复合材料波导器件具有轻质、高尺寸稳定性等优点,对提高天线系统性能具有重要意义.但树脂基复合材料导电性较差,需采用金属转移法对波导内腔表面进行金属化处理以满足电性能要求.然而,金属镀层与复合材料存在界面匹配性差、结合强度低等问题,影响复合材料波导器件的精度及性能.通常提高金属镀层与复合材料界面结合强度的方法主要针对已成型的复合材料表面进行机械粗化、化学粗化、等离子粗化等表面处理,这些方法难以应用于金属转移法制备的金属化复合材料结构.
为了提高金属转移法制备的金属镀层与复合材料界面结合力,本文借鉴分子自组装膜技术[1-5],选用4种烷基硫醇偶联剂对铜镀层进行表面自组装改性,并通过偶联剂分子链中的功能基团提高铜镀层与氰酸酯树脂基复合材料的界面相容性[6-10].通过表征改性前后铜镀层表面形貌、润湿性、化学键合、镀层与复合材料的结合强度,分析4种不同链长和端基的烷基硫醇对铜镀层与复合材料界面结合强度的影响.
1 实验 1.1 原材料硫醇偶联剂:3-巯基丙酸(HS(CH2)2COOH,MPA)纯度≥98%、11-巯基-十一烷酸(HS(CH2)11COOH,MUA)纯度≥95%、11-巯基-十一烷醇(HS(CH2)11OH,MUOL),纯度≥97%、正十二硫醇(HS(CH2)11CH3,DT),纯度≥98%,Aladdin试剂;无水乙醇和丙酮,分析纯,北京化工厂;去离子水;铜镀层(电镀在铝合金试片上)70 mm×80 mm,M40J/氰酸酯树脂预浸料,自制;室温胶黏剂,Redux 420.
1.2 铜镀层的自组装改性以无水乙醇为溶剂配置浓度约1 mmol/L的MPA、MUA、MUOL、DT自组装溶液.分别用无水乙醇、去离子水、丙酮对铜镀层进行超声清洗10 min,清洗后高纯氮气吹干;镀层浸入自组装溶液中,室温下自组装24 h,取出以无水乙醇漂洗多次,高纯氮气吹干.
1.3 复合材料金属化试片制备在铝合金试片的镀铜表面铺叠M40J/氰酸酯预浸料,采用真空袋-热压罐工艺固化,复合材料成型后去除铝合金得到铜镀层/复合材料.
1.4 性能测试及仪器采用德国ZEISS SIGMA型扫描电子显微镜观察改性前后镀层表面形貌;以座滴法测量改性前后铜镀层表面静态接触角,分析极性变化和自组装膜质量;采用红外光谱分析仪表征改性前后镀层表面基团的变化;按GB/T5210—2006规定的垂直拉开法在万能力学试验机上测定金属转移法制备的铜镀层/复合材料的界面结合强度.
2 结果与分析 2.1 铜镀层表面硫醇的自组装硫醇在纯铜表面形成自组装膜主要经过2个步骤:1)硫醇分子在基底表面快速吸附平铺;2)基底表面吸附饱和,慢速重组,分子链从无序状态缓慢形成二维晶体.此后过量的硫醇分子则会因氢键等分子相互作用形成多层组装膜.扫描电镜观察得到烷基硫醇处理前后铜镀层表面形貌如图 1,四种烷基硫醇改性之后的镀层表面出现白色膜层,认为是过量烷基硫醇形成多层自组装膜或微晶团簇[11-12],吸附在铜表面.
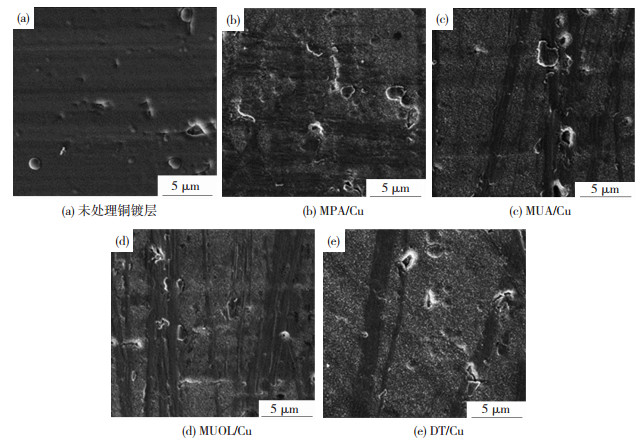
|
图 1 硫醇自组装改性前后铜镀层表面SEM图像 Figure 1 SEM images of copper coatings before and after surface treatment of thiol-based coupling agents:(a) untreated Cu; (b) MPA/Cu; (c)MUA/Cu; (d) MUOL/Cu; (e)DT/Cu |
多层自组装膜吸附时,外层分子组装有序性差,表面状态难以控制,且多层膜之间以非化学键连接,相互作用较弱,不利于铜镀层与复合材料的界面结合,因而除去多余的硫醇分子获得自组装单层膜至关重要.自组装单层膜和多层膜如图 2所示.
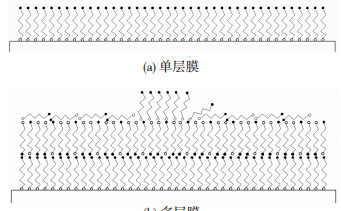
|
图 2 自组装膜示意图 Figure 2 Schematic diagram of SAMs: (a)monolayer; (b)multilayer |
为了制备单分子层的自组装膜层,分别对硫醇偶联剂处理后的铜镀层进行超声清洗,除去表面物理吸附的烷基硫醇分子.图 3为偶联剂处理后铜镀层表面的红外谱图.从图中可以看出,2 921 cm-1左右出现—CH2—的碳氢反对称伸缩振动峰,2 852cm-1处出现—CH2—的对称伸缩振动峰,1 732 cm-1处为C O伸缩振动峰,且3 100~3 500 cm-1之间有一个宽峰为缔合状态下(形成氢键)的—OH特征峰,并且2 584 cm-1处—SH的吸收峰消失[13-14],表明自组装膜在铜镀层表面形成化学连接.这是因为具有—SH的硫醇偶联剂能够自发地在纯铜表面组装成膜,形成Cu—S键合,作用机理可用式(1)表示[15]
| $ 2{\rm{Cu + 2HS}} - {\rm{R}} \to {\rm{2Cu}} - {\rm{S}} - {\rm{R + }}{{\rm{H}}_2} \uparrow . $ | (1) |
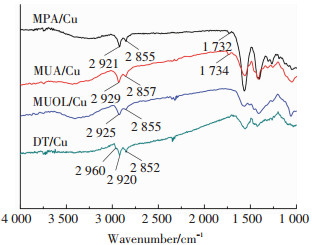
|
图 3 硫醇自组装改性后铜镀层表面红外光谱 Figure 3 FT-IR spectra of copper coatings treated by thiol-based coupling agents |
分子自组装膜技术可在铜镀层表面形成有机超薄膜,自组装膜高度有序,通过分子结构中的有机官能团影响镀层表面浸润性.图 4为静态接触角(去离子水)测试,未经处理的铜镀层表面接触角为85.5°,由MPA、MUA、MUOL改性后的镀层表面接触角均出现不同程度的降低,表明极性基团—COOH和—OH规则排列在膜层表面,导致表面接触角的降低.相反地,由DT改性后的镀层表面接触角增大到102.7°,因为形成自组装膜时,—SH与Cu反应,疏水性的碳链排列在镀层外侧.润湿理论[16-17]认为金属与聚合物之间的结合力是界面层分子或原子紧密接触、相互吸引产生的,良好的界面浸润性是形成紧密结合的前提.氰酸酯单体具有强极性,—COOH、—OH与—CH3的极性差异会影响氰酸酯树脂在铜表面的铺展润湿,最终影响界面结合.
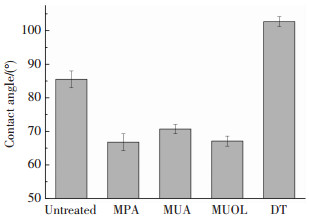
|
图 4 烷基硫醇处理前后铜镀层表面接触角 Figure 4 Contact angles of copper coatings before and after surface treatment of alkylthiols |
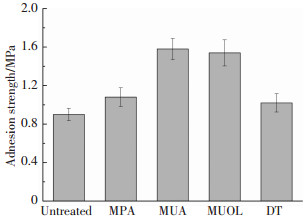
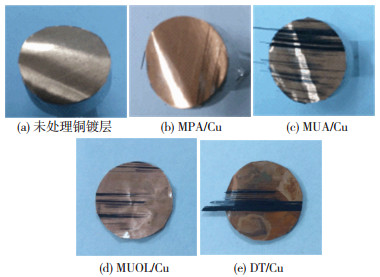
目前,非金属表面金属镀层的界面结合强度尚未有定量测试标准,参照GB/T5210—2006规定的垂直拉开试验测得自组装改性前后的界面结合强度如图 5所示.其中未经处理的铜镀层界面结合强度为0.9 MPa,5种烷基硫醇改性的界面结合强度分别为1.05、1.58、1.54、1.02 MPa,分别提高了约13%、75%、71%、12%.拉开试验后界面破坏形貌如图 6所示,未改性铜镀层表面光亮,改性铜镀层表面则均出现复合材料和纤维残留.
|
图 5 硫醇偶联剂改性前后界面结合强度 Figure 5 Adhesion strengths of copper coatings with and without surface treatment of thiol-based coupling agents |
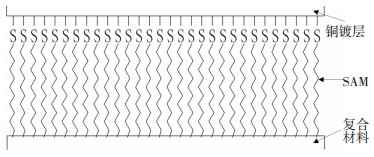
结果表明,具有反应活性基团(—COOH和—OH)的MPA、MUA、MUOL烷基硫醇能够提高镀层与复合材料的界面结合强度.氰酸酯树脂单体中含有两个或两个以上—OCN,—OCN的碳原子具有较强亲电性,能够与硫醇中的—COOH、—OH等亲核基团发生反应[18-19],形成“铜镀层-SAM-复合材料”的界面结构(见图 7),增强金属镀层与复合材料的界面结合力.
|
图 6 拉开试验后镀层结合面照片 Figure 6 Digital photos of copper coatings after pull-off test:(a) Untreated Cu; (b) MPA/Cu; (c)MUA/Cu; (d) MUOL/Cu; (e)DT/Cu |
|
图 7 “铜镀层-SAM-复合材料”的界面结构 Figure 7 Interfacial structure of "Cu-SAM-CFRP" |
同时,不具有反应活性基团的DT硫醇也能够使镀层和复合材料之间的界面结合力增强.相关研究表明[20],复合材料固化过程中,偶联剂的长烷基链段会与树脂交联网络产生分子链段的缠结,增大范德华接触,提高界面结合强度.MPA和MUA的具有相同的官能团—SH和—COOH,而具有长分子链的MUA改性后的镀层与复合材料的结合强度显著增高,表明链段的缠结作用对结合强度的提高有很大贡献;此外,MUOL与MUA分子组成相似,链长相当,因而结合强度提高也与MUA相当.
3 结论采用烷基硫醇自组装改性铜镀层,形成了铜镀层-SAM-复合材料的界面结构,可以提高金属转移法制备的铜镀层/氰酸酯树脂复合材料的界面结合强度,且具有长链和反应活性基团的烷基硫醇(MUA和MUOL),在铜镀层/氰酸酯树脂基复合材料界面中既有化学连接,又有分子链缠结作用,界面结合强度增强超过70%.
| [1] |
都颖, 陈海杰, 成中军, 等. 分子自组装法制备具有可控浸润性的铜表面[J]. 高等学校化学学报, 2014(1): 105–109.
DU Ying, CHEN Haijie, CHENG Zhongjun, et al. Preparation of controlled copper surfaces by molecular self-assembly[J]. Chemical Journal of Chinese Universities, 2014(1): 105–109. DOI: 10.7503/cjcu20130705 |
| [2] |
杜伟, 万俐, 丁毅, 等. 十八硫醇自组装膜对银的缓蚀作用[J]. 电镀与环保, 2012, 32(4): 35–37.
DU Wei, WAN Li, DING Yi, et al. Corrosion inhibition of Ntcadecanethiol self-assembly monolayers on silver[J]. Electroplating & Pollution Control, 2012, 32(4): 35–37. DOI: 10.3969/j.issn.1000-4742.2012.04.013 |
| [3] |
陈振宇, 黄礼平, 黄玲, 等. 硫醇分子自组装膜在铜表面的作用行为研究[J]. 表面技术, 2012, 41(2): 1–4.
CHEN Zhenyu, HUANG Liping, HUANG Ling, et al. Study on behavior of docecanethiol SAMs on pure copper surface[J]. Surface Technology, 2012, 41(2): 1–4. DOI: 10.3969/j.issn.1001-3660.2012.02.001 |
| [4] | VERICAT C, VELA M E, BENITEZ G, et al. ChemInform abstract:Self-assembled monolayers of thiols and dithiols on gold:New challenges for a well-known system[J]. ChemInform, 2010, 39(35): 1805–34. DOI: 10.1039/b907301a |
| [5] | JIMÉNEZ A, SARSA A, BLÁZQUEZ M, et al. A molecular dynamics study of the surfactant surface density of alkanethiol self-assembled monolayers on gold nanoparticles as a function of the radius[J]. Journal of Physical Chemistry C, 2010, 114(49): 21309–21314. DOI: 10.1021/jp1088977 |
| [6] | WONG C K Y, YUEN M M F, XU B. Thiol-based self-assembly nanostructures in promoting interfacial adhesion for copper-epoxy joint[J]. Applied Physics Letters, 2009, 94(26): 263102–263102. DOI: 10.1063/1.3167358 |
| [7] | DENAYER J, DELHALLE J, MEKHALIF Z. Aminealkylthiol and dithiol self-assembly as adhesion promoter between copper substrate and epoxy resin[J]. Applied Surface Science, 2011, 257(24): 10686–10691. DOI: 10.1016/j.apsusc.2011.07.080 |
| [8] |
何毅, 陈春林, 钟菲, 等. KH560改性纳米二氧化钛对环氧涂层性能影响[J]. 材料科学与工艺, 2014, 22(6): 101–106.
HE Yi, CHEN Chunlin, ZHONG Fei, et al. The impact of nano titanium dioxide modified by KH560 on epoxy coating performance[J]. Materials Science and Technology, 2014, 22(6): 101–106. DOI: 10.11951/j.issn.1005-0299.20140618 |
| [9] |
何德良, 高翔, 谢荟, 等. 铝锆有机金属偶联剂对环氧铝粉涂料的改性研究[J]. 湖南大学学报(自科版), 2011, 38(7): 59–63.
HE Deliang, GAO Xiang, XIE Hui, et al. Study of Al-rich epoxy coatings modified with Aluminium Zirconium organic metal coupling agent[J]. Journal of Hunan University (Natural Sciences), 2011, 38(7): 59–63. |
| [10] |
程国君, 于秀华, 唐忠锋, 等. 碳酸钙的偶联剂改性及对SBS力学性能的影响[J]. 材料科学与工艺, 2013, 21(1): 49–55.
CHENG Guojun, YU Xiuhua, TANG Zhongfeng, et al. Modification of calcium carbonate by using coupling agent and the influence to the mechanical properties of SBS[J]. Materials Science and Technology, 2013, 21(1): 49–55. DOI: 10.11951/j.issn.1005-0299.20130109 |
| [11] | PRADEEP T, SANDHYARANI N. An investigation of the structure and properties of layered copper thiolates[J]. Journal of Materials Chenistry, 2001, 11(11): 1294–1299. DOI: 10.1039/b009837j |
| [12] | CALDERÓN C A, OJEDA C, MACAGNO V A, et al. Interaction of oxidized copper surfaces with alkanethiols in organic and aqueous solvents.The mechanism of Cu2O reduction[J]. Journal of Physical Chemistry C, 2010, 114(9): 3945–3957. DOI: 10.1021/jp9045148 |
| [13] |
徐琴, 夏纲, 胡效亚, 等. 己硫醇单层保护金纳米团簇的合成及其性质[J]. 扬州大学学报自然科学版, 2002, 5(3): 27–31.
XU Qin, XIA Gang, HU Xiaoya, et al. Synthesis and properties of hexanethiol monolayer-protected gold nanoclusters[J]. Journal of Yangzhou University (Natural Science Edition), 2002, 5(3): 27–31. DOI: 10.3969/j.issn.1007-824X.2002.03.007 |
| [14] |
杨程, 成波, 滕乐金, 等. 金-烷基硫醇的自组装及其聚合物基复合材料的介电性能[J]. 材料工程, 2010(5): 34–37.
YANG Cheng, CHENG Bo, TENG Lejin, et al. Self-assembled gold-alkanethiols and dielectric properties of Au@S-R12/polymer composites[J]. Journal of Materials Engineering, 2010(5): 34–37. DOI: 10.3969/j.issn.1001-4381.2010.05.008 |
| [15] | SUNG M M, SUNG K, CHANG G K, et al. Self-assembled monolayers of alkanethiols on oxidized copper surfaces[J]. Journal of Physical Chemistry B, 2000, 104(10): 2273–2277. DOI: 10.1021/jp992995h |
| [16] | PALDEY S, DEEVI S C. Single layer and multilayer wear resistant coatings of (Ti, Al) N:A review[J]. Materials Science & Engineering A, 2003, 342(1-2): 58–79. DOI: 10.1016/s0921-5093(02)00259-9 |
| [17] | LEU M S, CHEN B F, CHEN S Y. The influence of magnetic solenoid filtration on the property of (Ti-Al) N coatings deposited in a cathodic arc deposition system[J]. Surface & Coatings Technology, 2001, 148(1): 25–29. DOI: 10.1016/S0257-8972(01)01332-9 |
| [18] | DANGER G, CHARLOT S, BOITEAU L, et al. Activation of carboxyl group with cyanate:Peptide bond formation from dicarboxylic acids[J]. Amino Acids, 2012, 42(6): 2331–2341. DOI: 10.1007/s00726-011-0975-2 |
| [19] | ZHAO Y, ZHANG D, GUO L. Shape-memory behavior of bisphenol A-type cyanate ester/carboxyl-terminated liquid nitrile rubber coreacted system[J]. Colloid and Polymer Science, 2014, 292(10): 2707–2713. DOI: 10.1007/s00396-014-3321-x |
| [20] |
贾近.炭纤维复合材料界面自组装结构的分子动力学模拟[D].哈尔滨工业大学, 2009. JIA Jin.Molecular dynamics simulation of interfacial self-assembly of carbon fiber reinforced plastics[D]Harbin Institute of Technology, 2009. |
 2018, Vol. 26
2018, Vol. 26


