When compared to standard machining techniques, Non-Traditional Machining (NTM) method is superior because it can generate surface finishes, dimensional precision, and complicated forms and sizes while minimizing heat and residual tensions[1]. Chemical energy is used in chemical machining. Instead of using physical force, this technique uses a controlled chemical reaction to precisely form metal into any size or shape. Chemical machining (CHM) predates all other non-traditional machining techniques. This approach eliminates material by using a controlled chemical assault using acids or alkalis, either selectively or simultaneously[2]. Machining temperature, milling time, milling solution concentration, and etchant type are the main parameters that affect the chemical milling process and, consequently, the finishing of a chemically machined alloy[3].
Titanium alloys, known for their resistance to corrosion, fatigue, temperature, and magnetic fields, are the subject of this investigation. These alloys are used in a wide range of industries. Titanium alloys are corrosion-resistant, lightweight, strong, and tough. The Ti-5Al-2.5Sn alloy has a high service temperature of 480 ℃ (896 °F), superior weldability and fabricability, and a stable microstructure[4].
El-Awadi et al.[5]tested stainless steel, copper, and aluminum sheets for metal removal rates (MRRs) at 50 ℃. They used etchants (FeCl3 and FeCl3+HNO3). They found that copper had the highest MRR, measuring 0.287 mm3/min, followed by aluminum with 0.738 and stainless steel with 0.224. At this temperature, the concentration of the FeCl3 solution in each and every metal was 33%. Zogheib et al.[6] investigated the effect of varying acid milling times on the surface roughness and flexural strength of a lithium disilicate-based glass ceramic.
Numerous research studies have investigated the multi-response optimization of process parameters in chemical milling for various alloys using the Taguchi method. The objective of these works is to increase process productivity by decreasing surface roughness and tool wear rates, while simultaneously increasing material removal rates[7-9].
Using chemical machining of stainless steel 304 by Shather[7] clarified how machining parameters affect surface roughness. The milling solution consisted of 5 mL HCl + 4 mL HNO3 + 4 mL HF + 5 mL H2SO4 + 82 mL H2O. The optimal combination of parameters at 3 min, 55 ℃, and 10% concentration that obtained by statistical analysis using Taguchi and ANOVA accorded the minimum surface roughness (SRa). Since the chemical machining process did not alter the crystalline structure, induce stresses, involve mechanical deformation or high temperatures, the hardness of all samples was unaffected. Additionally, the results indicate that increasing machining time increases SRa.
The study in Ref.[9] used Taguchi's method to analyze the effects of process parameters in Metal-Assisted Chemical Etching (M.A.C.E.) of silicon. The parameters included etching time, and etchant concentration, which were analyzed using Taguchi modeling. The data was analyzed using ANOVA, and graph modeling. As well, the study optimized the M.A.C.E. technique for silicon using the primary parameters of temperature, H2O2 concentration, HF concentration, and etching time. The results showed that SRa decreased by 52.5% and MRR increased by 26.7%. The optimal conditions were achieved at 60 ℃, with 3.22 mol/L HF, 0.14 mol/L H2O2, and an etching time of 90 min.
Improving SRa and MRR were the target outcomes of the research using examine CHM approach[10]. A face-centered central composite design (FCCCD) was used to optimize the etching time, concentration, and temperature. The significance of the parameters was confirmed by analyzing the variance. The optimized settings improved MRR by 17.8% and decreased surface roughness by 52.7% compared to the initial values.
Using a mixed acid FeCl3, Ibrahim[8] studied the relation between surface roughness, amount of aluminum alloy material removed, machining temperature, time, and solution concentration. Three distinct milling solution concentrations were used: 25%, 50%, and 75%. Time, temperature, and etchant concentration have the greatest influence on the finishing of a chemically machined aluminum alloy. Concentration is the most important parameter for maximum MRR and machining time for least surface roughness (SRa). Machine time, temperatures, and etchant concentration were the most important variables for finishing chemically machined aluminum alloy.
Chemical milling is widely used for the manufacturing of titanium components due to its flexibility and ability to obtain complex components. However, their high final machining costs limit the number of applications for which these materials are feasible. As well as under these conditions, titanium alloys exhibit a high degree of reactivity with oxygen, resulting in the formation of oxide on the metal's surface and an oxygen-enriched subsurface layer[2, 11]. The alpha-case is a continuous layer that is both hard and brittle, and it has a driving effect on all titanium alloys' significant mechanical properties[12-13].
Chemical milling was investigated by Deshmukh et al.[12]. Titanium work surfaces' alpha-case thickness is determined by the test pieces' oxygen concentration, temperature, and heating time. HF acid concentrations between 8% and 9% and etch milling times under 15 min were optimal. The removed alpha-case layer of Ti-6Al-4V alloy at 980 ℃ is thinner than the layer removed at 1030 ℃.
Using chemical milling, Nádai et al[2] modified the surface roughness of chemically milled titanium materials, especially nanoparticulate titanium discs and Grade 2 titanium discs that were 2 mm thick. The surface that was created was studied utilizing electron microscopy, reflected-light microscopy, and optical stereo. Milling at 30 ℃ for 30 s with a milling solution of hydrofluoric acid, 12 v/v% nitric acid, and distilled water is the perfect parameter.
Chemical milling with hydrofluoric-nitric acid solutions at 1∶3 and 1∶11 molar ratios was used to analyze the behavior of two different titanium alloys Ti-6Al-4V and Ti-6A-2Sn-4Zr-2Mo[13]. The rate of corrosion of Ti-6Al-4V was found to be faster than that of Ti-6Al-2Sn-4Zr-2Mo in solutions with a ratio of 1∶3. Corrosion rates for Ti-6Al-2Sn-4Zr-2Mo were found to be significantly higher in solutions with a ratio of 1∶11. The observations indicated that the α-layers corroded more rapidly in both alloys compared to the β-layers. The results showed that the chemical milling behavior of the two alloys tested is controlled by the microscopic corrosion behavior of the individual micro constituents.
Titanium alloy pre-treatment methods are crucial for surface treatment, coating pre-deposition, and surface modification. Vlčák[14] examined how chemical milling affected Ti-35Nb-7Zr-5Ta alloy surface properties and corrosion stability. The chemical milling procedures improved the corrosion stability of the native oxides and suboxides of the alloying elements. According to the research results, other surface parameters may not be as essential as charge transfer during corrosion (roughness, wettability).
The current objectives of this research include determining the optimum value, i.e., the maximum value of the material removal rate (MRR) and minimum surface roughness (SRa), of the Ti-5Al-2.5Sn alloy by varying the chemical milling parameters, including milling time, concentration, and temperature. Evaluate the impact of chemical milling parameters on the rate of material removal and surface roughness, which were investigated and estimated using ANOVA results.
1 Materials and Methods 1.1 Materials and Taguchi DesignIn this research, titanium alloy (Ti-5Al-2.5Sn alloy) was used to evaluate the effect of chemical milling parameters. The etchant concentration, immersion time, and temperature of the chemical milling were used as process parameters. All Ti-5Al-2.5Sn alloy test samples for the current study were cut to 10 mm in height and 20 mm in diameter. Cleaning and masking are important steps in the chemical machining process to remove oil, grease, dust, rust, or any other substance from the surface of the material. The samples were covered with masking material to preserve any components that did not need to be chemically produced. After the samples have undergone the chemical process, the mask material should be easily removable. The epoxy and its hardener in a 1∶1 ratio, were utilized for masking the samples. It took nearly 15-16 h to dry, after which the scribing process was performed to expose the desired area to the chemical milling solution. These samples were subjected to the chemical milling process using milling solution consisting of hydrofluoric acid (HF) and nitric acid (HNO3) that was added to distilled water in a predetermined ratio. Using the Taguchi technique, based on the theory of experimental design, researchers were able to identify the significance of parameters with their effect on the selected responses i.e. variables. Taguchi method is the effective technique used to optimize process parameters in a wide range of manufacturing applications such as machining processes[15-17]. Therefore, in this study, Taguchi method was chosen for the investigation because it is an efficient tool for both the planning and analysis of experiments. Table 1 shows the input CHM parameters with the selected levels. The Taguchi (L16) design (Table 2) has been used for performing the experimental runs of CHM process. To determine etching concentrations, hydrofluoric acid (HF) and nitric acid (HNO3) were added to distilled water in a predetermined ratio.
| Table 1 CHM input parameters required for the experimental work |
| Table 2 Taguchi (L16) design for performing experimental runs of CHM process |
1.2 Chemical Milling Process and Measurement
Chemical milling is the immersion of a part in a chemical bath and the chemical's action on the milled part. The time spent in the chemical bath determines the resulting milling depth[1]. In chemical milling, hydrogen fluoride and nitric acid solutions are used. Hydrogen fluoride is an odorous, flammable liquid or gas with no discernible hue. Nitric acid is a common laboratory reagent and a key industrial chemical used in the production of fertilizers and explosives. Handling hydrofluoric acid requires protective equipment due to its high reactivity. The hydrofluoric solution used in this study has a molecular weight of 20.01 g/mol and a density of 1.410-1.420 g/mL. Hydrofluoric acid and nitric acid mixtures etch titanium well but slowly and produce hydrogen gas. Hydrogen gas from HF alone can cause fires. Milling chemically is slow, and the surface finish may be poor[18]. Nitric acid accelerates the milling process, eliminates hydrogen gas, and smooths the surface. Therefore, in this study, a mixture of HF and HNO3 in 100 mL of distilled water was employed at four levels for each input CHM parameter, i.e. (concentrations, temperature, and machining time). The mixture is poured into the beaker on the stirrer apparatus, and an experiment-specific temperature is selected. The samples were submerged for the allotted time, then removed, thoroughly rinsed with distilled water, and dried in a dry oven. After machining, the maskant layer was removed and the weight of the specimens was measured to detect weight loss. Figs. 1(a) and (b) show the experimental setup for chemical milling. Material removal rate (MRR) covers all exposed surfaces, and the substances removed in chemical milling are removed faster than in conventional machining. The material removal rate was calculated experimentally using the weight difference method before and after chemical milling[5] in accordance with Eq.(1).
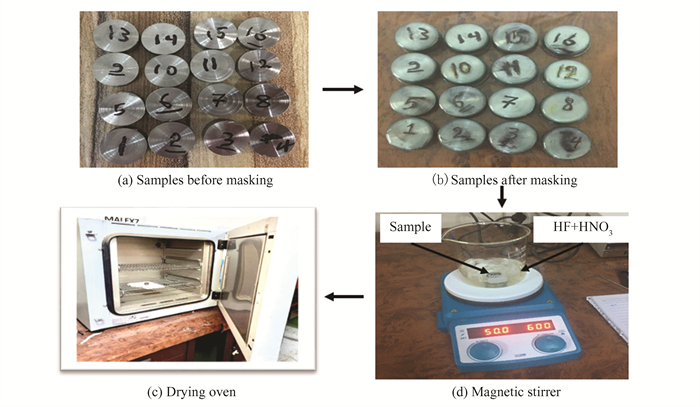
|
Fig.1 Experimental setup of chemical milling |
The MRR is defined as:
| $ \operatorname{MRR}=\frac{W_{\mathrm{b}}-W_{\mathrm{a}}}{t_{\mathrm{m}}} \quad(\mathrm{mg} \cdot \min ) $ | (1) |
where Wb represents the mass of workpiece before milling process (mg); Wa represents the mass of the workpiece after milling process (mg); tm represents the milling time (min).
The roughness of the unmasked surface was measured using the roughness tester model TR200 for four zones for each milled sample, and the average has been taken.
1.3 Analysis of Variance (ANOVA)ANOVA is a statistical method for determining the significance of parameters in the selected responses. Minitab 17 created an ANOVA table to determine which parameters were controlled for this research. According to the ANOVA analysis, the distribution of treatment data can be clarified to determine if it is normal or not. Assuming the distribution of treatment data was normal, the specimens were selected at random and independently. Specimens were independent[19-20].
The signal-to-noise ratio (S/N) of Taguchi is the logarithm of the desired output responses. The optimization ratios depend on the quality of the product or process[21].
The standard S/N ratios are nominally better (NTB), smaller better (STB), and higher better (HTB) [22-23]. In this study, the proper aim for MRR was selected as higher better (HTB) using the applicable S/N Eq.(2), while surface roughness (SRa) was calculated using the applicable S/N Eq.(3), i.e., smaller better (STB). S/N depends on the response aim (i.e. minimum or maximum) and is the ratio of the standard deviation to the mean. The objective function of optimization experiments using orthogonal design is well-balanced[21].
| $ \frac{\mathrm{S}}{\mathrm{~N}}=-10 \log \frac{1}{n}\left(\sum \frac{1}{z_i^2}\right) $ | (2) |
| $ \frac{\mathrm{S}}{\mathrm{~N}}=-10 \log \frac{1}{a}\left(\sum z_i^2\right) $ | (3) |
where a is the repetitions number; zi is the data of experiment.
2 Results and DiscussionChemical milling machining (CHM) was performed using Taguchi L16 and Minitab 17 software to investigate the machinability of the Ti-5Al-2.5Sn alloy in terms of surface roughness (SRa) and material removal rate (MRR). The results of MRR and SRa are presented in Table 3.
| Table 3 Predicted and measured roughness and material removal rate |
2.1 Material Removal Rate
The experimental data were analyzed to determine how chemical milling parameters (machining temperature, concentrations, and milling time) affect material removal rate (MRR). DOE-based Taguchi design was used to analyze all experimental data. All sixteen experiments used Ti-5Al-2.5Sn alloy as a workpiece. The difference in workpiece weight before and after chemical milling (ΔW) corresponds to the material removal rates (MRR). Table 3 presents the material removal rate values according to the runs of Taguchi design and based on Eq.(2). Fig. 2 shows residuals that are positioned close to the straight line, which corresponds to the regular distribution of the attained outcomes and normal error distribution. The comparison between measured and predicted values is based on the material removal rate design model and regression Eq.(4), as shown in Fig. 3. The measurements align with the forecasts.
| $ \begin{aligned} \text { MRR } & =0.00154+0.00662 \operatorname{Cont} \%- \\ & 0.00504 T+0.00186 E_t \end{aligned} $ | (4) |
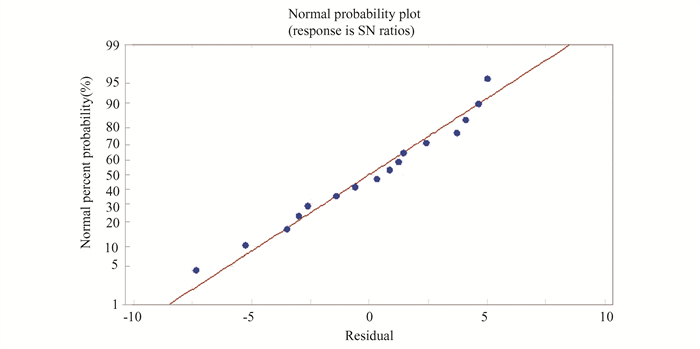
|
Fig.2 Normal probability plot of MRR |
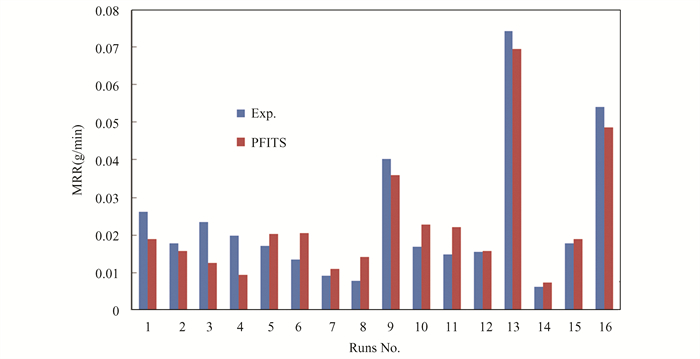
|
Fig.3 Compression between experiment and predicted values of MRR |
The optimum process parameters to achieve maximum temperature for MRR in the machined specimens are depicted in Fig. 4. In this graph, the MRR for the machined specimens increased as the temperature (T) decreased, indicating that the effect of temperature has an inverse relationship with the MRR. In other words, a decrease in machining temperature causes an increase in corrosion rate due to a powerful oxidizing agent and increased ion mobility. The graph demonstrates that the MRR increases as the temperature decreases from 45 to 60 ℃, with temperature having the least impact among the selected variables.
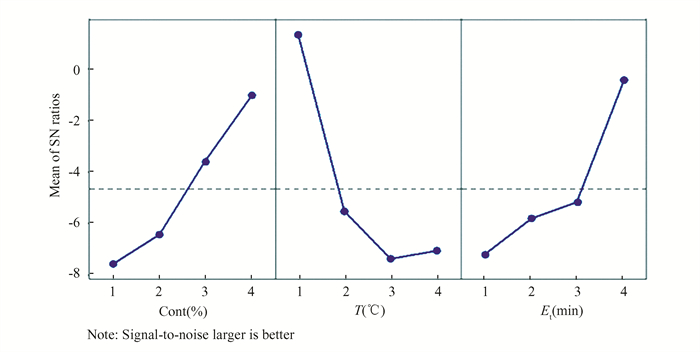
|
Fig.4 Main effect plot for S/N ratio for MRR |
The MRR for the machined specimens increased as the concentration percentage (cont.%) increased from 7.5% to 22.5% HF + 17% HNO3, as depicted in the graph. In chemical etching milling, the removal rate of etching is directly proportional to the etching concentration and the surrounding areas to be machined. The concentrations of etchant in chemical milling solutions are proportional to the amount of hydrogen ions in the chemical etchant. Higher etchant concentrations or hydrogen ion concentrations facilitate substrate chemical milling process. This applies to all types of alkaline and acidic chemical etchants. In addition, hydrofluoric and nitric acids, which form a highly acidic solution, are frequently used to etch titanium alloys. The greater the acid concentration, the more metal is removed, as indicated in the previous study[8].
The effect of milling time (Et) on the rate of material removal from workpieces is depicted in Fig. 4. The MRR increases as the milling time increases for all machined workpieces, as depicted in this graph. As the corrosion rate increases over time, the MRR rises from 15 to 60 min as the milling time increases.
The objective of response (MRR) is the larger-is-better rule which is used to measure the performance characteristic in this graph. So, the optimum chemical process parameters suggested to obtain the optimal material removal rate from Fig. 4 are (Cont%4T1Et4), i.e., at concentration level 4, milling temperature level 1, and milling time level 4. Thus, the maximum rate of material removal using HF and HNO3 acids was 0.0842 mg/min for experimental run number 13 at operating conditions of machining temperature (45 ℃), concentrations (22.5% HF +17% HNO3), and milling time (60 min). The figure illustrates the average signal-to-noise ratios for each parameter in terms of characteristic response. The larger-is-better rule is used to measure the performance characteristics in this graph. In addition, Fig. 4 suggests that the optimal material removal rate can be obtained at concentration level 4, milling temperature level 1, and milling time level 4.
The results of the analysis of variance (ANOVA) for the rate of material removal from specimens are presented in Table 4, according to the Taguchi design. The table illustrates the relationship between quantity of material removed from a specimen and the three chemical milling parameters: temperature, concentration, and chemical milling time. The F-values demonstrate that concentration has an effect of 65.05%, temperature of 5.67 ℃, and time of 8.10 min respectively. It appears that the chemical milling solution concentration is the most important parameter with a percentage of contribution (PCR) 81%, followed by milling duration with a PCR of 10%, and then machining temperature with a PCR of 7%. Among the selected variables, chemical milling temperature had the least effect on the MRR, which increases as the temperature drops from 45 to 60 ℃. This may be due to the difficulty in installing the magnetic stirrer device used to measure temperature during the experimental work.
| Table 4 Analysis of variance for SN ratios MRR |
The response is displayed in Table 5 according to the maxim "larger is better". This table explains the relative impact of each parameter on the material removed rate. The rankings in Table 5 indicate the extent to which each parameter contributes to the overall response. The concentration, ranked first among the parameters, has the greatest effect, followed by the etching chemical milling time and machining temperature, which are ranked second and third, respectively. The following confirms the arrangement in this table: Etching at higher concentrations has the greatest impact, followed by longer etching durations and higher temperatures. It is possible to make educated guesses regarding the effect of various parameters on surface roughness. One of the most important parameters determining surface roughness is the rate of material removal. When the material rate is lower, roughness values tend to be higher.
| Table 5 Response for Signal to Noise Ratios |
2.2 Surface Roughness
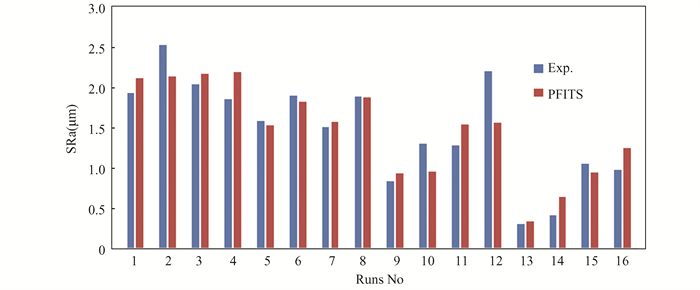
Surface roughness refers to deviations in the surface texture and is often quantified by measuring the average roughness height (SRa), which is the arithmetic mean of the surface deviations from the mean line. This study found that the concentration and temperature of the chemical milling solution have a significant effect on the surface roughness of the machined specimens. Table 3 presents the surface roughness value according to Taguchi design runs. Fig. 5 depicts the data points that fall along a straight line on the probability plot, indicating that the data is normally distributed. Fig. 6 shows the comparison between measured and predicted values based on the average surface roughness and regression Eq.(5); it indicates that the predicted values are close to the experimental values, which would suggest a good agreement between the two.
| $ \begin{aligned} & \mathrm{SRa}=2.551-0.4537 {\text { Cont }} \%+0.1638 \;T- \\ & \quad 0.1397 \;E_{\mathrm{t}} \end{aligned} $ | (5) |

|
Fig.5 Normal probability plot of SRa |
|
Fig.6 Comparison between experiment and predicted values of SRa |
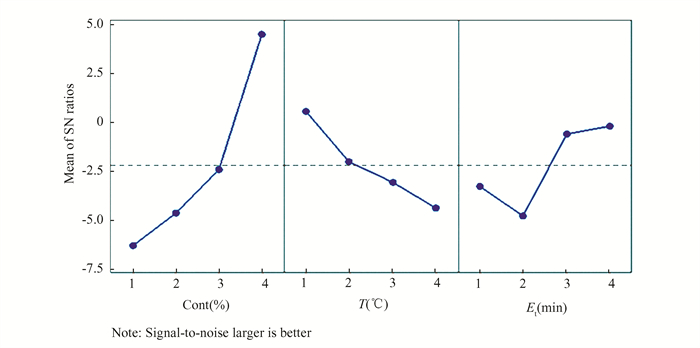
Fig. 7 depicts the effect plot for the signal-to-noise ratios in terms of characteristic responses that can help visualize the optimum chemical milling parameters (Cont%, T, and Et) to achieve the best surface finish. The main effect plot for SN ratio (SRa) shows that the concentration of the chemical milling solution significantly affects on the surface roughness of machined workpieces, with increasing concentration leads to a smoother surface finish. As the concentration expands from level 1 to level 4, the SRa decreases. In addition, the SRa value at level 1 is the highest (6.3503 μm), and the SRa value at level 4 is the lowest (4.4884 μm). The main effect plot for T also shows that as the temperature increases, the SRa slightly decreases, indicating that higher temperatures lead to smoother surfaces. The effect of milling time (Et) is relatively small, while the effect of temperature (T) is more pronounced. With the longest milling time, and considering the effect of the concentration of solution on the temperature, the lowest roughness is achieved. Thus, the optimum chemical milling parameters are at (cont%4T1Et4), i.e., the optimal process parameter settings are a higher concentration of the chemical milling solution (Cont%), a lower machining temperature (T), and a longer etching time (Et), which result in a smoother surface finish.
|
Fig.7 The main effect plot for SN ratio (SRa) |
According to the Taguchi design, the results of the analysis of variance (ANOVA) for the average roughness height (SRa) are presented in Table 6. The positions in Table 7 indicate the contribution of each parameter to the overall response. It can be seen that the concentration (Cont%) has a significant effect on the roughness of the surface (SRa) with a PCR of 67%. The SRa value at levels 2 and 3 decreases gradually. The effect of time (Et) on SRa is statistically significant, with an F-value of 5.98 and PCR of 15%. The effect of temperature (T) is relatively small compared to the other parameters, such as concentration (Cont%) and milling time. The ANOVA results show that the F-value for T is 5.34, with a PCR of 13%, which is significant but lower than the values obtained for Cont% and Et. The rankings in Table 7 indicate the extent to which each parameter contributed to the overall responses. The concentration, ranked first among the parameters, has the greatest effect, followed by machining temperature and then chemical milling time, which are ranked second and third, respectively.
| Table 6 Analysis of variance for SN ratios for SRa |
| Table 7 Signal to Noise Ratios for SRa |
3 Conclusions
From the experimental work, the following conclusions can be observed:
1) The Taguchi method was used to predict MRR values with high accuracy.
2) The MRR is directly proportional to both concentration rate and milling time. However, the MRR increases as the temperature decreases.
3) The SRa decreases directly with a proportional increase in both concentration rate and milling time. However, SRa decreases as the temperature decreases.
4) The concentration of the chemical milling solution has the greatest influence on the responses (MRR and SRa), followed by duration of the milling process and machining temperature. The results indicate that the contribution percentages of the concentration of the etching acids on the both responses (MRR and SRa) are 81% and 67% respectively.
5) The optimal combination of chemical milling process parameters for achieving higher material removal and a smoother surface finish is a higher concentration of the chemical milling solution, a low temperature, and a longer milling time, i.e., a concentration of (22.5% HF + 17% HNO3), milling temperature of 45 ℃, and 1 h of milling time. These parameters resulted in a maximum MRR of 0.0842 mg/min and a minimum SRa of 0.30 μm.
| [1] |
Puthumana G, Anusree T G. Analysis of chemical machining for practical applications. BEST: International Journal of Management, Information Technology and Engineering, 2014, 2(3): 77-86. (  0) 0) |
| [2] |
Nádai L., Katona B, Terdik A, et al. Chemical etching of titanium samples. Periodica Polytechnica Mechanical Engineering, 2013, 57(2): 53-57. DOI:10.3311/PPME.7046 (  0) 0) |
| [3] |
Abdulhusein N, Ibrahim A, Huayier A. Influence of machining parameters on surface roughness in chemical machining of Silicon Carbide (SiC). Engineering and Technology Journal, 2022, 40(6): 879-884. DOI:10.30684/etj.v40i6.1879 (  0) 0) |
| [4] |
Speight J. Lange's Handbook of Chemistry. New York: McGraw-Hill Education, 2005.
(  0) 0) |
| [5] |
El-Awadi G A, Enb T A, Abdel-Samad S, et al. Chemical machining for stainless steel, aluminum and copper sheets at different etchant conditions. Arab Journal of Nuclear Science and Applications, 2016, 94(2): 132-139. (  0) 0) |
| [6] |
Zogheib L V, Della Bona A, Kimpara E T, et al. Effect of hydrofluoric acid etching duration on the roughness and flexural strength of a lithium disilicate-based glass ceramic. Braz Dent J, 2011, 22(1): 45-50. DOI:10.1590/S0103-64402011000100008 (  0) 0) |
| [7] |
Shather S K, Ibrahim A. Influence of machining parameters on surface roughness in chemical machining of stainless steel 304. Engineering and Technology Journal, 2015, 33(6): 1377-1388. DOI:10.30684/etj.33.6A.8 (  0) 0) |
| [8] |
Ibrahim A F, Mustafa A M. Experimental investigation of machining parameters on material removal rate and surface roughness in chemical machining. IOP Conference Series: Materials Science and Engineering, 2019, 518(3): 032029. DOI:10.1088/1757-899X/518/3/032029 (  0) 0) |
| [9] |
Nordin N M, Azhari A W, Halin D S C, et al. Application of taguchi's design of experiments in optimization of metal assisted chemical etching process. IOP Conference Series: Materials Science and Engineering. Bristol: IOP Publishing, 2019, 572(1): 012059. DOI:10.1088/1757-899X/572/1/012059 (  0) 0) |
| [10] |
Serapio R K L, Antilano E T, Soreda A S. Application of face-centered central composite design for the optimization of chemical etching process of QFN-mr package using Alkaline-Based chemical solution. 2020 IEEE 22nd Electronics Packaging Technology Conference (EPTC). Piscataway: IEEE, 2020: 413-418. DOI:10.1109/EPTC50525.2020.9315087
(  0) 0) |
| [11] |
Umanath K, Devika D. Optimization of electric discharge machining parameters on titanium alloy (Ti-6Al-4v) using Taguchi parametric design and genetic algorithm. MATEC Web of Conferences, 2018, 172: 04007. DOI:10.1051/matecconf/201817204007 (  0) 0) |
| [12] |
Deshmukh V, Kadam R, Joshi S S. Removal of alpha case on titanium alloy surfaces using chemical milling. Machining Science and Technology, 2017, 21(2): 257-278. DOI:10.1080/10910344.2017.1284558 (  0) 0) |
| [13] |
Sefer B, Dobryden I, Almqvist N, et al. Chemical milling of cast Ti-6Al-4V and Ti-6Al-2Sn-4Zr-2Mo alloys in Hydrofluoric-Nitric Acid solutions. Corrosion, 2017, 73(4): 394-407. DOI:10.5006/2277 (  0) 0) |
| [14] |
Vlčák P, Fojt J, Koller J, et al. Surface pre-treatments of Ti-Nb-Zr-Ta beta titanium alloy: The effect of chemical, electrochemical and ion sputter etching on morphology, residual stress, corrosion stability and the MG-63 cell response. Results in Physics, 2021, 28: 104613. DOI:10.1016/j.rinp.2021.104613 (  0) 0) |
| [15] |
Kara F, Bulan N, Akgün M, et al. Multi-objective optimization of process parameters in milling of 17-4 PH stainless steel using Taguchi-based gray relational analysis. Engineered Science, 2023, 26: 961. DOI:10.30919/es961 (  0) 0) |
| [16] |
Shihab S K, Mubarak E M, Al-Kalali R H. Influence and optimization of surface roughness on surface integrity during turning using grey relational analysis. Journal of Harbin Institute of Technology (New Series), 2021, 28(2): 38-46. DOI:10.11916/j.issn.1005-9113.2019063 (  0) 0) |
| [17] |
Nas E, Kara F. Optimization of EDM machinability of hastelloy C22 super alloys. Machines, 2022, 10(12): 1131. DOI:10.3390/machines10121131 (  0) 0) |
| [18] |
Zhakeyev A, Wang P, Zhang L, et al. Additive manufacturing: unlocking the evolution of energy materials. Advanced Science, 2017, 4(10): 1700187. DOI:10.1002/advs.201700187 (  0) 0) |
| [19] |
Akgün M, Özlü B, Kara F. Effect of PVD-TiN and CVD-Al2O3 coatings on cutting force, surface roughness, cutting power, and temperature in hard turning of AISI H13 steel. Journal of Materials Engineering and Performance, 2023, 32(3): 1390-1401. DOI:10.1007/s11665-022-07190-9 (  0) 0) |
| [20] |
Kara F, Bayraktar F, Sava F, et al. Experimental and statistical investigation of the effect of coating type on surface roughness, cutting temperature, vibration and noise in turning of mold steel. Journal of Materials and Manufacturing, 2023, 2(1): 31-43. DOI:10.1016/j.measurement.2020.1084 (  0) 0) |
| [21] |
Ibrahim A F, Huayier A F, Abdulhusein N A. Influence of machining parameters on material removal rate in chemical machining of silicon carbide (SIC). Journal of Physics: Conference Series, 2021, 1818(1): 012217. DOI:10.1088/1742-6596/1818/1/012217 (  0) 0) |
| [22] |
Slebi-Acevedo C J, Silva-Rojas I M, Lastra-González P, et al. Multiple-response optimization of open graded friction course reinforced with fibers through CRITIC-WASPAS based on Taguchi methodology. Construction and Building Materials, 2020, 233: 117274. DOI:10.1016/j.conbuildmat.2019.117274 (  0) 0) |
| [23] |
Zhang F, Wang M, Yang M. Successful application of the Taguchi method to simulated soil erosion experiments at the slope scale under various conditions. Catena (Amst), 2021, 196: 104835. DOI:10.1016/j.catena.2020.104835 (  0) 0) |
 2024, Vol. 31
2024, Vol. 31


