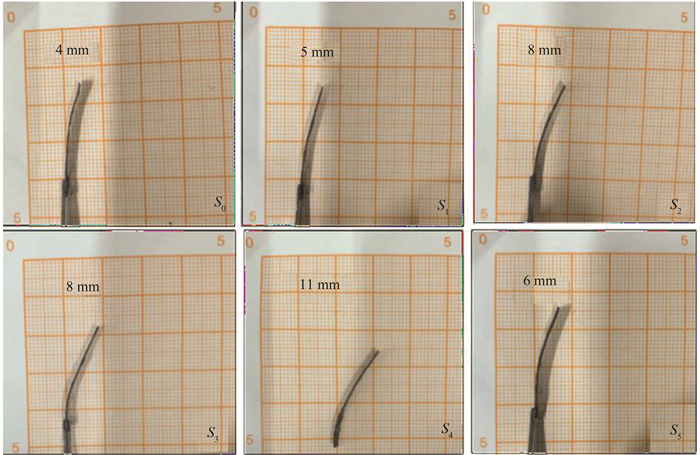
In addition, the final deflection state diagrams of samples with different NaCl doping ratios at 5 V are shown in Fig. S4. The test point for testing the deflection differed from the deflection at the farthest end of the deflection, which was due to the fact that the infrared test point was chosen to measure the farthest end of the point that was 3 mm inward. The trend of both deflections is basically the same, reaching the maximum deflection at sample S4, which coincides with the maximum output force trend above.
However, it was also found that the bending repeatability of the actuator was poor, probably because air bubbles appeared on the surface and inside the actuator at the end of the output force test and displacement test, which resulted in the almost loss of braking performance of the prototype, so the output force and displacement deflection performance of the CSIA prototypes and its reusability required improvement.
As can be seen from Fig. 1(a) and (c), when the maximum value of output force and displacement deflection both appear in sample S4, CSIA sample shows a trend of initially increasing and then decreasing from S0 to S5. In general, there was a positive correlation between output force and displacement deflection.
Researchers[30] pointed out that the deflection mechanism of the ion activation aceration mechanism of the cellulose backbone was that, under the action of electric field, the charge was distributed at the cathode and the anode to form an electric double layer. The positively charged cation -NH3+ was bound by the polymer CS backbone and fixed with the action of Van der Waals force. The anion -CH3COO- moved towards the anode side of the electrode, and accumulates with increasing concentration over time, while the cation moved in the direction of the cathode. Based on the above, the cathodic deflection of the actuator in a weakly acidic solution biogel was used, and this cathodic deflection phenomenon was mainly the result of internal ion movement.
As shown in Fig. 1(d), the CS molecule contains a large number of -NH2. In the aqueous acetic acid solution, the backbone chain hydrolysis of CS, many free amino groups on the internal ions bind H+ in the solution, so that chitosan became -NH3+ polyelectrolyte, while the remaining anions were in a free state to dissolve -CH3COO- of CS. As shown in Fig. 1(e), in the CSIA actuate membranes after NaCl doping, Na+ ions concentrated near the cathode electrode membrane, because the ion radius of Na+ ions (0.97Å) was less than -CH3COO- ion radius (4.50 Å).When the concentration on both sides reached to a certain amount, the ion concentration difference on both sides was inside the actuate membranes under the action of Van der Waals force to make the surface of the electrode showing stress and bending strain. Since the ionic radius of the anion was significantly larger than the ionic radius of the cation, the volume difference of the actuate membranes was generated, so that actuator exhibited cathodic deflection. When voltage was applied across the electrode, ion migration changed from the previous disordered state to ordered. The ion migration rate was enhanced. Then, the ion concentration gradient was quickly established, which correspondingly reduced the resistance of the actuator membrane and enhanced the internal ion concentration and mechanical properties of the ion actuator sample.
2.2 Effect of NaCl on Electrochemical Performance of CSIAThe electrochemical performance of CSIA with different doping ratios was tested by cyclic voltammetry, and the cyclic voltammetry characteristic curves at different scanning speeds are shown in Fig. 2(a)-(c), taking the specific capacitance at 50 mV·s-1 scanning speed as an example, with the increase of the doping rate of NaCl, the specific capacitance of the actuate membranes initially increased to S4 and then decreased to S5. Specifically, the capacitance of CS was up to 0.07719 F·g-1 in S4, which was 1.58 times higher than the 0.0299 F·g-1 of the capacitance of CSIA S0. The specific capacitance value indicated the efficiency of ion migration in the sample, which might be due to the over crosslinking of the CSIA of the NaCl doped to interrupt the channel of ion migration in the actuate membranes, making its internal structure dense and uniform, thereby affecting the ion migration rate. In addition, the specific capacitance trend of the actuate membranes under different doping ratios and different scanning speeds is shown in Fig. 2(d)-(e). The specific capacitance of the actuate membranes gradually decreased as the scanning rate increased, which indicated that the scanning speed increase rate was faster than the area increases ratios of the CV curve. Finally, the relationship between the resistance of the actuate membrane and the specific capacitance was shown in Fig. 2(f). The two generally showed an inverse ratio, in the experimental group of S4. The specific capacitance reached the maximum, the resistance reached the minimum, which corresponded to the output force of S4, reflecting that the smaller the resistance, the larger the specific capacitance, the better the mechanical properties of the corresponding ion actuator.
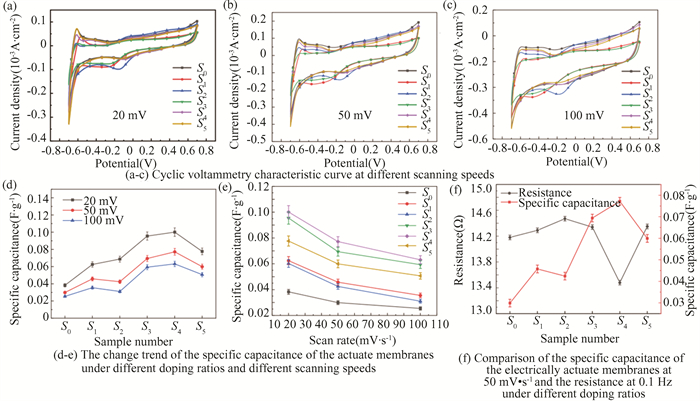
|
Fig.2 Characteristic map of electrochemically relevant parameters |
The relationship between the real resistance and virtual reactance of the CSIA actuate membranes is shown in Fig. 3(a) and (b), and it can be seen that there is a good linear relationship between them. Curve fitting was performed by Z-view software, and the equivalent circuit model is shown in Fig. S4(c). The line obtained by linear fitting intersects the x-axis, and the abscissa value of the intersection point was the resistance value of the actuate membrane of CSIA[31]. The resistance of the actuate membranes of CSIA is shown in Fig. 3(c). The resistance of the CSIA sample Rs could reflect the conductivity of the sample to a certain extent. Taking 0.1-100000 Hz as an example, the resistance of the CSIA actuate membranes showed a trend of first decreasing and then increasing, with the resistance of the actuate membrane at S4 being 13.48 Ω, which was 4.937 % lower than that of the control group of S0.
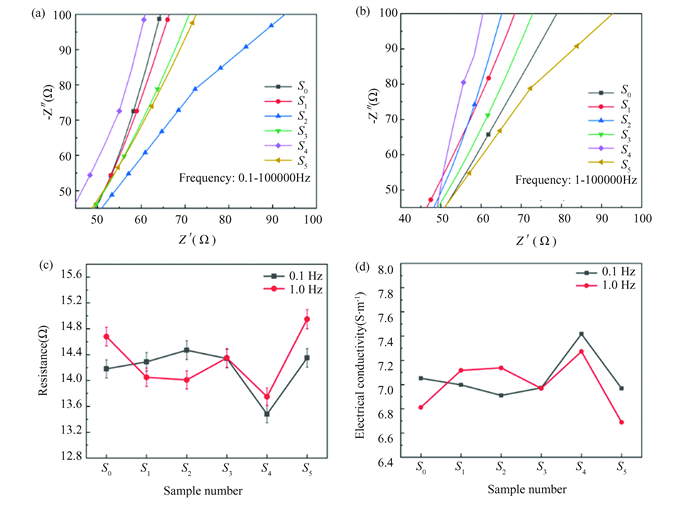
|
Fig.3 (a-b) Z-view fitted plot of different NaCl doping ratios at the low frequencies 0.1 Hz and 1 Hz; (c) Resistance of different NaCl doping ratios at the low frequencies 0.1 Hz and 1 Hz; (d) Electrical conductivity for different NaCl doping ratios at the low frequencies 0.1 Hz and 1 Hz |
This showed that the CSIA sample achieved better conductive effect at S4, and appropriate NaCl cross-linking doping could improve the electrochemical performance of the CSIA.However, excessive doping would intensify and change the molecular structure arrangement inside the actuate membrane of CSIA. This causes the ion channels to become clogged and crowded, increasing the resistance, and decreasing the electrochemical performance of CSIA.
As shown in Fig. 3(d), we can observe the change in the conductivity of CSIA, and the best CSIA sample is more intuitive. When the measured conductivity changed from S0 to S5, the conductivity of CSIA shows a changing curve, and reaching its maximum at S4.
2.3 Effect of NaCl on Surface Morphology and Functional Groups of CSIA 2.3.1 Electron microscopy scan of CSIAThe micromorphology of the substrate surface and the side section under doped samples of CSIA S0-S5 is shown in Fig. 4. As can be seen from Fig. 4(a)-(f), the surface of the CSIA actuate membranes in the control group is smooth, and with the increase of NaCl doping ratio, the particle size and roughness of the surface of the actuate membranes gradually increase. The particle size and roughness of CSIA increased significantly at S5. In addition, the interface change between the electrode membranes and the actuate membranes of the CSIA doped with NaCl is shown in Fig. 4(g)-(i).

|
Fig.4 (a-f) Microscopic morphology of substrate surface of samples S0-S5 at 20 m; (g-h) Microscopic morphology of sample CSIA at different sizes; (i) Cross-sectional micromorphology of CSIA samples without NaCl doped |
Researchers had found that semi-interpenetrating network systems generally exhibit surprising properties that were superior to either of the two single polymers alone[32]. After observation and comparison, it was found that the undoped CSIA was smooth contact, while the interface of the NaCl-doped CSIA had an obvious three-dimensional physical cross-network structure. When a voltage was applied to the electrode, the ion migration changed from the previous disordered state to an ordered state, and ion mobility was improved. Then, the ion concentration gradient was rapidly established, which correspondingly reduced the resistance of the actuator membrane and increased the internal ion concentration and mechanical properties of the ion actuator sample.
This showed that the doping of NaCl had obvious effect on the internal structure of CSIA, which might be helpful to the performance of CSIA, but excessive doping could also be counterproductive.
2.3.2 Fourier transform infrared spectroscopy of CSIAThe FTIR spectra of CSIA sample S0-S5 are shown in Fig. 5. At 3376.79 cm-1 and 2929.28 cm-1, they matched to the telescopic vibration peaks of -OH and -NH2, respectively. The flexural vibration peaks were caused by -CH2 and -CH3 groups near 1566.79 cm-1 and 1411.96 cm-1. A tensile vibration peak was caused by the -CH2-O-CH2 group near 1045.12 cm-1, and bending vibration of -CH2-O-CH2 group appeared around 854.10 cm-1. From this functional group, it could be preliminarily inferred that it is a functional group in the structure of CSIA, and the peaks of S1-S5 in the experimental group were basically the same as those in the control group, indicating that the CSIA of NaCl-doped had no significant effect on its functional groups.
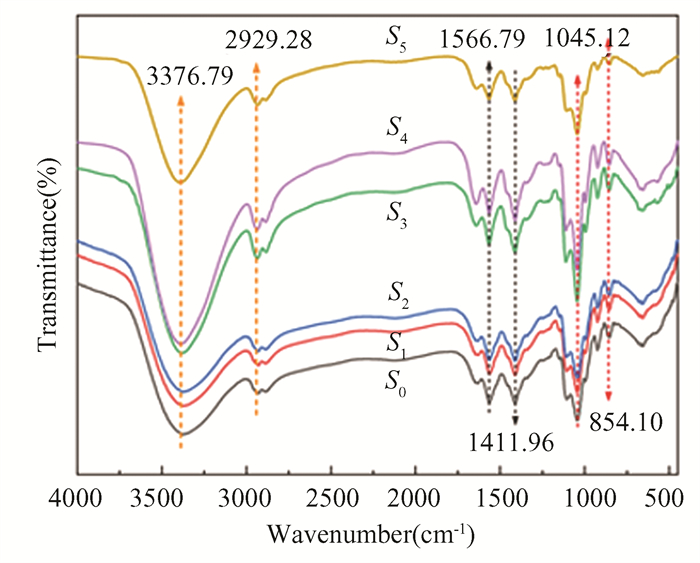
|
Fig.5 FTIR spectra of CSIA samples of S0-S5 in the range of 4000-400 cm-1 |
3 Conclusions
In order to explore the effect of sodium chloride on the enhanced performance of chitosan-based ion actuator (CSIA), sodium chloride doped CSIA was prepared by sol-gel method. Therefore, the effect of sodium chloride on CSIA was investigated. The mechanical properties of CSIA was tested by establishing an output force test platform while testing its porosity. The electrochemical performance was measured using an electrochemical workstation. And the surface morphology and functional groups were measured by scanning electron microscopy and infrared spectrogram, respectively. Then, the results indicated that the molar concentration of the sodium chloride was the best at 0.06836 mol·L-1 for CSIA. Its mechanical properties could reach an output force of 2.939 mN and a deflection displacement of 4.025 mm, with the maximum porosity of 12.98 % at the same time. The specific capacitance of the electrochemical performance was up to 0.07719 F·g-1, and the minimum resistance reached 13.48 Ω. If the doping ratio of NaCl continued to increase, its output force, porosity and specific capacitance decreased to varying degrees. It turned out that over doping would block the internal three-dimensional structure of the CSIA, the ion channel would decrease, and the corresponding resistance would increase, resulting in a decrease in the output force performance. Through scanning electron microscopy, it was shown that excessive doping of CSIA led to an increase in compactness and cracks between the surface and interface of the membrane. The infrared showed that NaCl had no significant effect on the CSIA internal functional groups. The effective internal ion concentration and significantly reduced internal stress provided excellent performances under the appropriate voltage conditions. The doping of inorganic ion sodium chloride improved the internal electron transport efficiency of chitosan ion actuator, and advanced the mechanical properties of the actuator. This work ultimately showed that the appropriate sodium chloride mass ratio had a great effect on the performance of CSIA. In future research, the addition of glycerol to the experiment reduced the internal stress generated in the CSIA itself, and increasing the water content of CSIA enhanced the working life and storage conditions of the ion actuator.
AcknowledgmentThe author wishes to acknowledge College of Material Science and Chemical Engineering, Harbin Engineering University, for providing the required facilities. The authors would also like to thank Prof. Yanzhuo Lv, Dr. Yan Xu, Dr. Xiaoli Zhao, and Weikun Jia for providing the necessary facilities to conduct the experiments. The authors would also like to thank Prof. Yueming Ren for providing paper revisions. This work was supported by Key Laboratory of Engineering Bionics, Ministry of Education, Jilin University.
The authors commit that the design, data presentation, and citation of the study are in accordance with the standard COPE ethical guidelines and appropriate approvals and consents have been obtained.
Supporting Information Text S1 Preparation of Experimental MaterialsForce measurement software (FA1004) was produced by Shanghai Shangping Instrument Co., Ltd. (Shanghai, China). Vacuum drying oven (DZF-6050) was produced by Shanghai Boxun Medical Biological Instrument Co., Ltd. (Shanghai, China). Electrochemical workstation (CHI760E) was produced by Shanghai Chenhua Instrument Co., Ltd. Infrared spectroscopy (FT-IR200) was produced by Tianjin Gangdong Technology Development Co., Ltd. Scanning electron microscope (S-240) was produced by Cambridge Instrument Company.
Chitosan (CS), sodium chloride (NaCl), acetic acid (HAC), multi-walled carbon nanotubes (MCNT), glycerol were provided by Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). All chemicals were of analytic grade and used without further treatment for purification and the deionized water was self-produced.
In this experiment, the ion actuator investigated was a new type of responsive electronic material, which was composed of electrode membranes and actuate membranes.In which the actuate membranes were mainly composed of multi-walled carbon nanotubes and chitosan, and the actuate membranes were composed of sodium chloride with different doping ratios, chitosan, and acetic acid. Sodium chloride doped CSIA were prepared by the sol-gel method[26].
Preparation of chitotan-based ion actuator as shown in Fig. S1. The CSIA preparation process consists of the following steps. First of all, 40 mL of acetic acid solution with a volume fraction of 2% was poured into six 250 mL beakers placed in a water bath at 50 ℃ to keep them warm. Secondly, 1.2 g of chitosan was added to the beaker slowly and the solution was thoroughly dissolved with magnetron stirring. Thirdly, different amounts of NaCl were added to different beakers in turn when the chitosan was completely dissolved. According to the experimental design, the doping ratios of NaCl added to the actuate membrane mixtures were detailed in Table S1. When the mixtures were completely dissolved, 2 mL of glycerol was injected into the solution to obtain actuate membrane mixtures. Eventually, after ultrasonic defoaming for 15 min, the hydrogel was poured into a 50 mm℅50 mm mold, placed in a drying oven, and dried at 50 ℃ for 12 h.

|
S1 Preparation of chitotan-based ion actuator |
| Table S1 Ratio table of different CSIA raw materials |
For the electrode film made of MCNT, take two sets of CS base film solution as adhesive, apply them between the electrode film and the base film with a brush, form a sandwich structure, and physically press the parts under the condition of external pressure closure for about 10 h to make the MCNT electrode film and the CS base film bonded together.
According to the experimental design, the doping ratio of NaCl added to the actuate membrane mixture is detailed in Table S1. Samples of CSIA with different NaCl qualities were numbered.
Notes:
The doping ratios of NaCl were determined by the mass ratio of NaCl to the chitosan solution. For all groups, the chitosan ingredient was 1.2 g, glycerol was 2 mL, and deionized water was 40 mL. The formula for calculating the molar concentration of NaCl proportion gel solution is shown below.
| $ C=\frac{m}{M \times V} $ | (S1) |
where C is the molar concentration of the NaCl ratio gel solution in mol·L-1, m is the mass of NaCl doping, V is the gel solution volume of 40 mL and M is the molar fraction of NaCl with a value of 58.5 g·mol-1.
The output force test platform is shown in Fig. S2(a). It mainly included test software, DC power supply, gripping device, analyzing balance, etc. The test process involved providing a stable and controllable voltage to the actuator through the DC power supply, and the actuator under the support of the gripping device was measured by the analyzing balance in terms of the weight of the bending actuator, which was then analyzed and converted into output force by the analyzing balance data transmission to the computer. The displacement test platform is shown in Fig. S2(b), which mainly includes test software, infrared sensor, grasping device, DC power supply, etc. The test process was to provide a stable and controllable voltage to the actuator through the DC power supply, which is gradually pressurized from 0 to 5 V, so that the actuator, with the infrared sensor supported by the grasping device, transmit the test data to the computer. The amount of the deflected displacement was obtained through data combing, and the maximal deflected displacement state could be measured after the end of the test.
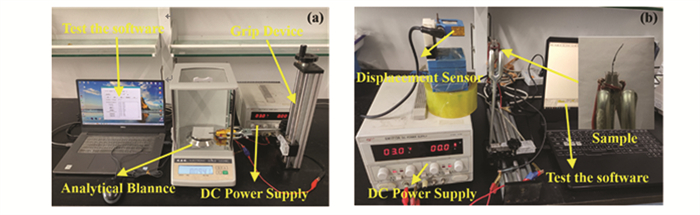
|
S2 (a) Output force test platform, and (b) Displacement test device platform |
When measuring the displacement, choose select the infrared laser test point located 3 mm from the sample endpoint. When the sample deflects, the farthest end would be offset to one side, but when the offset angle was too large, it will lead to the loss of the laser measurement point, resulting in the inability to continue the measurement. So choosing to rely on the end point within a certain distance was better. After actual measurement, the choice of 3 mm was found to be optimal for this distance.
Text S2 Computational DetailsThe final deflection state diagram of the sample with different NaCl doping ratios at 5 V is shown in Fig. S3. After the displacement test, the sample was removed and placed in a square with a scale value for comparative measurement. The displacement amount identified in the figure referred to the vertical distance between the farthest end of the deflection end of the sample and the clamping end.
|
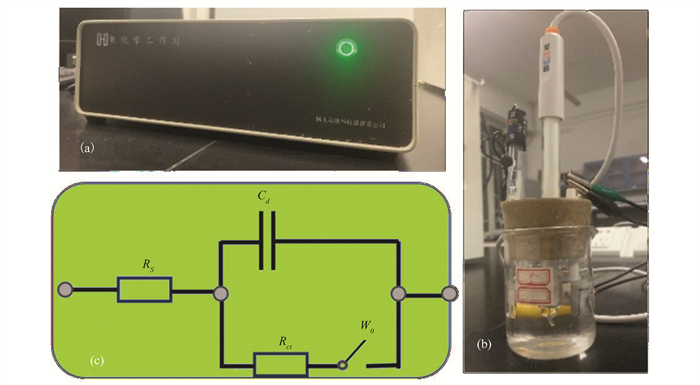
S3 (a) Electrochemical workstation; (b) three-electrode system; (c) Z-view analog circuitry |
The electrochemical tests were conducted using the electrochemical workstation, as shown in Fig. S4(a). The three-electrode system (Fig. S4(b)) was employed, consisting of a saturated calomel electrode as reference electrode, and platinum electrode as auxiliary electrode and working electrode.
|
S4 Diagram of the final deflection state of samples with different NaCl doping ratios at 5 V |
The equivalent circuit model is indicated in Fig. S4(c). It was used to study the electrochemical impedance data[29].This analog circuit used Rs to represent the resistance of the electrolyte solution, Rct to denote the charge transfer resistance, Cd to show the electric double-layer capacitance, and Wo to represent the open potential. Data fitting was performed using Z-view software. The reverse extension of the data in the figure was connected to the x-axis, and its intercept was the measured resistance value.
| Table S2 Performance parameters of the sample |
| [1] |
Lasprilla A J, Martinez G A, Lunelli B H, et al. Poly-lactic acid synthesis for application in biomedical devices - a review. Biotechnology Advances, 2012, 30(1): 321-328. DOI:10.1016/j.biotechadv.2011.06.019 (  0) 0) |
| [2] |
Gu G Y, Zhu J, Zhu L M, et al. A survey on dielectric elastomer actuators for soft robots. Bioinspir Biomim, 2017, 12(1): 011003. DOI:10.1088/1748-3190/12/1/011003 (  0) 0) |
| [3] |
Yuk H, Lin S, Ma C, et al. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nature Communications, 2017, 8: 14230. DOI:10.1038/ncomms14230 (  0) 0) |
| [4] |
Henke E M, Schlatter S, Anderson I A. Soft dielectric elastomer oscillators driving bioinspired robots. Soft Robot, 2017, 4(4): 353-366. DOI:10.1089/soro.2017.0022 (  0) 0) |
| [5] |
Hirose M, Ogawa K. Honda humanoid robots development. Philos Trans A Math Phys Eng Sci, 2007, 365: 11-19. DOI:10.1098/rsta.2006.1917 (  0) 0) |
| [6] |
Kawaharazuka K, Nishiura M, Koga Y, et al. Automatic grouping of redundant sensors and actuators using functional and spatial connections: Application to muscle grouping for musculoskeletal humanoids. IEEE Robotics and Automation Letters, 2021, 6: 1981-1988. DOI:10.1109/lra.2021.3060715 (  0) 0) |
| [7] |
Kim S, Laschi C, Trimmer B. Soft robotics: A bioinspired evolution in robotics. Trends in Biotechnology, 2013, 31(5): 287-294. DOI:10.1016/j.tibtech.2013.03.00 (  0) 0) |
| [8] |
Tampieri A, Sandri M, Landi E, et al. HA/Alginate hybrid composites prepared through bio-inspired nucleation. Acta Biomater, 2005, 1(3): 343-351. DOI:10.1016/j.actbio.2005.01.001 (  0) 0) |
| [9] |
Wang Y, Fan C, Hu H, et al. Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnology Advances, 2016, 34: 997-1017. DOI:10.1016/j.biotechadv.2016.06.001 (  0) 0) |
| [10] |
Marakana P G, Dey A, Saini B. Isolation of nanocellulose from lignocellulosic biomass: Synthesis, characterization, modification, and potential applications. Journal of Environmental Chemical Engineering, 2021, 9(6): 106606. DOI:10.1016/j.jece.2021.106606 (  0) 0) |
| [11] |
Dunlop M J, Acharya B, Bissessur R. Isolation of nanocrystalline cellulose from tunicates. Journal of Environmental Chemical Engineering, 2018, 6(4): 4408-4412. DOI:10.1016/j.jece.2018.06.056 (  0) 0) |
| [12] |
Wang H, Gurau G, Rogers R D. Ionic liquid processing of cellulose. Chemical Society Reviews, 2012, 41: 1519-1537. DOI:10.1039/c2cs15311d (  0) 0) |
| [13] |
Caccavo D, Cascone S, Lamberti G, et al. Hydrogels: experimental characterization and mathematical modelling of their mechanical and diffusive behaviour. Chemical Society Reviews, 2018, 47: 2357-2373. DOI:10.1039/c7cs00638a (  0) 0) |
| [14] |
Schmedlen R H, Masters K S, West J L. Photocross lindable polyvinyl alcohol jydrogels that can be modified with cell adhision peptides for use in tissue engineering. Biomaterials, 2002, 23(22): 4325-4332. DOI:10.1016/s0142-9612(02)00177-1 (  0) 0) |
| [15] |
Zhai M L, Liu N, Li J, et al. Radiation preparation of PVA-g-NIPAAm in a homogeneous system and its application in controlled release. Radiation Physics and Chemistry, 2000, 57(3-6): 481-484. DOI:10.1016/s0969-806x(99)00476-4 (  0) 0) |
| [16] |
Peppas N A, Van Blarcom D S. Hydrogel-based biosensors and sensing devices for drug delivery. Journal of Controlled Release, 2016, 240: 142-150. DOI:10.1016/j.jconrel.2015.11.02 (  0) 0) |
| [17] |
Ohtaa M, Handaa A, Iwatab H, et al. Poly-vinyl alcohol hydrogel vascular models for in vitro aneurysm simulations the key to low friction surfaces. Technology and Health Care, 2004, 12(3): 225-233. DOI:10.3233/thc-2004-12302 (  0) 0) |
| [18] |
Yi Y, Xie C, Liu J, et al. Self-adhesive hydrogels for tissue engineering. Journal of Materials Chemistry B, 2021, 9: 8739-8767. DOI:10.1039/d1tb01503f (  0) 0) |
| [19] |
Dolatabadi R, Mohammadi A, Walker R B. A novel three-dimensional printed device with conductive elements for electromembrane extraction combined with high-performance liquid chromatography and ultraviolet detector. Journal of Separation Science, 2022, 45: 3187-3196. DOI:10.1002/jssc.202200028 (  0) 0) |
| [20] |
Dolatabadi R, Zaheri M, Ebrahimi S, et al. A study on determination of theophylline in plasma and urine sample using electromembrane extraction combined with high-performance liquid chromatography-ultraviolet. Chemical Papers, 2021, 76: 681-690. DOI:10.1007/s11696-021-01889-0 (  0) 0) |
| [21] |
Amiri A, Mazaheri H. Study on the behavior of a temperature-sensitive hydrogel micro-channel via FSI and non-FSI approaches. Acta Mechanica, 2020, 231: 2799-2813. DOI:10.1007/s00707-020-02673-z (  0) 0) |
| [22] |
Niroumandi S, Shojaeifard M, Baghani M. PH-sensitive hydrogel-based valves: A transient fully-coupled fluid-solid interaction study. Journal of Intelligent Material Systems and Structures, 2021, 33: 196-209. DOI:10.1177/1045389x211011671 (  0) 0) |
| [23] |
Nourian A H, Amiri A, Moini N, et al. Synthesis, test, calibration and modeling of a temperature-actuated hydrogel bilayer. Smart Materials and Structures, 2020, 29: 105001. DOI:10.1088/1361-665X/ab9f46 (  0) 0) |
| [24] |
Arbabi N, Baghani M, Abdolahi J, et al. Finite bending of bilayer pH-responsive hydrogels: A novel analytic method and finite element analysis. Composites Part B: Engineering, 2017, 110: 116-123. DOI:10.1016/j.compositesb.2016.11.006 (  0) 0) |
| [25] |
Ilyas R A, Sapuan S M, Bayraktar E. Bio and synthetic based polymer composite materials. Polymers (Basel), 2022, 14(18): 3778. DOI:10.3390/polym14183778 (  0) 0) |
| [26] |
Bokov D, Turki Jalil A, Chupradit S, et al. Nanomaterial by Sol-Gel method: synthesis and application. Advances in Materials Science and Engineering, 2021, 2021: 1-21. DOI:10.1155/2021/5102014 (  0) 0) |
| [27] |
Antonietti M, Fechler N, Fellinger T P. Carbon aerogels and monoliths: control of porosity and nanoarchitecture via Sol-Gel routes. Chemistry of Materials, 2014, 26: 196-210. DOI:10.1021/cm402239e (  0) 0) |
| [28] |
Zhang M, Yang D Y, Li J T. Supercapacitor performances of MnO2 and MnO2/ reduced graphene oxide prepared with various electrodeposition time. Vacuum, 2020, 178: 109455. DOI:10.1016/j.vacuum.2020.109455 (  0) 0) |
| [29] |
Panwar V, Ko SY, Park J-O, et al. Enhanced and fast actuation of fullerenol/PVDF/PVP/PSSA based ionic polymer metal composite actuators. Sensors and Actuators B: Chemical, 2013, 183: 504-517. DOI:10.1016/j.snb.2013.04.037 (  0) 0) |
| [30] |
Kim J., Wang N, Chen Y. Effect of chitosan and ions on actuation behavior of cellulose-chitosan laminated films as electro-active paper actuators. Cellulose, 2007, 14: 439-445. DOI:10.1007/s10570-007-9134-z (  0) 0) |
| [31] |
Sun Z, Yang L, Zhang D. High performance, flexible and renewable nano-biocomposite artificial muscle based on mesoporous cellulose/ ionic liquid electrolyte membrane. Sensors and Actuators B: Chemical, 2019, 283: 579-589. DOI:10.1016/j.snb.2018.12.073 (  0) 0) |
| [32] |
Myung D, Waters D, Wiseman M. Progress in the development of interpenetrating polymer network hydrogels. Polymers for Advanced Technologies, 2008, 19: 647-657. DOI:10.1002/pat.1134 (  0) 0) |
 2024, Vol. 31
2024, Vol. 31


