2. 上海纺织化学清洁生产工程技术研究中心,上海 201620
2. Shanghai Engineering Research Center for Clean Production of Textile Chemistry, Shanghai 201620, China
随着现代工业化发展和人口的快速增长,环境污染问题日益严重,已成为迫切需要解决的世界性问题[1-3]。各大行业每年都要产生大量废水,特别是纺织品行业,其废水排放量每年都呈翻倍趋势增长,已在世界范围内引起高度关注[4-6]。废水成分复杂,其中有机染料在大多数情况下具有较稳定的化学性质、高色度值和高化学需氧量(COD)和生物降解性差等特点,从而难以处理[7-8]。若工业废水未经适当处理即排放,可能导致水体富营养化,给生态系统和人类健康带来极大危害[9-11]。
目前,人们关于环境污染问题已提出众多解决方法,包括膜过滤法[12-13]、生物处理[14-15]、电化学法[16-17]、催化氧化及吸附法[18-20]等,但是这些方法的处理效果都不尽如人意。光电催化技术是一种操作简单、无二次污染、低能耗且高效的催化降解污染物的方法。在光电的条件下,催化剂表面能够产生电子-空穴对(e--h+),电子-空穴对在合适的带隙宽度条件下即可转移和分离,从而催化剂表面形成的活性氧簇(ROS)可将有机污染物氧化降解为二氧化碳和水[21-22]。
光电催化材料种类丰富,包括金属有机框架[23-24]、金属氧化物半导体[25-26]、硫化物半导体[27-28]等。金属有机骨架(MOFs)是一种新型纳米级多孔聚合材料,这类材料是以金属(簇)为节点,有机配体为连接体,通过强配位键桥连形成开放式的具有永久孔道的晶型骨架[29-30],它具有比表面积大、孔隙率高、稳定性优异等特有的物理化学性。MoS2是一种二维分层纳米半导体材料,每个单元中都有S-Mo-S的结构,各层之间通过微弱的范德华力连接在一起[31-32]。此外,与纳米颗粒状的MoS2相比,花状多级结构的MoS2具有更大的比表面积和独特的缺陷富集晶体结构,能够暴露更多的活性边缘位点,从而在光照条件下,其表面产生的载流子能够有效转移和分离[33-34]。
本文借助简单易行的水热反应,将花状多级结构的MoS2成功负载至Ti-MOFs表面,构建了MoS2/Ti-MOFs复合结构,并对样品的形貌、结构、组分、光电化学及光电催化性能做了系统研究。
1 实验 1.1 MoS2/Ti-MOFs的制备 1.1.1 Ti-MOFs的制备将2-氨基对苯二甲酸(NH2-BDC,0.181 g)缓缓加入到N,N-二甲基甲酰胺/甲醇(体积比9 ∶1)的混合溶剂中,待完全溶解后加入钛酸异丙酯(0.194 mL),并在室温下温和搅拌30 min,然后将其转移至50 mL的聚四氟乙烯内衬中,置于150 ℃的烘箱中保持24 h[35]; 反应结束后,反应釜自然冷却,将得到的黄色产物分别用N,N-二甲基甲酰胺、甲醇洗涤3次; 最后,将洗净后的样品置于60 ℃的真空烘箱中干燥12 h,获得黄色粉末产物。
1.1.2 MoS2/Ti-MOFs的制备MoS2/Ti-MOFs的制备过程示意图如图 1所示。将硫脲(1.066 g,14 mmol)和四水合钼酸铵(1.236 g,1 mmol)溶解在去离子水中,然后将一定量的Ti-MOFs添加到上述溶液中形成悬浮液,将悬浮液转移至50 mL的聚四氟乙烯内衬中,并将反应釜置于180 ℃的烘箱中保持12 h; 反应结束后,待冷却至室温,将悬浮液离心,并取其沉淀,再对所得沉淀分别用水、乙醇洗涤3次; 最后,将产物置于60 ℃的真空烘箱内干燥12 h,收集黑色粉末产物。改变Ti-MOFs含量,即MoS2质量分数分别为60%、20%、10%、5%时,所得产物记为MoS2/Ti-MOF-40、MoS2/Ti-MOF-80、MoS2/Ti-MOF-90、MoS2/Ti-MOF-95。
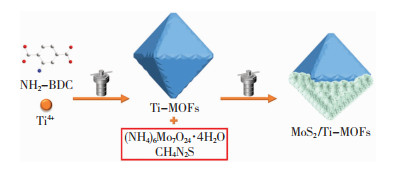
|
图 1 MoS2/Ti-MOFs制备过程示意图 Fig.1 Schematic diagram of preparation process for MoS2/Ti-MOFs |
红外光谱仪(FTIR,Thermo Scientific,Nicolet 6700)用于分析样品所含的官能团; X射线衍射仪(XRD,PANalytical,X’pert-Pro MRD)、冷场发射电子显微镜(SEM,Hitachi,SU8010)和透射电子显微镜(TEM,FEI Tecnai F-20)分别用于表征样品的晶体结构和微观形貌; 配置有单色的Al Kα X射线源的光电子能谱分析仪(XPS,KRATOS,Axis Ultra HAS)用于分析样品所含化合态; 紫外-可见分光光度计(UV-vis DRS,Shimadzu,UV- 2600) 用于测试样品的紫外-可见吸收光谱(UV-vis DRS)和Tauc plot图,探究催化剂的光响应性和带隙宽度; 电化学工作站(Metrohm Autolab,M204)用于分析样品的光电化学性能。
1.3 光电化学性能测试使用配置有传统三电极电解池的电化学工作站为光电极施加一定的电压,涂覆有制备的催化剂的导电玻璃(FTO)为工作电极,铂箔和Ag/AgCl(3.0 mmol的KCl溶液)分别作为对电极和参比电极。在光电化学测试之前,将N2流鼓入Na2SO4溶液(0.2 mmol,pH=6.8)中30 min以去除溶解于电解质溶液中的氧气。配置有紫外滤光片(λ>420 nm)的300 W氙灯(Trusttech,PLS-SXE 300)作为可见光光源,光源与电极之间的距离设置为15 cm,光照射到电极表面的光强为100 mW/cm2。在0.30 V(vs. Ag/AgCl)的偏压条件下记录光电流-时间曲线(I-t),光源的开/关循环时间设置为30 s。在0.1~105 Hz的频率范围内获取电化学阻抗谱(EIS),交流电压振幅为5 mV,偏压设置为0 V (vs.Ag/AgCl)。线性扫描伏安曲线(LSV)测试时,扫描速率为20 mV/s,电压范围设置为-0.5~1.0 V (vs.Ag/AgCl)。
1.4 有机污染物催化降解实验光电催化降解有机污染物实验装置与光电化学性能测试相同,装置示意图如图 2所示。将10 mg样品分散在含全氟磺酸(Nafion)的乙醇溶液中,并取分散液涂覆至FTO的导电面制备光电极,以50 mL的2,4,6-TCP(10 mg/L,初始pH值为4.2)作为电解质,进行光电催化降解实验。利用紫外分光光度计测试光电催化过程中2,4,6-TCP的吸光度,并将其换算为浓度,计算得到模拟有机污染物的降解速率。
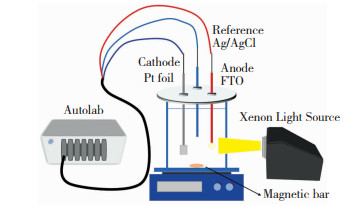
|
图 2 光电催化降解有机物示意图 Fig.2 Schematic diagram of photoelectrocatalytic degradation of organic pollutants |
Ti-MOFs和MoS2/Ti-MOFs-80的FTIR如图 3所示,400和800 cm-1之间的特征峰属于Ti-MOFs的O—Ti—O[36]; 位于1 253、1 335、1 420和1 634 cm-1处的特征峰归因于Ti-MOFs中芳香胺的C—N伸缩振动[37]; 位于1 033,1 577和1 699 cm-1处的特征峰分别归因于Ti-MOFs中C=C和C=O伸缩振动[38]; 位于1 415和1 541 cm-1处的特征峰分别归因于2-氨基对苯二甲酸中羧酸根的对称和不对称伸缩振动,而位于3 381 cm-1处的特征峰归因于N—H键的振动[38]。在MoS2/Ti-MOFs-80的FTIR光谱中,位于1 035、1 420和1 634 cm-1处较弱的特征峰分别对应于苯环、C—N键的伸缩振动。MoS2/Ti-MOFs-80含有Ti-MOFs的特征峰,表明MoS2的修饰不会破坏Ti-MOFs的化学结构。此外,它们在895、941和1 113 cm-1处显示相似的Mo—O振动和不对称S=O伸缩振动[39],表明经过水热反应过程,MoS2原位沉积在Ti-MOFs表面,不同元素之间能够充分接触且深入杂化。
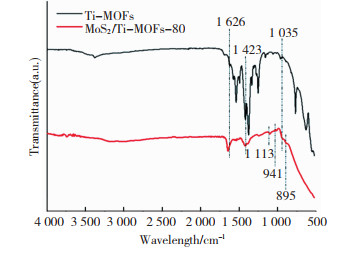
|
图 3 Ti-MOFs与MoS2/Ti-MOFs-80的FTIR Fig.3 FTIR of Ti-MOFs and MoS2/Ti-MOFs-80 |
图 4给出了Ti-MOFs和MoS2/Ti-MOFs的XRD谱图,位于6.7°、9.8°、11.9°、17.7°、19.5°和25°的特征峰分别归因于Ti-MOFs的(101)、(002)、(211)、(301)、(004)和(101)晶面[22]。MoS2/Ti-MOF的XRD谱图中不仅可以观察到Ti-MOFs的所有特征峰,还可以观察到位于11.1°、33.4°和54.4°的特征峰,分别归因于MoS2的(001)、(100)和(110)晶面结构(JCPDS 37 -1492)[33, 39]。XRD测试结果进一步证实MoS2/Ti-MOFs复合材料的成功制备,且水热反应过程引入MoS2不会破坏Ti-MOFs的化学结构。
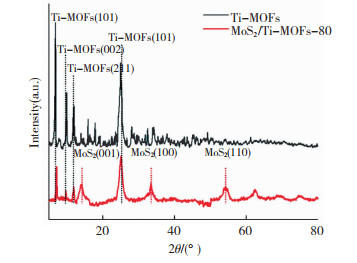
|
图 4 Ti-MOFs和MoS2/Ti-MOFs的XRD谱图 Fig.4 XRD patterns of Ti-MOFs and MoS2/Ti-MOFs |
图 5(a)给出了材料的微观形貌图,由图中可见,制备的Ti-MOFs为多面体,粒径约200~500 nm。MoS2/Ti-MOFs-80的SEM表明花状多级结构的MoS2均匀包覆在Ti-MOFs表面(图 5(b)),MoS2/Ti-MOFs的TEM图(图 5(c))进一步证实了MoS2在Ti-MOFs表面的均匀分布。MoS2/Ti-MOFs的HRTEM图(图 5(d))表明,晶格条纹间距0.13 nm对应于Ti-MOFs的(101)晶面[40],晶格条纹间距0.273、0.624 nm分别对应于MoS2的(100)及(002)晶面[41],该结果进一步证实了MoS2/Ti-MOFs的成功制备,MoS2与Ti-MOFs的不同元素之间能够充分接触且深入杂化。
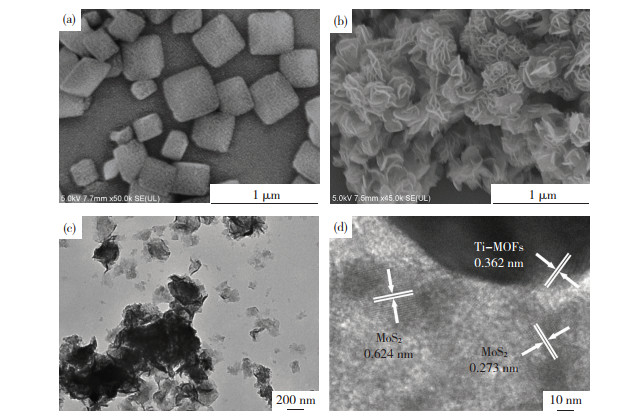
|
图 5 Ti-MOFs的SEM(a)以及MoS2/Ti-MOFs-80的SEM(b)、TEM(c)、HRTEM(d) Fig.5 SEM image of Ti-MOFs (a) and SEM (b), TEM (c), and HRTEM (d) images of MoS2/Ti-MOFs-80 |
进一步利用XPS测试分析制备材料的电子结构和化学组分。XPS全谱图(图 6)表明MoS2/Ti-MOFs含有C、N、O、Ti、Mo和S元素,其原子百分比分别为20.39%、4.36%、40.88%、15.46%、11.30%和7.61%。此外,在C1s的分峰谱图中,位于284.0、285.6、288.1 eV处的特征峰分别归因于2-氨基对苯二甲酸的C=C、C—N、C=O键振动(图 7(a))[40]。在N1s分峰谱图中,位于399.7、402.5 eV的特征峰分别归因于Ti-MOFs中—NH2的N原子和带正电荷的N离子(—N = +,—NH = +) (图 7(b))[40]。图 7(c) 所示的位于458.5和464.3 eV处的特征峰分别对应于Ti2p3/2和Ti2p1/2,意味着Ti—O中的Ti为Ti4+[42]。在O1s的分峰谱图中,位于532.6、531.1、529.9 eV的3个特征峰分别归因于—OH、C=O和Ti—O键振动(图 7(d))[42]。在Mo3d分峰谱图中,位于228.1、231.7 eV的特征峰分别对应于Mo3d5/2和Mo3d3/2跃迁(图 7e)[39]。S2p分峰谱图中,在161.2、162.5 eV处观察到的两个特征峰分别对应于S2p3/2和S2p1/2(图 7(f))[42]。
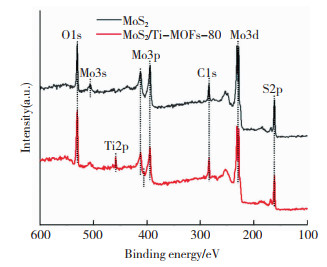
|
图 6 MoS2与MoS2/Ti-MOFs-80的XPS全谱图 Fig.6 XPS spectra of MoS2 and MoS2/Ti-MOF-80 |
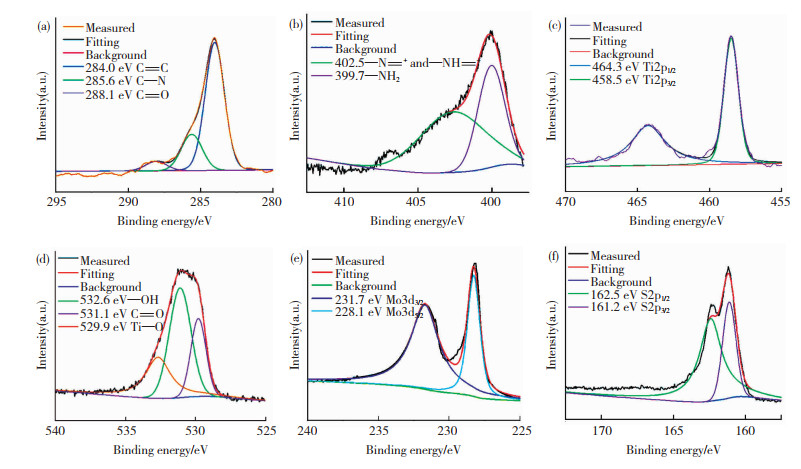
|
图 7 不同元素高分辨率峰拟合的XPS谱图 Fig.7 High-resolution peak-fitting XPS spectra of different elements: (a) C1s; (b) N1s; (c) Ti2p; (d) O1s; (e) Mo3d; (f) S2p |
Ti-MOFs和MoS2/Ti-MOFs在紫外光区域均表现出优异光吸收能力,在可见光区域,MoS2/Ti-MOFs仍然表现较强的光吸收性,而Ti-MOFs对可见光的吸收极其微弱(图 8(a))。此外,Ti-MOFs在290和390 nm处的吸收特征峰可能归因于有机金属框架的Ti-O转变[43]。MoS2/Ti-MOFs表现出显著增强的可见光响应性,表明花状多级结构的MoS2作为光敏化剂,可有效促进Ti-MOFs对可见光的吸收。
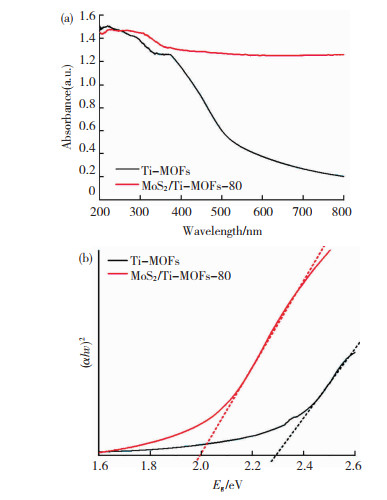
|
图 8 Ti-MOFs与MoS2/Ti-MOFs-80的紫外-可见漫反射光谱(a)和光学带隙宽度(b) Fig.8 UV-vis DRS (a) andoptical band gap(b)of Ti-MOFs and MoS2/Ti-MOFs-80 |
MoS2/Ti-MOFs优异的可见光响应性可能归因于复合材料的窄带隙宽度。半导体材料的带隙能根据Tauc plot法进行测算,计算公式如下[44]
| $ [(\alpha h \nu)]^{n}=A\left(h \nu-E_{\mathrm{g}}\right) $ | (1) |
式中:α为光吸收指数; h为普朗克常量; v为频率; A是常数; Eg为半导体材料带隙宽度。
指数n与半导体类型有关,即半导体为直接带隙半导体,n=1/2; 半导体为间接带隙半导体,n=2。如图 8(b)所示,MoS2/Ti-MOFs-80的带隙宽度为1.99 eV,明显小于Ti-MOFs带隙宽度(2.28 eV)。较窄的带隙宽度有利于半导体材料对可见光的吸收,因此带隙能测试结果进一步证实了MoS2的修饰可有效增强Ti-MOFs的可见光响应性。
2.6 光电化学性能分析图 9所示的线性扫描伏安曲线、电化学阻抗谱(EIS)、I-t曲线测试用于研究催化剂的光电化学性能。如图 9(a)所示,在可见光照射下,MoS2/Ti-MOFs的饱和光电流密度较Ti-MOFs明显增大,且MoS2/Ti-MOFs-80的饱和光电流密度高达0.290 mA/cm2(0.3 V (vs.Ag/AgCl)),几乎是Ti-MOFs(0.033 mA/cm2)的9倍,表明MoS2的负载可有效增强Ti-MOFs对可见光的响应性,并且通过调控MoS2在Ti-MOFs表明的负载量及分布可有效优化样品的光电响应性。
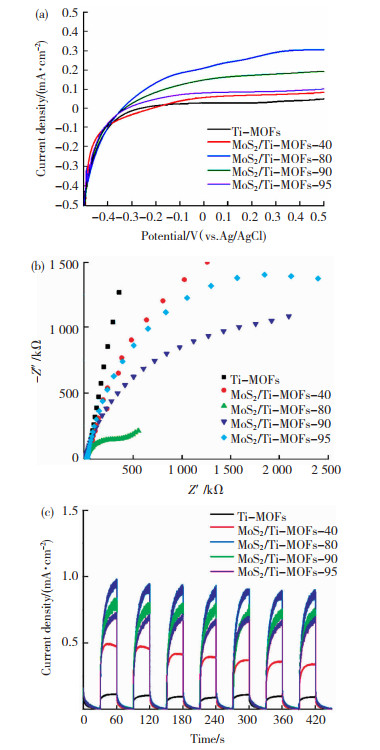
|
图 9 Ti-MOFs与MoS2/Ti-MOFs在可见光照射下的LSV曲线(a),EIS Nyquist谱图(b)和I-t曲线(c) Fig.9 LSV curves (a), EIS Nyquist plots (b), and I-t curves (c) of Ti-MOFs and MoS2/Ti-MOFs under visible light irradiation |
EIS测试用于进一步探究电极/电解质界面处的电荷转移动力学。如图 9(b)所示,在可见光照射下,MoS2/Ti-MOFs的EIS谱图较Ti-MOFs表现更小的弧半径,表明MoS2的引入可有效增加载流子的转移通道,促进电子-空穴对的分离和转移。此外,在所有样品中,MoS2/Ti-MOFs-80的EIS谱图显示的弧半径最小,意味着MoS2负载量为20%时,制备的催化剂表界面产生的光生电荷的转移和分离效率最高。
此外,从I-t曲线(图 9(c))可以看出,在施加0.30 V (vs. Ag/AgCl)的偏压条件下,MoS2/Ti-MOFs的光电流密度较Ti-MOFs明显增大,且光电流密度随MoS2沉积量的不同表现出明显差异,其中MoS2/Ti-MOFs-80表现最大的光电流密度。此外,经过450 s的循环测试,合成的催化剂的光电流密度略有下降。以上结果进一步证实,MoS2用于修饰Ti-MOFs可有效增强其可见光响应性; 光电流密度与时间关系的循环测试表明,合成的催化剂具有较好的光电化学稳定性。
2.7 光电催化降解模拟有机污染物分析在进行光电催化降解实验之前,先将初始质量浓度为10 mg/mL的2,4,6-TCP溶液置于黑暗中搅拌30 min,以达到吸附平衡。在之后的可见光照射下进行光电催化降解过程中,每隔1 h取3 mL悬浮液进行离心分离,得到的上层清液用于测试有机物的吸光度。有机物的降解效率可根据式(2)计算得到[22]
| $ \eta \%=1-C_{t} / C_{0} \times 100 \% $ | (2) |
式中:C0为有机物初始浓度; Ct为光电催化实验进行到t min时的有机物浓度。
如图 10(a)所示,经过6 h的光照后,MoS2/Ti-MOFs-80光电极对2,4,6-TCP的去除效率达到82.59%,为Ti-MOFs(32.15%)的2.6倍,且高于其他成分的MoS2/Ti-MOFs对2,4,6-TCP的去除效率; 在相同条件下,MoS2对2,4,6-TCP的去除效率为52.52%,低于合成的MoS2/Ti-MOFs对2,4,6-TCP的去除效率。结果表明,MoS2具有明显的光电催化活性,将其用做助催化剂可有效增强Ti-MOFs的光电催化能力。
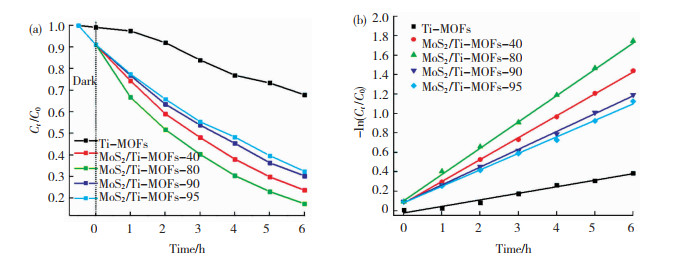
|
图 10 在可见光照射下,Ti-MOFs与MoS2/Ti-MOFs分别对2,4,6-TCP光电催化降解的速率-时间曲线(a)及对应的动力学线性模型(b) Fig.10 Photoelectrocatalytic degradation rate curves of 2, 4, 6-TCP by Ti-MOFs and MoS2/Ti-MOFs under visible light irradiation (a)and corresponding kinetic models (b) |
为进一步探究催化剂对有机物污染物的去除效果,我们对2,4,6-TCP降解的动力学曲线进行了拟合,所根据的一级动力学方程如下[32]
| $ k=-t / \ln \left(C_{0} / C_{t}\right) $ | (3) |
其中k为催化反应动力学常数。计算得到相关的线性拟合系数R2,几种材料的R2值均大于0.98,说明其线性拟合关系较好,符合一级动力学方程。如图 10(b)所示,MoS2/Ti-MOFs-80作为光电极时,有机物催化降解的动力学常数达到1.77 h-1,为Ti-MOFs(0.39 h-1)的4.5倍,且高于MoS2(0.74 h-1)、MoS2/Ti-MOFs-40(1.13 h-1)、MoS2/Ti-MOFs-90(1.19 h-1)及MoS2/Ti-MOFs- 95 (1.44 h-1)作为光电极对应的有机物催化降解动力学常数。上述结果表明,MoS2作为助催化剂能够有效增强Ti-MOFs的光电催化活性,且MoS2负载量为20%时,即MoS2/Ti-MOFs-80光电催化活性最强。MoS2负载量过大或者过小,合成的催化剂的催化活性均减弱,原因可能在于MoS2负载量过低时,不能够为载流子的转移和分离提供足够的通道及带隙宽度,而过量的MoS2则会堆积在Ti-MOFs表面,抑制了载流子的转移和分离。
为了验证光电催化的优越性,我们以MoS2/Ti-MOFs-80作为催化剂,分别在黑暗、电催化(0.30 V(vs. Ag/AgCl))、光催化条件下对2,4,6-TCP进行降解,并将降解速率与光电催化降解速率进行对比(图 11(a))。MoS2/Ti-MOFs-80在黑暗、电催化(0.30 V(vs. Ag/AgCl))、光催化条件下对2,4,6-TCP的降解速率分别为5.80%、50.23%和72.51%,对应的催化降解速率常数分别为0.060、0.698和1.291 h-1(图 11(b))。上述结果表明,MoS2/Ti-MOFs-80作为催化剂,光电催化降解有机物的效率明显优于电催化、光催化降解有机物的效率。光电催化过程优于电催化、光催化过程可归因于施加电压和提供光照的协同效应。
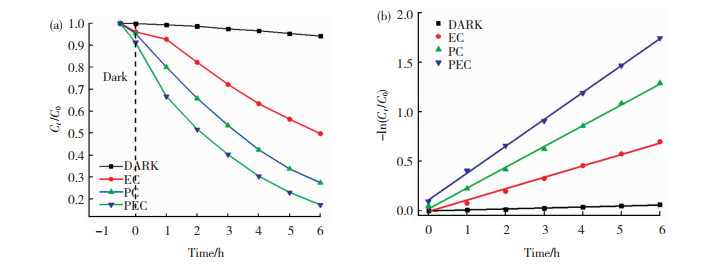
|
图 11 MoS2/Ti-MOFs-80在不同条件下对应的2,4,6-TCP降解速率-时间曲线(a)及对应的动力学线性模型(b) Fig.11 Degradation rate curves of 2, 4, 6-TCP by MoS2/Ti-MOFs-80 under different conditions (a)and corresponding kinetic models (b) |
为了进一步证实合成的催化剂的光电催化稳定性,我们进行了5次循环(每个循环周期为6 h) 的光电催化降解模拟有机污染物实验。研究结果表明,在经过5次循环的光电催化降解实验后,2,4,6-TCP的降解速率仍保持在80%以上(图 12(a)),说明制备的MoS2/Ti-MOFs-80具有较好的光电催化稳定性。此外,经过5次循环光电催化降解实验后,MoS2/Ti-MOFs-80的微观形貌(图 12(b))几乎无改变,进一步证实光电催化过程对合成的催化剂的结构、活性几乎无影响。
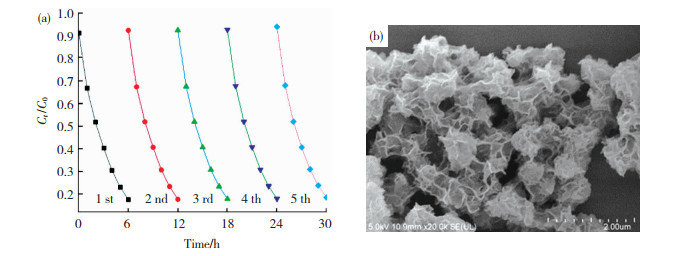
|
图 12 MoS2/Ti-MOFs-80在可见光照射下连续5次循环时2,4,6-TCP光电催化降解的速率-时间曲线(a)和SEM图(b) Fig.12 Photoelectrocatalytic degradation rate curves of 2, 4, 6-TCP by MoS2/Ti-MOFs-80 for five consecutive cycling runs (a) and SEM image (b) |
1) 借助简单易行的水热反应,将花状多级结构的MoS2成功负载至Ti-MOFs表面,制备了MoS2/Ti-MOFs复合材料。
2) 在可见光照射下,MoS2/Ti-MOFs-80的光电流密度达到0.290 mA/cm2,MoS2/Ti-MOFs较Ti-MOFs表现出显著增强的光电化学性能。
3) MoS2/Ti-MOFs-80对有机污染物的光电催化降解效率较Ti-MOFs显著提高; 模拟有机污染物降解循环实验证实合成的催化剂具有较好的光电催化稳定性。
4) MoS2/Ti-MOFs的成功制备为开发基于钛金属有机框架的催化剂提供了新思路,促进了废水处理技术的发展,具有潜在的应用前景。
| [1] |
BILDIRICI M, GÖKMENOĜLU S. Environmental pollution, hydropower energy consumption and economic growth: evidence from G7 countries[J]. Renewable and Sustainable Energy Reviews, 2017, 75: 68-85. DOI:10.1016/j.rser.2016.10.052 |
| [2] |
PETER S. Reduction of CO2 to chemicals and fuels: a solution to global warming and energy crisis[J]. ACS Energy Letters, 2018, 3(7): 1557-1561. DOI:10.1021/acsenergylett.8b00878 |
| [3] |
YIN Zhihong, ZHU Liandong, LI Shuangxi, et al. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions[J]. Bioresource Technology, 2020, 301: 122804. DOI:10.1016/j.biortech.2020.122804 |
| [4] |
KANG Le, DU Huiling, DU Xian, et al. Study on dye wastewater treatment of tunable conductivity solid-waste-based composite cementitious material catalyst[J]. Desalination and Water Treatment, 2018, 125: 296-301. DOI:10.5004/dwt.2018.22910 |
| [5] |
NARESH D, ANAND K, IFFAT N, et al. A review on advanced physico-chemical and biological textile dye wastewater treatment techniques[J]. Reviews in Environmental Science and Bio/Technology, 2020, 19(3): 543-560. DOI:10.1007/s11157-020-09543-z |
| [6] |
SENTHIL K P, VARJANI S, SUGANYA S. Treatment of dye wastewater using an ultrasonic aided nanoparticle stacked activated carbon: kinetic and isotherm modelling[J]. Bioresource Technology, 2018, 250: 716-722. DOI:10.1016/j.biortech.2017.11.097 |
| [7] |
YASEEN D, SCHOLZ M. Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review[J]. International Journal of Environmental Science and Technology, 2018, 16(2): 1193-1226. DOI:10.1007/s13762-018-2130-z |
| [8] |
HUANG Zhihui, LI Yuzhen, CHEN Wenjun, et al. Modified bentonite adsorption of organic pollutants of dye wastewater[J]. Materials Chemistry and Physics, 2017, 202: 266-276. DOI:10.1016/j.matchemphys.2017.09.028 |
| [9] |
GUILHERME C B, JOJA C S, JULIANO C C, et al. Assessment of several advanced oxidation processes applied in the treatment of environmental concern constituents from a real hair dye wastewater[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 2794-2802. DOI:10.1016/j.jece.2018.04.041 |
| [10] |
NIDHEESH P V, ZHOU MINGHUA, MRHMET O. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes[J]. Chemosphere, 2018, 197: 210-227. DOI:10.1016/j.chemosphere.2017.12.195 |
| [11] |
KATHERESAN V, KANSEDO J, LAU Y. Efficiency of various recent wastewater dye removal methods: a review[J]. Journal of Environmental Chemical Engineering, 2018, 6(4): 4676-4697. DOI:10.1016/j.jece.2018.06.060 |
| [12] |
WU Ming, SUN Lijuan, MIAO Kesong, et al. Detection of sudan dyes based on inner-filter effect with reusable conjugated polymer fibrous membranes[J]. ACS Applied Materials & Interfaces, 2018, 10(9): 8287-8295. DOI:10.1021/acsami.8b00164 |
| [13] |
HU Ying, YUE Min, YUAN Fanshu, et al. Bio-inspired fabrication of highly permeable and anti-fouling ultrafiltration membranes based on bacterial cellulose for efficient removal of soluble dyes and insoluble oils[J]. Journal of Membrane Science, 2021, 621: 118982. DOI:10.1016/j.memsci.2020.118982 |
| [14] |
DEEPIKA B, NEETA R S, JOGINDER S, et al. Biological methods for textile dye removal from wastewater: a review[J]. Critical Reviews in Environmental Science and Technology, 2017, 47(19): 1836-1876. DOI:10.1080/10643389.2017.1393263 |
| [15] |
LODHA B, CHAUDHARI S. Optimization of fenton-biological treatment scheme for the treatment of aqueous dye solutions[J]. Journal of Hazardous Materials, 2007, 148(1-2): 459-66. DOI:10.1016/j.jhazmat.2007.02.061 |
| [16] |
SHUKLA S, OTURAN M A. Dye removal using electrochemistry and semiconductor oxide nanotubes[J]. Environmental Chemistry Letters, 2015, 13(2): 157-172. DOI:10.1007/s10311-015-0501-y |
| [17] |
NIE Chunhong, DONG Jing, SUN Pingping, et al. An efficient strategy for full mineralization of an azo dye in wastewater: a synergistic combination of solar thermo-and electrochemistry plus photocatalysis[J]. RSC Advances, 2017, 7(58): 36246-36255. DOI:10.1039/C7RA05797K |
| [18] |
WANG Hou, YUAN Xingzhong, WU Yan, et al. In situ synthesis of In2S3@MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis[J]. Applied Catalysis B: Environmental, 2016, 186: 19-29. DOI:10.1016/j.apcatb.2015.12.041 |
| [19] |
WANG Chaohai, Kim J H, TANG Jing, et al. Large-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents[J]. Angewandte Chemie International Edition, 2020, 59(5): 2066-2070. DOI:10.1002/anie.201913719 |
| [20] |
OVEISI M, ASLI M A, MAHMOODI N M, et al. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems[J]. Journal of Hazardous Materials, 2018, 347: 123-140. DOI:10.1016/j.jhazmat.2017.12.057 |
| [21] |
YANG Cao, YOU Xia, CHENG Jianhua, et al. A novel visible-light-driven In-based MOF/graphene oxide composite photocatalyst with enhanced photocatalytic activity toward the degradation of amoxicillin[J]. Applied Catalysis B: Environmental, 2017, 200: 673-680. DOI:10.1016/j.apcatb.2016.07.057 |
| [22] |
WANG Qingjuan, WANG Guanlong, LIANG Xiaofei, et al. Supporting carbon quantum dots on NH2-MIL-125 for enhanced photocatalytic degradation of organic pollutants under a broad spectrum irradiation[J]. Applied Surface Science, 2019, 467-468: 320-327. DOI:10.1016/j.apsusc.2018.10.165 |
| [23] |
ZHANG Suoying, LI Hang, LIU Pengfei, et al. Directed self‐assembly of MOF‐derived nanoparticles toward hierarchical structures for enhanced catalytic activity in CO oxidation[J]. Advanced Energy Materials, 2019, 9(48): 1901754. DOI:10.1002/aenm.201901754 |
| [24] |
WANG Dengke, HUANG Renkun, LIU Wenjun, et al. Fe-based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways[J]. ACS Catalysis, 2014, 4(12): 4254-4260. DOI:10.1021/cs501169t |
| [25] |
WANG Qun, CAI Jingsheng, BIESOLD-MCGEE G, et al. Silk fibroin-derived nitrogen-doped carbon quantum dots anchored on TiO2 nanotube arrays for heterogeneous photocatalytic degradation and water splitting[J]. Nano Energy, 2020, 78: 105313. DOI:10.1016/j.nanoen.2020.105313 |
| [26] |
HAN Yu, YUE Dongting, KAN Miao, et al. [Mo3S13]2- modified TiO2 coating on non-woven fabric for efficient photocatalytic mineralization of acetone[J]. Applied Catalysis B: Environmental, 2019, 245: 190-196. DOI:10.1016/j.apcatb.2018.12.060 |
| [27] |
RAMA K C, JEONG Y D, MISOOK K. Hydrothermal growth of two dimensional hierarchical MoS2 nanospheres on one dimensional CdS nanorods for high performance and stable visible photocatalytic H2 evolution[J]. Applied Surface Science, 2018, 433: 240-248. DOI:10.1016/j.apsusc.2017.09.260 |
| [28] |
ZHAO Tianyu, XING Zipeng, XIU Ziyuan, et al. Oxygen-doped MoS2 nanospheres/CdS quantum dots/g- C3N4 nanosheets super-architectures for prolonged charge lifetime and enhanced visible-light-driven photocatalytic performance[J]. Applied Materials & Interfaces, 2019, 11(7): 7104-7111. DOI:10.1021/acsami.8b21131 |
| [29] |
JAVIER T, ARNAU C, CIVAN A, et al. Colloidal metal-organic framework particles: the pioneering case of ZIF-8[J]. Chemical Society Review, 2019, 48(23): 5534-5546. DOI:10.1039/C9CS00472F |
| [30] |
WANG Sibo, WANG Xinchen. Multifunctional metal-organic frameworks for photocatalysis[J]. Small, 2015, 11(26): 3097-3112. DOI:10.1002/smll.201500084 |
| [31] |
CHEN Ming, DAI Yu, WANG Jingjing, et al. Smart combination of three-dimensional-flower-like MoS2 nanospheres/interconnected carbon nanotubes for application in supercapacitor with enhanced electrochemical performance[J]. Journal of Alloys and Compounds, 2017, 696: 900-906. DOI:10.1016/j.jallcom.2016.12.077 |
| [32] |
FU Shuai, YUAN Wei, YAN Yunhui, et al. Highly efficient visible-light-induced photoactivity of Z-Scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1436-1445. DOI:10.1039/C7EN00713B |
| [33] |
MUTHURASU A, MARUTHAPANDIAN V, KIM H Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction[J]. Applied Catalysis B: Environmental, 2019, 248: 202-210. DOI:10.1016/j.apcatb.2019.02.014 |
| [34] |
ZHANG Xianghua, HUANG Xiaohai, XUE Maoquan, et al. Hydrothermal synthesis and characterization of 3D flower-like MoS2 microspheres[J]. Materials Letters, 2015, 148: 67-70. DOI:10.1016/j.matlet.2015.02.027 |
| [35] |
HU Shen, LIU Min, LI Keyan, et al. Solvothermal synthesis of NH2-MIL-125(Ti) from circular plate to octahedron[J]. Cryst Eng Comm, 2014, 16: 9645. DOI:10.1039/c4ce01545b |
| [36] |
ZHANG Shiyu, DU Meng, XING Zipeng, et al. Defect-rich and electron-rich mesoporous Ti-MOFs based NH2-MIL-125(Ti) @ZnIn2S4/CdS hierarchical tandem heterojunctions with improved charge separation and enhanced solar-driven photocatalytic performance[J]. Applied Catalysis B: Environmental, 2020, 262: 118202. DOI:10.1016/j.apcatb.2019.118202 |
| [37] |
YANG Zhiwang, XU Xueqing, LIANG Xixi, et al. Construction of heterostructured MIL-125/Ag/g-C3N4 nanocomposite as an efficient bifunctional visible light photocatalyst for the organic oxidation and reduction reactions[J]. Applied Catalysis B: Environmental, 2017, 205: 42-54. DOI:10.1016/j.apcatb.2016.12.012 |
| [38] |
REDA M A, DAVID M T, MOHAMED. Engineering highly effective and stable nanocomposite photocatalyst based on NH2-MIL-125 encirclement with Ag3PO4 nanoparticles[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2018, 351: 50-58. DOI:10.1016/j.jphotochem.2017.10.011 |
| [39] |
PENG Weijun, WANG Wei, HAN Guihong, et al. Fabrication of 3D flower-like MoS2/graphene composite as high performance electrode for capacitive deionization[J]. Desalination, 2020, 473: 114191. DOI:10.1016/j.desal.2019.114191 |
| [40] |
LIU Hong, ZHANG Jiang, AO Dan. Construction of heterostructured ZnIn2S4@NH2-MIL-125(Ti) nanocomposites for visible-light-driven H2 production[J]. Applied Catalysis B: Environmental, 2018, 221: 433-442. DOI:10.1016/j.apcatb.2017.09.043 |
| [41] |
ZHENG Lingxia, HAN Sancan, LIU Hui, et al. Hierarchical MoS2 nanosheet@TiO2 nanotube array composites with enhanced photocatalytic and photocurrent performances[J]. Small, 2016, 12(11): 1527-1536. DOI:10.1002/smll.201503441 |
| [42] |
ZHANG Shiyu, DU Meng, KUANG Junyan, et al. Surface-defect-rich mesoporous NH2-MIL-125(Ti)@Bi2MoO6 core-shell heterojunction with improved charge separation and enhanced visible light-driven photocatalytic performance[J]. Journal of Colloid and Interface Science, 2019, 554: 324-334. DOI:10.1016/j.jcis.2019.07.021 |
| [43] |
WANG Zixi, HUANG Jianying, MAO Jiajun, et al. Metal-organic frameworks and their derivatives with graphene composites: preparation and applications in electrocatalysis and photocatalysis[J]. Journal of Materials Chemistry A, 2020, 8(6): 2934-2961. DOI:10.1039/C9TA12776C |
| [44] |
YANG Yang, ZHANG Chen, HUANG Danlian, et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation[J]. Applied Catalysis B: Environmental, 2019, 245: 87-99. DOI:10.1016/j.apcatb.2018.12.049 |
 2022, Vol. 30
2022, Vol. 30


