2. 东华大学 化学化工与生物工程学院,上海201620;
3. 复旦大学 高分子科学系,上海200433
2. College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, China;
3. Department of Macromolecular Science, Fudan University, Shanghai 200433, China
刺激响应聚合物是指一类具有“智能”行为的大分子体系,即当外界环境如温度、pH、光、压力、电场强度、磁场强度、离子强度或添加物浓度等改变时,大分子会做出相应的链构象或分子结构上的转变,进而表现为外在的可检测到的宏观性质变化[1].刺激响应聚合物通常伴随着表观的相转变现象.由于这一独特的刺激响应性质,刺激响应聚合物在智能器件、药物控释、纳米材料、化学传感和生物技术等领域表现出了极为广泛的应用前景[2-5].
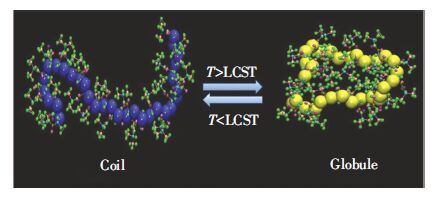
在刺激响应聚合物的大家庭中,温敏水溶性聚合物有着极其重要的地位.温敏水溶性聚合物有低临界溶解温度(LCST)型与高临界溶解温度(UCST)型之分.其中,LCST型聚合物被研究得最为广泛.如图 1所示,LCST型聚合物相转变又被称为线团-胶束(coil-to-globule)转变,在水溶液中低温下溶解而高温下由于疏水相互作用发生分子链聚集或塌缩从而形成稳定的胶束,降温后又可重新恢复到原状[6-8].UCST型聚合物的相变情况则与此相反[9].与常见高分子随温度升高溶解性增加不同,LCST型聚合物随温度升高溶解性急剧下降.由于很多水溶性聚合物的LCST比较接近生理温度(~37 ℃),因而,LCST型聚合物在生物医学领域应用极为广泛,如载药及药物控制释放[10]、组织工程[11]、生物分子响应[12-13]、蛋白质吸附[14]、有机-无机复合材料[2]等.
|
图 1 LCST型聚合物coil-to-globule转变示意图[8] Figure 1 Schematic coil-to-globule transition of LCST-type polymers[8] |
温敏水溶性聚合物转变机制的研究对于探讨这类聚合物材料的响应行为及其应用有着至关重要的意义.除了常见的浊度分析、量热分析、光散射、小角X射线衍射、显微镜观测等,分子光谱,尤其是红外光谱对于链段构象或基团相互作用极为敏感,近年来逐渐发展成为研究温敏聚合物的重要分析手段.但由于一维分子光谱的分辨能力有限,分子光谱对温敏聚合物体系的研究并不特别深入.这一方面是因为温敏聚合物体系较为复杂,涉及到聚合物与水及聚合物自身之间的多重相互作用;另一方面,一维光谱中谱峰重叠的现象较为严重.
为了解决一维分子光谱的低分辨率及谱峰重叠问题,Noda于1986年首次提出了二维相关分析的概念[15],后于1989年将其发展为广义二维相关光谱[16-17],即能够对任意外扰作用下的系列谱图进行相关分析处理,从而获得了极为广泛的应用进展.由于不同基团对外界扰动的响应不同,二维相关光谱将谱峰信息在二维尺度上进行延展,对隐藏在一维谱峰下不明显的峰数目和峰位置进行了很好的区分.二维相关光谱还可以辨识在可控外扰下各基团的运动顺序,从而广泛用于研究分子内和分子间的结构或构象变化.
本文首先对二维相关光谱及其衍生外扰相关移动窗口技术进行简要描述,接着重点介绍近些年来一维及二维相关光谱对温敏水溶性聚合物体系的研究进展.为便于讨论,本文将温敏聚合物体系分为了LCST型均聚物、LCST型共聚物和混合物、LCST型聚合物凝胶和LCST型聚合物刷.由于二维相关光谱对UCST型聚合物体系研究相对较少[18],本文不多做介绍.最后,对本领域的未来发展重点进行了展望.
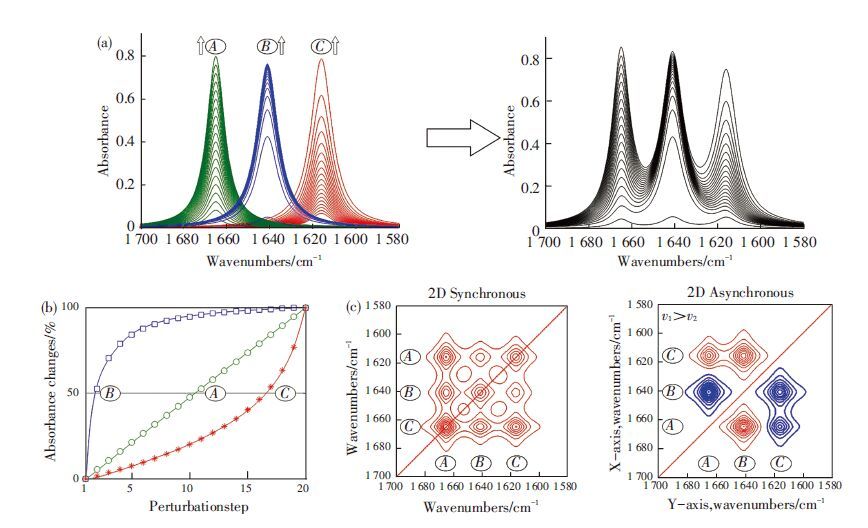
1 二维相关光谱及外扰相关移动窗口技术 1.1 二维相关光谱二维相关光谱的谱图获取及多维光谱分析如图 2所示.二维相关光谱本质上是一种数学处理,是对复杂矩阵变换的一种简化表达,通常由等高线图表示,颜色深浅或等高线密度代表该位置处的强度高低.二维相关分析的数学表达本文不做详细介绍,可参考其他文献表述[16, 19].
|
图 2 二维相关光谱的谱图获取及二维光谱分析[20] Figure 2 Acquisition of two-dimensional correlation spectroscopy and two-dimensional spectral analysis: (a) simulated three peak variations and their overlaid dynamic spectra;(b) peak intensity variations as a function of perturbation variable;(c) generated 2D synchronous and asynchronous spectra (red,positive; blue,negative)[20] |
由图 2可以看出,二维相关光谱在谱图上是由同步谱和异步谱2张谱组成[20].同步谱是关于主对角线对称的.位于主对角线上的峰称为自动峰,自动峰总是正峰,它的强度大小代表了该处吸收峰对于外扰的敏感程度.主对角线之外的峰称为交叉峰,交叉峰可正可负,它的出现表明官能团之间存在对外扰的协同响应.交叉峰为正表示2个官能团的峰强度随外扰的变化而升高或降低的方向相同,反之则相反.
异步谱是关于主对角线反对称的,它没有自动峰,只在对角线之外存在交叉峰,代表了官能团之间是否存在强的化学作用、直接相连或成对现象.异步谱可大大提高谱图的分辨率.如图 2所示,尽管3个相邻谱峰有所叠加,在异步谱中它们可以被完全分辨出来,这在实际体系中尤为实用.异步谱交叉峰亦有正负之分,其符号可用来判断分子基团的运动次序.判断规则又被称为Noda规则.简单说来,对于2个吸收峰v1 >v2,如果同步谱与异步谱符号相同,则波数较大的v1先变化,反之,符号相反则波数较小的v2先变化.例如,图 2中A、B、C 3个峰强度均随外扰变化增加,因而在同步谱上相关峰均为正峰,表现为同步变化.异步谱(因谱图反对称,只分析左上角谱峰)上,A与B相关峰为负,A与C相关峰为正,B与C相关峰为正.根据Noda规则判断,B变化最先,A次之,C最后.这一顺序与图 2(b)的强度变化曲线完全一致,而二维光谱的优势在于它反映的是一段光谱范围所有位置的变化情况.
1.2 外扰相关移动窗口技术基于同样外扰下的动态光谱,除了能够从中获取关于谱峰的数目、位置及变化次序等方面的信息外,通常还需要对光谱随外界扰动的变化情况做一个整体的了解.特别是一些具有转变点的相变体系,相变点的确定通常对二维相关光谱分析的区域选择至关重要[21].移动窗口技术由此发展而来.
移动窗口(Moving window)最初由M. Thomas提出[22],本质上基于二维同步谱的power spectra(即对角线上的切线谱)的变化.它允许选定一个合适的窗口大小,然后逐点移动,通过power spectra的变化情况便可以反映出所研究光谱区域随外扰的变化快慢,从而确定转变点的位置.
2006年S. Morita[23]将外扰变量也引入到了相关方程,提出了外扰相关移动窗口二维相关光谱(perturbation correlation moving window two-dimensional correlation spectroscopy,简称PCMW2D).PCMW2D谱图开始有了同步与异步之分,如图 3所示.同步谱与原来的移动窗口谱图几乎完全相同,但同时引入了符号的变化来反映一维谱图的变化方向.异步谱通过二阶导数转换,可反映出谱图变化更为精细的信息.

|
图 3 外扰相关移动窗口二维相关光谱(PCMW2D)谱图及判断规则(外扰增量的情况下) Figure 3 Perturbation correlation moving window two dimensional correlation (PCMW2D) spectra and the determination rule (in case of perturbation increment) |
PCMW2D谱图的判断规则如图 3所示:在外扰变量为增量的情况下,同步谱为正表示光谱强度增加,同步谱为负表示光谱强度减小;异步谱为正表示光谱强度变化为一凸形变化,异步谱为负表示光谱强度变化为一凹形变化.
对于有明显相转变的温敏聚合物体系,光谱强度变化通常表现为S形或反S形.如图 3所示的例子,PCMW2D同步谱可以确定LCST的转变温度,而异步谱的峰确定的是S或反S形曲线的拐点,即相转变温度区间(相关信息汇总在图 3左栏最下图).由此可以看出,PCMW2D非常适合研究温敏聚合物体系的相变行为.
2 二维相关光谱及外扰相关移动窗口技术研究温敏水溶性聚合物的相变行为 2.1 LCST型均聚物LCST型均聚物是最为简单的水溶性聚合物相转变体系,同时也是研究其他复杂体系的基础.近十年来,二维相关光谱及PCMW2D被大量用以研究了线性LCST型均聚物体系,相关体系类型、LCST转变温度及文献引用见表 1.
| 表 1 二维相关光谱研究的典型LCST型均聚物体系 Table 1 Two dimensional correlation spectroscopy study of typical LCST type Homo system |
其中,PNIPAM是最为典型的LCST型聚合物,也是目前研究最多的温敏水溶性聚合物.PNIPAM的LCST约为32 ℃,转变温度受分子量和浓度的影响不大.低温时,PNIPAM与水存在强烈的氢键和水合作用.随着温度的升高,PNIPAM与水之间的氢键逐渐解离,而PNIPAM自身酰胺键之间的分子间氢键逐渐形成,导致PNIPAM分子链发生塌缩进而形成稳定的胶束.基于变温红外的PCMW2D很好地跟踪了这一过程,确定了PNIPAM的相转变温度和温度区间分别为31.2 ℃和29.1~31.1 ℃[26].二维相关分析发现,升温过程中,甲基首先发生了两步的脱水过程,主链的塌缩次之,酰胺键的氢键变化最后发生[24].降温过程的顺序与之相反.加入少量的乙醇会降低PNIPAM的LCST,从二维分析结果上来看,乙醇的加入主要通过抑制疏水基团的水合过程削弱了PNIPAM的相变[25].
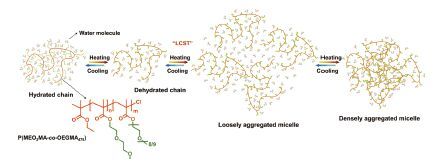
聚甲基丙烯酸寡聚乙二醇酯(POEGMA)是近年来研究非常热门的LCST型聚合物.POEGMA实际上是由不同PEG长度的甲基丙烯酸寡聚乙二醇酯单体无规共聚形成,但由于不同结构单元的相似性,将其划分到了均聚物的范畴.POEGMA的LCST可以通过改变不同OEGMA单体的比例在27~60 ℃内可调[39].笔者所在的课题组研究了P(MEO2MA-co-OEGMA475)在重水中升温及降温过程中的分子链构象变化[32].与PNIPAM不同,P(MEO2MA-co-OEGMA475)不存在分子间的缔和作用,在红外光谱变化上表现为低于LCST时剧烈变化而高于LCST时缓慢变化,在动态光散射上则表现为相转变前不存在分子链的预塌缩过程.PCMW确定了该聚合物的转变温度和转变温度区间分别为32.5 ℃和28.5~37 ℃.二维相关分析表明,在P(MEO2MA-co-OEGMA475)的相转变过程中,分子链存在着“水合链-脱水合链-松散聚集胶束-密集聚集胶束”4个阶段,如图 4所示.降温过程与之相反.当其中1个OEGMA单体长度加长后,如P(MEO2MA-co-PEGMA2080)又表现出了多步聚集的相转变行为[33].
|
图 4 P(MEO2MA-co-OEGMA475)相转变过程中的分子链构象变化[32] Figure 4 Schematic conformational changes of P(MEO2MA-co-OEGMA475) chains during phase transition[32] |
LCST共聚物由2种和多种不同类型的单体共聚生成.无规共聚物的结构相对简单,多用来做比较分析.嵌段聚合物和共混物由于不同聚合物链之间迥异的亲疏水性或温敏性,对它们的研究有助于理解不同聚合物之间复杂的相互作用,从而为设计更为多样的温敏自组装体系提供一定的理论指导.
PEO-PPO-PEO三嵌段共聚物是采用二维相关分析方法研究较早的一类LCST型共聚物.二维相关分析法辨别了PEO-PPO-PEO在LCST相转变过程中各嵌段的运动次序,发现EO嵌段的旁式-顺式构象转变首先发生,亚甲基脱水次之,之后C—O—C与水氢键逐渐解离,最后疏水基团成核形成核壳结构胶束[40].
此后,二维相关分析研究了其他类型的嵌段共聚物,如PNIPAM与聚离子液体嵌段共聚物poly(NIPAM-b-BVImBr)[41]、 PNIPAM-b-PEO[42]、POEGMA-b-P4VP[43]、PVCL-b-PEO[44]等.这些共聚物的特点是均含有1个LCST型的聚合物嵌段和1个亲水聚合物嵌段.升高温度会诱导LCST型聚合物嵌段发生亲水-疏水转变,疏水段发生聚集导致核壳结构胶束的形成.二维相关分析可以分辨不同嵌段的基团运动,从而揭示在这个转变过程中不同嵌段所发挥的作用及氢键变化情况.此外,更为复杂的非线性三嵌段共聚物如由PNIPAM、Poly(acrylic acid)(PAA)、Poly(N-vinylpyrrolidone) (PVP)组成的不同拓扑结构的四臂共聚物的LCST型相转变行为也被加以研究,其中,PNIPAM的LCST相转变引起的微环境变化导致即使本身无温敏效应的PAA嵌段在红外光谱上也表现出了类似的相转变行为[45-46].
如果1个嵌段聚合物由2种不同的LCST型聚合物组成,在发生相转变时2个嵌段会因体系不同发生或协同或独立的相互作用.以PNIPAM-b-PVL为例,相转变过程中PNIPAM和PVCL段协同作用导致只有1个LCST被检测到[47].而与之类似,PNIPAM/PVCL共混物和无规共聚物由于2个嵌段协同聚集,也只表现为1个LCST.一维及二维光谱分析发现,无规和嵌段共聚物较共混物相转变更加剧烈,而PNIPAM与PVCL单元由于水合程度不同在相变过程中会发生聚集的竞争效应,导致最终胶束的结构有所不同[48].
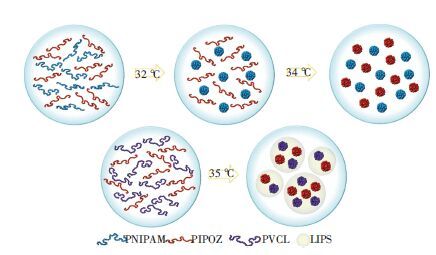
笔者所在课题组还比较了PIPOZ/PVCL及PIPOZ/PNIPAM共混体系的相转变行为.发现由于PIPOZ与PVCL的强烈相互作用,该体系只存在1个LCST相转变,但PIPOZ与PNIPAM的相互作用较弱则表现为2个LCST相转变(图 5)[49].此外,其他的共混体系如PNIPAM与聚离子液体P[P4,4,4,4][SS]共混,P[P4,4,4,4][SS]的温敏性被抑制而不再表现出LCST相转变行为[50].
|
图 5 PIPOZ/PNIPAM与PIPOZ/PVCL共混物的相转变机理[49] Figure 5 Phase transition mechanisms of PIPOZ/PNIPAM and PIPOZ/PVCL mixtures[49] |
LCST型聚合物凝胶是应用极为广泛的一类温敏聚合物材料[3].由于交联结构的存在,聚合物凝胶会表现出与水溶液中截然不同的相转变性质.LCST型聚合物凝胶在升温过程中会发生溶胀-收缩的体积变化,因而相应的转变温度又被称为体积相转变温度.
由于LCST型聚合物凝胶较高的聚合物浓度,非常适合原位红外跟踪分析.例如,笔者所在课题组利用二维相关光谱研究了PNIPAM的本体凝胶的体积相转变行为,发现与水溶液中不同,PNIPAM凝胶的C—H及C=O存在着很多中间态.升温过程中,PNIPAM凝胶网络首先发生塌缩进而导致水分子扩散出凝胶网络,而降温过程与之相反[51].稍后又研究了PNIPAM-co-AA共聚物水凝胶的体积相转变行为,发现AA结构单元的引入导致了该凝胶的降温过程不能完全回复,原因在于AA结构单元之间形成了难以解离的氢键相互作用[52].
微凝胶的体积相转变由于被限定在了纳米尺度,水的扩散距离较短,因而在温度变化引起的体积收缩或溶胀过程中可以被忽略.如图 6所示.
|
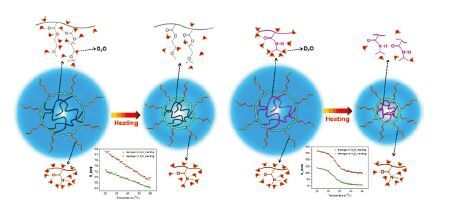
图 6 POEGMA/PDMA及PNIPAM/PDMA核壳结构微凝胶的体积相转变机理[53] Figure 6 Volume phase transition mechanisms of POEGMA/PDMA and PNIPAM/PDMA core-shell structured microgels[53] |
L. Hou 等合成了分别以POEGMA和PNIPAM为核,poly(N,N'-dimethylacrylamide) (PDMA)为壳的核壳结构微凝胶,并利用原位红外光谱研究了这两类微凝胶的体积相转变机理[53].二维相关分析表明,POEGMA/PDMA微凝胶中POEGMA的CO连续缓慢脱水导致了微凝胶的线性体积变化,而PNIPAM/PDMA微凝胶中PNIPAM的氢键剧烈转化导致了微凝胶的反S形体积变化.
此外,二维相关光谱还被用以研究了PNIPAM与poly(2-hydroxyethyl methacrylate) (PHEMA)互穿网络微凝胶[54-55]、聚离子液体交联的POEGMA微凝胶[56]及金纳米粒子负载的PVCL微凝胶[57]等体系的体积相转变行为.
2.4 LCST型聚合物刷温敏性聚合物刷具有非常特殊的表面结构,一端在基板表面化学连接,而另一端在溶剂中自由伸展.温度变化时,聚合物刷会发生可控的塌缩或伸展,引起表面性质如亲疏水性的显著变化.这一性质使得温敏聚合物刷在超疏水表面或可控细胞吸附方面有着非常重要的应用[11, 58].
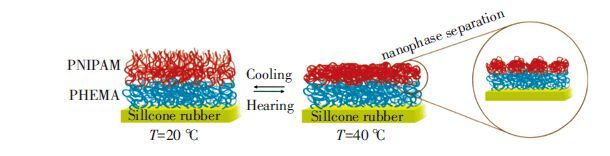
二维相关光谱也被用于研究LCST型聚合物刷的链构象转变.如图 7所示,K. Jalili等利用原位红外光谱跟踪了高密度PHEMA-b-PNIPAM聚合物刷的LCST相转变行为[59].与之前介绍的非线性三嵌段PNIPAM-PAA-PVP共聚物[45]类似,在该体系中,无温度敏感性的PHEMA段受PNIPAM相转变的影响也表现出了“假”的亲疏水性变化.顶层PNIPAM嵌段和底层PHEMA嵌段的LCST分别被确定为33和33.5 ℃.二维相关分析辨别了升降温过程中所有相关基团的运动次序.温度高于LCST时,PNIPAM段塌缩成了很小的团簇紧靠着密集排列的PHEMA层,从而整体呈现“海岛”状的表面形貌.
|
图 7 PHEMA-b-PNIPAM聚合物刷在升降温过程中的分子链构象变化[59] Figure 7 Conformational changes of PHEMA-b-PNIPAM brushes during heating and cooling[59] |
得益于二维相关光谱与外扰相关移动窗口等先进分子光谱技术的发展,人们得以从分子层面上更加深入地了解温敏聚合物体系的刺激响应机制.这一方面有助于人们考察温敏水溶性聚合物的温度敏感性,另一方面也可以启发人们设计合成新型的温敏响应聚合物材料.但同时,不可否认的是,尽管二维相关光谱技术在温敏聚合物尤其是LCST型聚合物体系中取得了长足进步,但随着越来越多新型温敏聚合物及更为复杂的多组分体系的发现,基于二维相关分析的多维分子光谱技术在这类体系中的应用还远远不足.在未来的研究工作中,二维相关分析工作者应关注以下几点:
首先,现有的被用于二维相关光谱研究的LCST型聚合物体系多集中于本文所介绍的存在形式,即多为溶液和凝胶.但实际上,LCST型聚合物材料的应用范围极为广泛,除了文中介绍的聚合物刷之外,还有诸如薄膜、微胶囊、有机-无机杂化材料、乳液等多种形态.这就需要研究工作者在今后的工作中进一步拓展二维相关分析的应用范围.
其次,目前针对温敏聚合物的二维相关光谱研究多基于中红外光谱,但中红外光谱在很多体系中有着一定的局限性,比如,聚合物浓度低导致谱峰强度不足,透射液体池厚度不易控制,水干扰严重等.对于一些不适合中红外光谱表征的体系,可设法采用其他的分子光谱技术进行跟踪,如拉曼光谱、近红外光谱等.
再次,二维相关光谱分析工作者常常过于关注二维分析的结果而忽视了一维光谱常规分析的重要性.实际上,只有当二维光谱分析的结果与一维光谱分析相一致时才能够被认为是确凿无误的.这也是本文中提倡多维分子光谱分析的原因.
最后,基于二维相关光谱分析的结果涉及到了较深层次的分子结构的变化,除了在机理解释上力求合理之外,还须尽量做到与其他分析手段的结果相互验证.这样也有助于人们更加合理地理解二维相关光谱分析的结果.
| [1] | STUART M A C, HUCK W T S, GENZER J, et al. Emerging applications of stimuli-responsive polymer materials[J]. Nature Materials, 2010, 9 (2) : 101–113. DOI: 10.1038/nmat2614 |
| [2] | MOLINA M, ASADIAN-BIRJAND M, BALACH J, et al. Stimuli-responsive nanogel composites and their application in nanomedicine[J]. Chemical Society Reviews, 2015, 44 (17) : 6161–6186. DOI: 10.1039/C5CS00199D |
| [3] | MOTORNOV M, ROITER Y, TOKAREV I, et al. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems[J]. Progress in Polymer Science, 2010, 35 (1/2) : 174–211. |
| [4] | MA X, TIAN H. Stimuli-responsive supramolecular polymers in aqueous solution[J]. Accounts of Chemical Research, 2014, 47 (7) : 1971–1981. DOI: 10.1021/ar500033n |
| [5] | MURA S, NICOLAS J, COUVREUR P. Stimuli-responsive nanocarriers for drug delivery[J]. Nature Materials, 2013, 12 (11) : 991–1003. DOI: 10.1038/nmat3776 |
| [6] | BAJPAI A, SHUKLA S K, BHANU S, et al. Responsive polymers in controlled drug delivery[J]. Progress in Polymer Science, 2008, 33 (11) : 1088–1118. DOI: 10.1016/j.progpolymsci.2008.07.005 |
| [7] | SCHMALJOHANN D. Thermo-and pH-responsive polymers in drug delivery[J]. Advanced Drug Delivery Reviews, 2006, 58 (15) : 1655–1670. DOI: 10.1016/j.addr.2006.09.020 |
| [8] | DESHMUKH S A, KAMATH G, SUTHAR K J, et al. Non-equilibrium effects evidenced by vibrational spectra during the coil-to-globule transition in poly(N-isopropylacrylamide) subjected to an ultrafast heating-cooling cycle[J]. Soft Matter, 2014, 10 (10) : 1462–1480. DOI: 10.1039/C3SM51750K |
| [9] | ROY D, BROOKS W L A, SUMERLIN B S. New directions in thermoresponsive polymers[J]. Chemical Society Reviews, 2013, 42 (17) : 7214–7243. DOI: 10.1039/c3cs35499g |
| [10] | QIU Y, PARK K. Environment-sensitive hydrogels for drug delivery[J]. Advanced Drug Delivery Reviews, 2001, 53 (3) : 321–339. DOI: 10.1016/S0169-409X(01)00203-4 |
| [11] | WISCHERHOFF E, UHLIG K, LANKENAU A, et al. Controlled cell adhesion on PEG-based switchable surfaces[J]. Angewandte Chemie International Edition, 2008, 47 (30) : 5666–5668. DOI: 10.1002/anie.v47:30 |
| [12] | MIYATA T, URAGAMI T, NAKAMAE K. Biomolecule-sensitive hydrogels[J]. Advanced Drug Delivery Reviews, 2002, 54 (1) : 79–98. DOI: 10.1016/S0169-409X(01)00241-1 |
| [13] | ASHER S A, ALEXEEV V L, GOPONENKO A V, et al. Photonic crystal carbohydrate sensors: Low ionic strength sugar sensing[J]. Journal of the American Chemical Society, 2003, 125 (11) : 3322–3329. DOI: 10.1021/ja021037h |
| [14] | YANG W, TANG Z, LUAN Y, et al. Thermoresponsive copolymer decorated surface enables controlling the adsorption of a target protein in plasma[J]. ACS Applied Materials & Interfaces, 2014, 6 (13) : 10146–10152. |
| [15] | NODA I. Two-dimensional infrared spectroscopy of synthetic and biopolymers[J]. Bull Am Phys Soc, 1986, 31 : 520. |
| [16] | NODA I. Generalized two-dimensional correlation method applicable to infrared, Raman, and other types of spectroscopy[J]. Applied Spectroscopy, 1993, 47 (9) : 1329–1336. DOI: 10.1366/0003702934067694 |
| [17] | NODA I. Two-dimensional infrared spectroscopy[J]. Journal of the American Chemical Society, 1989, 111 (21) : 8116–8118. DOI: 10.1021/ja00203a008 |
| [18] | HOU L, WU P. Understanding the UCST-type transition of P(AAM-co-AN) in H2O and D2O: Dramatic effects of solvent isotopes[J]. Soft Matter, 2015, 11 (35) : 7059–7065. DOI: 10.1039/C5SM01745A |
| [19] | NODA I. Two-dimensional correlation analysis useful for spectroscopy, chromatography, and other analytical measurements[J]. Analytical Sciences, 2007, 23 (2) : 139–146. DOI: 10.2116/analsci.23.139 |
| [20] | CZARNIK-MATUSEWICZ B, PILORZ S, ASHTON L, et al. Potential pitfalls concerning visualization of the 2D results[J]. Journal of Molecular Structure, 2006, 799 (1/2/3) : 253–258. |
| [21] | WANG M, SUN S, WU P. Spectral insight into intensity variations in phase-transition processes using two-dimensional correlation analysis[J]. Applied Spectroscopy, 2010, 64 (12) : 1396–1406. DOI: 10.1366/000370210793561529 |
| [22] | THOMAS M, RICHARDSON H H. Two-dimensional FT-IR correlation analysis of the phase transitions in a liquid crystal, 4’-n-octyl-4-cyanobiphenyl (8CB)[J]. Vibrational Spectroscopy, 2000, 24 (1) : 137–146. DOI: 10.1016/S0924-2031(00)00086-2 |
| [23] | MORITA S, SHINZAWA H, NODA I, et al. Perturbation-correlation moving-window two-dimensional correlation spectroscopy[J]. Applied Spectroscopy, 2006, 60 (4) : 398–406. DOI: 10.1366/000370206776593690 |
| [24] | SUN B, LIN Y, WU P, et al. A FTIR and 2D-IR spectroscopic study on the microdynamics phase separation mechanism of the poly (N-isopropylacrylamide) aqueous solution[J]. Macromolecules, 2008, 41 (4) : 1512–1520. DOI: 10.1021/ma702062h |
| [25] | SUN S, WU P. Role of water/methanol clustering dynamics on thermosensitivity of poly(N-isopropylacrylamide) from spectral and calorimetric insights[J]. Macromolecules, 2010, 43 (22) : 9501–9510. DOI: 10.1021/ma1016693 |
| [26] | LAI H J, WU P Y. A infrared spectroscopic study on the mechanism of temperature-induced phase transition of concentrated aqueous solutions of poly(N-isopropylacrylamide) and N-isopropylpropionamide[J]. Polymer, 2010, 51 (6) : 1404–1412. DOI: 10.1016/j.polymer.2010.01.036 |
| [27] | GUO Y, SUN B, WU P. Phase separation of poly(vinyl methyl ether) aqueous solution: A near-infrared spectroscopic study[J]. Langmuir, 2008, 24 (10) : 5521–5526. DOI: 10.1021/la7038398 |
| [28] | SUN B J, LAI H J, WU P Y. Integrated microdynamics mechanism of the thermal-induced phase separation behavior of poly(vinyl methyl ether) aqueous solution[J]. The Journal of Physical Chemistry B, 2011, 115 (6) : 1335–1346. DOI: 10.1021/jp1066007 |
| [29] | SUN S, WU P. Infrared spectroscopic insight into hydration behavior of poly(N-vinylcaprolactam) in water[J]. The Journal of Physical Chemistry B, 2011, 115 (40) : 11609–11618. DOI: 10.1021/jp2071056 |
| [30] | LAI H J, CHEN G T, WU P Y, et al. Thermoresponsive behavior of an LCST-type polymer based on a pyrrolidone structure in aqueous solution[J]. Soft Matter, 2012, 8 (9) : 2662–2670. DOI: 10.1039/c2sm06779j |
| [31] | LI T, TANG H, WU P. Molecular evolution of poly(2-isopropyl-2-oxazoline) aqueous solution during the liquid-liquid phase separation and phase transition process[J]. Langmuir, 2015, 31 (24) : 6870–6878. DOI: 10.1021/acs.langmuir.5b01009 |
| [32] | SUN S, WU P. On the thermally reversible dynamic hydration behavior of oligo(ethylene glycol) methacrylate-based polymers in water[J]. Macromolecules, 2013, 46 (1) : 236–246. DOI: 10.1021/ma3022376 |
| [33] | ZHANG B, TANG H, WU P. In depth analysis on the unusual multistep aggregation process of oligo(ethylene glycol) methacrylate-based polymers in water[J]. Macromolecules, 2014, 47 (14) : 4728–4737. DOI: 10.1021/ma500774g |
| [34] | LI W, WU P. Unusual thermal phase transition behavior of an ionic liquid and poly(ionic liquid) in water with significantly different LCST and dynamic mechanism[J]. Polymer Chemistry, 2014, 5 (19) : 5578–5590. DOI: 10.1039/C4PY00593G |
| [35] | WANG G, WU P. In-depth study of the phase separation behaviour of a thermoresponsive ionic liquid and a poly(ionic liquid) in concentrated aqueous solution[J]. Soft Matter, 2015, 11 (26) : 5253–5264. DOI: 10.1039/C5SM00603A |
| [36] | JING Y, WU P. Study on the thermoresponsive two phase transition processes of hydroxypropyl cellulose concentrated aqueous solution: From a microscopic perspective[J]. Cellulose, 2013, 20 (1) : 67–81. DOI: 10.1007/s10570-012-9816-z |
| [37] | WANG H, SUN S, WU P. Thermodynamics of hyperbranched poly(ethylenimine) with isobutyramide residues during phase transition: An insight into the molecular mechanism[J]. The Journal of Physical Chemistry B, 2011, 115 (28) : 8832–8844. DOI: 10.1021/jp2008682 |
| [38] | SUN S, WANG H, WU P. Dynamic self-aggregation and disaggregation behavior of thermoresponsive hyperbranched polyethylenimine with peripheral NIPAM groups: An infrared spectroscopic study[J]. Soft Matter, 2013, 9 (10) : 2878–2888. DOI: 10.1039/c2sm27544a |
| [39] | LUTZ J F, HOTH A. Preparation of ideal PEG analogues with a tunable thermosensitivity by controlled radical copolymerization of 2-(2-methoxyethoxy) ethyl methacrylate and oligo (ethylene glycol) methacrylate[J]. Macromolecules, 2006, 39 (2) : 893–896. DOI: 10.1021/ma0517042 |
| [40] | JIA L, GUO C, YANG L, et al. Mechanism of PEO-PPO-PEO micellization in aqueous solutions studied by two-dimensional correlation FTIR spectroscopy[J]. Journal of Colloid and Interface Science, 2010, 345 (2) : 332–337. DOI: 10.1016/j.jcis.2010.01.060 |
| [41] | WANG Z, LAI H, WU P. Influence of PIL segment on solution properties of poly(N-isopropylacrylamide)-b-poly(ionic liquid) copolymer: Micelles, thermal phase behavior and microdynamics[J]. Soft Matter, 2012, 8 (46) : 11644–11653. DOI: 10.1039/c2sm26172c |
| [42] | WANG Q, TANG H, WU P. Aqueous solutions of poly(ethylene oxide)-poly(N-isopropylacrylamide): Thermosensitive behavior and distinct multiple assembly processes[J]. Langmuir, 2015, 31 (23) : 6497–6506. DOI: 10.1021/acs.langmuir.5b00878 |
| [43] | DAI Y, WU P. Exploring the influence of the poly(4-vinyl pyridine) segment on the solution properties and thermal phase behaviours of oligo(ethylene glycol) methacrylate-based block copolymers: The different aggregation processes with various morphologies[J]. Physical Chemistry Chemical Physics, 2016, 18 (31) : 21360–21370. DOI: 10.1039/C6CP04286D |
| [44] | WANG Q, TANG H, WU P. Dynamic phase transition behavior and unusual hydration process in poly(ethylene oxide)-b-poly(N-vinylcaprolactam) aqueous solution[J]. Journal of Polymer Science Part B: Polymer Physics, 2016, 54 (3) : 385–396. DOI: 10.1002/polb.v54.3 |
| [45] | SUN S, ZHANG W, ZHANG W, et al. Dynamic self-aggregation behavior of a PNIPAM-based nonlinear multihydrophilic block copolymer revealed by two-dimensional correlation spectroscopy[J]. Soft Matter, 2012, 8 (14) : 3980–3987. DOI: 10.1039/c2sm06908c |
| [46] | SUN S, WU P, ZHANG W, et al. Effect of structural constraint on dynamic self-assembly behavior of PNIPAM-based nonlinear multihydrophilic block copolymers[J]. Soft Matter, 2013, 9 (6) : 1807–1816. DOI: 10.1039/C2SM27183D |
| [47] | HOU L, WU P. LCST transition of PNIPAM-b-PVCL in water: Cooperative aggregation of two distinct thermally responsive segments[J]. Soft Matter, 2014, 10 (20) : 3578–3586. DOI: 10.1039/c4sm00282b |
| [48] | HOU L, WU P. Comparison of LCST-transitions of homopolymer mixture, diblock and statistical copolymers of NIPAM and VCL in water[J]. Soft Matter, 2015, 11 (14) : 2771–2781. DOI: 10.1039/C5SM00026B |
| [49] | LI T, TANG H, WU P. Remarkable distinctions in the heat-induced phase transition processes of two poly(2-isopropyl-2-oxazoline)-based mixed aqueous solutions[J]. Soft Matter, 2015, 11 (15) : 3046–3055. DOI: 10.1039/C5SM00186B |
| [50] | WANG G, WU P. Unusual phase transition behavior of poly(N-isopropylacrylamide)-co-poly(tetrabutylphosphonium styrenesulfonate) in water: Mild and linear changes in the poly(N-isopropylacrylamide) part[J]. Langmuir, 2016, 32 (15) : 3728–3736. DOI: 10.1021/acs.langmuir.6b00392 |
| [51] | SUN S, HU J, TANG H, et al. Chain collapse and revival thermodynamics of poly(N-isopropylacrylamide) hydrogel[J]. The Journal of Physical Chemistry B, 2010, 114 (30) : 9761–9770. DOI: 10.1021/jp103818c |
| [52] | SUN S, HU J, TANG H, et al. Spectral interpretation of thermally irreversible recovery of poly(N-isopropylacrylamide-co-acrylic acid) hydrogel[J]. Physical Chemistry Chemical Physics, 2011, 13 (11) : 5061–5067. DOI: 10.1039/c0cp01939a |
| [53] | HOU L, MA K, AN Z, et al. Exploring the volume phase transition behavior of POEGMA-and PNIPAM-based core-shell nanogels from infrared-spectral insights[J]. Macromolecules, 2014, 47 (3) : 1144–1154. DOI: 10.1021/ma4021906 |
| [54] | ZHANG B, SUN S, WU P. Synthesis and unusual volume phase transition behavior of poly(N-isopropylacrylamide)-poly(2-hydroxyethyl methacrylate) interpenetrating polymer network microgel[J]. Soft Matter, 2013, 9 (5) : 1678–1684. DOI: 10.1039/C2SM27355A |
| [55] | ZHANG B, TANG H, WU P. The unusual volume phase transition behavior of the poly(N-isopropylacrylamide)-poly(2-hydroxyethyl methacrylate) interpenetrating polymer network microgel: Different roles in different stages[J]. Polymer Chemistry, 2014, 5 (20) : 5967–5977. DOI: 10.1039/C4PY00653D |
| [56] | ZHOU Y, TANG H, WU P. Volume phase transition mechanism of poly[oligo(ethylene glycol)methacrylate] based thermo-responsive microgels with poly(ionic liquid) cross-linkers[J]. Physical Chemistry Chemical Physics, 2015, 17 (38) : 25525–25535. DOI: 10.1039/C5CP03676C |
| [57] | HOU L, WU P. The effect of added gold nanoparticles on the volume phase transition behavior for PVCL-based microgels[J]. RSC Advances, 2014, 4 (74) : 39231–39241. DOI: 10.1039/C4RA06471B |
| [58] | MASUDA T, HIDAKA M, MURASE Y, et al. Self-oscillating polymer brushes[J]. Angewandte Chemie International Edition, 2013, 52 (29) : 7468–7471. DOI: 10.1002/anie.201301988 |
| [59] | JALILI K, ABBASI F, MILCHEV A. Surface microdynamics phase transition and internal structure of high-density, ultrathin PHEMA-b-PNIPAM diblock copolymer brushes on silicone rubber[J]. Macromolecules, 2013, 46 (13) : 5260–5278. DOI: 10.1021/ma4003962 |
 2017, Vol. 25
2017, Vol. 25


