TiO2是一种低价、无毒的光催化剂和多相催化剂载体[1-5]。由于TiO2难以制成多孔材料且比表面积较低,使其在催化领域的应用受到限制[6-8]。SiO2无毒且化学稳定性高,易于制备成多孔高比表面积材料[9-11]。将TiO2物种分散于SiO2骨架得到的TiO2-SiO2复合材料(TiO2-SiO2 Composite Material,TSCM)兼具来自SiO2骨架的大比表面积和易调变孔道结构,又有来自TiO2物种的高催化活性,是近年来受到高度重视的新型催化功能材料。直接用作催化剂时,TSCM能够表现出高于单一组分的TiO2材料的活性。与经典的钛硅分子筛材料(如TS-1)相比,TSCM可以提供更多活性中心,同时更灵活地调节Ti活性位点结构[5]。TSCM作为载体材料的特点在于活性中心可以灵活地负载于SiO2或TiO2上。
TSCM的催化应用性能受到其所含TiO2物种的晶相和SiO2骨架的孔道结构的显著影响[8, 12-15]。TSCM的孔道特性和形貌来自于其SiO2骨架。同时,SiO2骨架对TiO2粒子起到包覆-支撑作用,而且对TiO2粒子的生长和相变有限制作用。但是,目前研究者对TSCM的制备中如何精细地、可控地调变TiO2物种晶相以及SiO2骨架特性等结构特征因素,仍然缺少系统地分析,并且,这些结构特征因素对TSCM的催化应用性能所产生的效应尚缺乏相应的清晰了解。
为此,本文总结、分析了TSCM所含TiO2物种的晶相、SiO2骨架的结构特性对其催化应用的影响,并对TSCM制备中,如何可控地调变其TiO2物种的晶相、SiO2骨架的结构展开了分析,了解其结构控制的关键步骤及关键控制因素,以期为开发基于TSCM材料的高效催化功能材料提供参考。
1 TSCM的合成TSCM中TiO2物种在SiO2骨架上的分散性与制备方法和参数有关。TSCM的合成按照制备步骤可分为一步法和两步法。
常见的一步法有溶胶-凝胶(sol-gel)法和共沉淀法。图 1所示的sol-gel法合成TSCM中,钛源和硅源同时水解得到TSCM,其TiO2纳米粒子分布在SiO2的多孔骨架中,分散度较高,容易获得更多的Ti—O—Si位点[16-17]。一步法合成TSCM时,不需要预先水解钛源或硅源,操作流程少,更容易得到多孔-大比表面积材料和丰富的Ti—O—Si界面活性物种。但是,前驱体的种类、钛醇盐和硅醇盐水解速率、pH值、Ti/Si比等条件对TSCM中TiO2物种在SiO2骨架上的分散性有着重要影响[18]。有时还需考虑加入螯合剂和改性剂降低钛醇盐的水解速率,促进形成TiO2纳米粒子均匀分布在SiO2多孔骨架中的TSCM材料,因此,合成条件的选择和参数优化是很大的挑战。
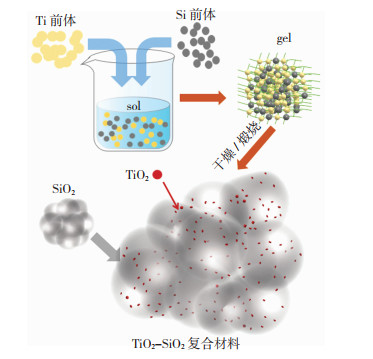
|
图 1 Sol-gel法合成TSCM Fig.1 Sol-gel synthesis of TSCM |
常见的两步法有浸渍法和化学气相沉积等。两步法合成TSCM时,需先制得单一组分TiO2或SiO2材料,然后在其上覆盖/负载SiO2或TiO2,形成的TSCM在结构上类似负载型催化剂,先制得的TiO2或SiO2起到了“载体”作用。这时TiO2与SiO2之间的范德华力键合作用更明显。如图 2所示的两步法合成TSCM中,SiO2包覆于TiO2纳米粒子表面得到了TiO2@SiO2核-壳型材料[19]。两步法合成TSCM时,TSCM表面的均匀性和完整性取决于Ti/Si比:Ti/Si比适中时,TiO2在SiO2核心上形成由非常细小的锐钛矿相(7 nm)TiO2组成的壳层,表现出较高的催化活性;Ti/Si比较小时,TiO2不能在SiO2上形成均匀的表面涂层;Ti/Si比较大时,TiO2容易在SiO2表面聚集[20]。因此,调节制备参数可以得到不同形貌的TSCM,但两步法操作流程较为复杂,并且得到的TSCM中TiO2活性中心的物种形态和分布规律与一步法有显著区别。
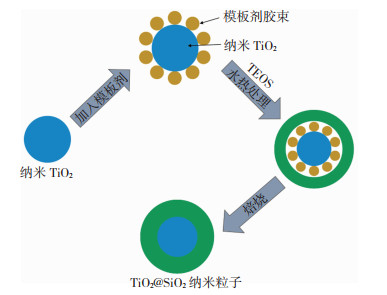
|
图 2 TiO2@SiO2核-壳型TSCM合成 Fig.2 Preparation of TiO2@SiO2 core-shell TSCM |
TiO2常见晶相有锐钛矿(Anatase)、金红石(Rutile)、板钛矿(Brookite),它们的催化性能各异[2, 21-22]。为了在合成TSCM时实现对TiO2物种的晶相控制,研究者尝试了不同制备方法(表 1),期望合成出含单一锐钛矿相TiO2物种的Anatase-SiO2、含单一金红石相TiO2物种的Rutile-SiO2或同时含金红石相和锐钛矿相TiO2物种的混合晶相A/R-SiO2材料。
TiO2粒子的催化性能由其表面性质决定。对TSCM的相关文献深入分析发现,其中TiO2物种的晶相归属基本都是依据XRD数据,更多地反映了TSCM中TiO2物种的体相结构特性,缺少对TSCM所含TiO2物种的表面结构更敏感的Raman等光谱数据支撑;在一些文献中还存在XRD数据分析错误的情况,导致对TSCM中所含TiO2物种的晶相归属出现偏差,从而削弱了后续构-效关系分析的结果。在TSCM制备中,TiO2物种的晶相调控机制仍有待深入研究,合成出具有高纯度单一晶相TiO2物种的TSCM、在混合TiO2晶相的TSCM材料中实现不同TiO2晶相的比例可调仍是难点。
2.1 Anatase-SiO2材料锐钛矿相TiO2缺陷和位错丰富,具有更多的氧空位,电子捕获能力强[27-28]。文献报道的TSCM中,所含TiO2物种大多归属于锐钛矿相。锐钛矿相TiO2的禁带宽度为3.2 eV,能够吸收波长在387.5 nm以下的近紫外光,故而Anatase -SiO2可直接用作光催化剂,其中SiO2和TiO2物种之间形成Ti—O—Si键,表现出比单一TiO2材料更好的催化性能[29-30]。Ren[31]在CO甲烷化反应中发现Ni/Anatase-SiO2催化剂中的锐钛矿相TiO2促进了Ni的分散,增强了反应中的电子转移,提高了催化剂的活性。在高温下会发生从锐钛相到金红石相(A→R)的相变,此相变过程起始于TiO2粒子的界面,逐渐扩展到整个团聚体(图 3(a)),相变过程与粒子团聚过程伴生[32-33]。TSCM中的TiO2受到SiO2骨架的包覆/支撑,其晶相稳定性在较大的温度范围内得以提高。Bedilo等[34]发现TSCM中TiO2纳米粒子需达到一定的临界尺寸(~12 nm)才会发生A→R相变,在1 000 ℃时才出现少量金红石相TiO2,而单一组分的TiO2材料在600 ℃时就出现大量的金红石相TiO2,如图 3(b)和(c)所示。Li等[35]分别以sol-gel法和水热辅助sol-gel法合成了Anatase-SiO2,发现水热处理增加了Anatase-SiO2中Ti—O—Si位点含量,提高了Anatase-SiO2的稳定性,经1 000 ℃煅烧后TiO2仍能保持在锐钛矿相。值得注意的是,对于薄膜型Anatase-SiO2材料,热水处理可以促进前体中的Ti—O—Si键水解,形成锐钛矿相纳米晶体[36-37]。
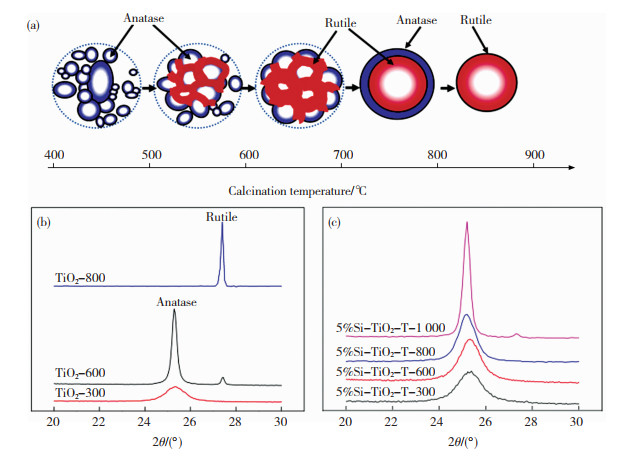
|
图 3 温度对TiO2相变的影响: (a) TiO2纳米粒子的相变与聚集[32];(b)单一组分TiO2及(c)TSCM中TiO2物种的晶相变化[34] Fig.3 Effect of temperature on TiO2 phase transition: (a) phase transition and aggregation of TiO2 nano-particles[32]; phase transition of TiO2 species in (b) single-component TiO2 and (c) TSCM[34] |
金红石相TiO2的禁带宽度为3.0 eV,可见光活性较低,通常不直接作为光催化剂,多用作紫外屏蔽剂[38-39]。然而,TSCM中的金红石相TiO2物种在用作催化剂和催化剂载体时,可以表现出不同于单一组分TiO2材料的结构效应。理论上可以对Anatase-SiO2进行高温煅烧,使TiO2发生A→R相变,进而得到Rutile-SiO2[33],但高温下TSCM的孔道结构会崩塌[40];Li等[41]发现,只有在800 ℃下长时间煅烧Anatase-SiO2才能够得到Rutile-SiO2,且比表面积急剧减少。为了不经过高温煅烧步骤就得到Rutile-SiO2材料,Yener等[26]采用两步法将TiCl4在稻壳灰上沉积-水解得到Rutile-SiO2,但需在接近水沸腾的条件下水解TiCl4,反应条件较为苛刻。张海东等[42]以TiCl4为钛源,正硅酸乙酯为硅源,使用水热辅助的sol-gel法在400 ℃煅烧,得到晶相单一的Rutile -SiO2,其比表面积高达331~560 m2/g。
2.3 Brookite-SiO2材料板钛矿相TiO2为斜方晶系,因其晶相结构的不稳定性而较少作为催化剂使用[43],有关Brookite-SiO2材料的报道极少。近期的研究发现,板钛矿相TiO2也可以表现出一定的催化活性[44]。Arier[45]尝试以钛酸四丁酯、正硅酸乙酯为原料,采用sol-gel法合成了Brookite-SiO2,但未有数据支撑其在TSCM中合成了板钛矿相TiO2。因此,Brookite-SiO2的制备和应用仍然是一个充满挑战的领域。
2.4 A/R-SiO2材料同时含有锐钛矿晶相和金红石晶相TiO2物种的A/R-SiO2类型TSCM材料的重要性不亚于具有单一晶相TiO2物种的TSCM材料。TiO2具有混晶效应,A/R-SiO2型TSCM在催化、太阳能电池、传感器等方面都表现出广泛的应用前景[46]。Zhang等[47]发现,不同晶相TiO2形成的“异相结”可显著提高催化剂的活性。Bao等[48]以钛酸四丁酯为钛源,采用溶剂热法合成了具有三维层状海胆中空球结构的A/R-SiO2材料,其A/R比为13.7/86.3,将光生电子聚集在金红石相TiO2和SiO2内壳,而光生空穴聚集在锐钛矿相TiO2上,很好地抑制了电子和空穴的复合。张海东等[49]以钛酸四丁酯和正硅酸乙酯为原料,采用sol-gel法制得的A/R-SiO2材料中,锐钛相和金红石相TiO2的比例(A/R)在13.7/1~99/1之间可调,这些材料在可见光下具有很高的催化活性。Song等[23]将异丙醇钛浸渍在SiO2微球上,使用不同的醇溶剂,合成出A/R比不同但孔结构相近的A/R-SiO2,用其作为Fe@A/R-SiO2催化剂的载体,在F-T反应中发现,较低的A/R比有利于提高活性中心的分散度,增强催化活性。
3 TSCM中SiO2骨架的结构控制SiO2比TiO2更容易形成丰富的孔道结构和更高的比表面积,并且对均匀分散的TiO2粒子可以起到包覆和支撑作用,因此合成TSCM时,SiO2骨架决定了TSCM的孔道特性和形貌结构[17]。
3.1 孔道特性SiO2骨架的孔道尺寸可以在微孔、介孔、大孔的广泛范围内进行调变[50]。Long等[21]以一步法合成的TSCM的比表面积为405.6 m2/g,且孔道相互交织成多级连续互通结构,降低了反应中的传质阻力,在大分子有机物的光催化氧化反应中表现出高活性。Yener等[26]以TiCl4和稻壳灰为原料制备TSCM,发现微孔和介孔共存时,其比表面积和孔结构均匀性随着介孔的生成增高。Yang等[9]发现,介孔TSCM可暴露出更多的活性位,并且能将产物快速传输出孔道,在环氧化反应中表现出高活性和选择性。
3.2 形貌光催化过程中,TSCM的形貌对电子-空穴分离有重要影响[48]。核壳结构微球型TSCM具有较大的折射率差值、较大的比表面积和微球纳米结构壁面,对提高催化活性有利[22]。Tang等[51]发现核壳型TSCM中,SiO2均匀沉积在TiO2表面,阻止了TiO2浸出,增加了催化剂的重复性。Ekka等[52]发现,两步法制备的核壳结构微球型TSCM的结构稳定性高于sol-gel法制备的球形TSCM。当TSCM为薄膜形貌时,可获得较大的比表面积,具有更高的催化活性[53]。Cui等[54]采用液相合成-电纺丝法制备了MnO2@SiO2-TiO2纳米纤维膜,作为载体的TSCM具有高孔隙率、大长径比、高柔性和良好的力学性能,其分层结构增加了催化剂的比表面积,为催化氧化反应提供了更多的活性位点。
3.3 结构导向剂TSCM制备中使用的结构导向剂是决定其孔道特性的重要控制因素[55]。结构导向剂通常为离子型和非离子型(表 2)。Wang等[56]以十六烷基三甲基溴化铵(CTAB)为结构导向剂,通过sol-gel法合成的TSCM比表面积可达到918.9 m2/g。Smeets等[5]通过气溶胶辅助的sol-gel法,以F127和四丙基氢氧化铵为混合结构导向剂,成功得到了不同孔径的TSCM。郑亚超等[57]比较了P123、F127、N-十六烷基乙二胺(HEDA)等不同结构导向剂对TSCM比表面积和孔径的影响,发现P123或F127得到的TSCM比表面积更大,且P123更有利于获得均匀分布的介孔结构,从而表现出更好的吸附和光催化性能。
| 表 2 TSCM合成中的结构导向剂 Table 2 Structure-directing agents in synthesis of TSCM |
与钛硅分子筛的催化活性来自于其骨架中的Ti4+位点不同,TSCM的催化活性来自于其所含的TiO2氧化物纳米粒子,在环境催化领域应用广泛[48]。通常认为TiO2和SiO2界面上的Ti—O—Si键位是TSCM的活性位[58]。而Ti—O—Si键的含量受到TiO2的晶相和晶粒大小的影响,同时Ti—O—Si键也可以控制TiO2颗粒生长(图 4),从而影响催化性能[59]。Rasalingam等[60]认为,TSCM中的Ti—O—Si键位促进了有机物的氧化,在光催化反应中表现出良好的催化活性。Mahesh等[61]将Ag沉积在核壳型TSCM制得Ag-SiO2 @TiO2催化剂,发现SiO2提供AB-1染料的吸附位点(图 5),增加了催化剂表面AB-1染料的浓度,而Ag物种在TiO2上以Ag0和Ag+形式存在,抑制了电子-空穴的复合,因此,Ag-SiO2@TiO2催化剂在光催化氧化反应中的活性远高于SiO2@TiO2催化剂。

|
图 5 Ag-SiO2 @ TiO2及其光催化降解有机染料 Fig.5 Ag-SiO2@TiO2 and its photocatalytic degradation of dye |
除光催化外,TSCM也可用于其他催化反应。TSCM表面的酸性位点、较大比表面积和孔道结构,为反应物分子提供了更多的吸附位点,从而提高了催化反应活性及选择性。Sadegh等[62]对比了TSCM、TiO2、SiO2纳米粒子催化芳基亚甲基吡咯酸和二甲基酮缩合反应的催化活性,发现TSCM的催化性能最好。Bazyari等[58]认为,TSCM表面暴露的Lewis酸位点吸附了二苯并噻吩,提高了二苯并噻吩氧化脱硫效率。
4.2 TSCM载体材料TiO2可以表现出显著的金属-载体强相互作用(Strong Metal-Support Interaction,SMSI)。TSCM作为催化剂载体负载金属活性中心时,使用浸渍法,嫁接法,光沉积法,沉积-沉降法等不同制备方法,可以将金属粒子有选择地分别优先负载在TiO2物种、SiO2骨架上,或同时负载在TiO2和SiO2物种上,从而影响金属中心的粒子大小、分散性和价态[19, 63]。Huang等[64]发现,Cu-ZnO/SiO2-TiO2催化剂中具有高度分散的铜物种且存在SMSI,促进了电子从TiO2向铜物种转移,在加氢反应中具有较高的催化活性。Mohamed等[65]发现,浸渍法得到的Ag/TiO2-SiO2催化剂中,Ag粒子同时分散在TiO2和SiO2物种上,而光沉积制备的Ag/TiO2-SiO2催化剂中,Ag则更多地沉积在TiO2上,从而表现出更高的催化活性。Park等[66]通过原子沉积法制备的夹层NiO/TiO2/SiO2催化剂,TiO2在高湿环境下实现对甲苯的选择性吸附,从而在高湿环境下表现出高甲苯催化燃烧活性。
5 总结与展望TiO2-SiO2复合材料(TSCM)兼具来自SiO2骨架的大比表面积、易调变孔道结构和由TiO2物种提供的高活性中心,可直接用作催化剂或作为催化剂载体材料,其性能受到其所含的TiO2物种的晶相和SiO2骨架的孔道结构的显著影响。TSCM用作催化剂时,其活性高于单一的TiO2材料,通常认为其活性位是Ti—O—Si键位和表面酸性位点。TSCM用作催化剂载体时,由于其中TiO2物种的SMSI作用表现出较强的载体效应。
通过精细调控硅源、钛源、制备参数,可以得到TiO2物种晶相单一的Anatase-SiO2和Rutile -SiO2材料。但Rutile-SiO2的制备需要使用TiCl4作为钛源,仍需探索其他更方便、来源更广泛、价格更低廉的钛源用于合成Rutile-SiO2。精准调控混合晶相A/R-SiO2材料中金红石和锐钛矿的比例,可有效抑制光生空穴和电子的复合,因此,对A/R-SiO2材料中两种晶相的比例调控是未来的研究重点。TSCM中TiO2物种的晶相调控机理复杂,大多数研究仅停留在方法优化阶段,但对TiO2物种的晶相转变机制研究较少。原位Raman光谱等表征技术对TSCM合成过程中TiO2物种的晶相转变进行实时监测,可以为TSCM材料中TiO2物种的可控合成研究提供有力的工具。
TSCM的SiO2骨架决定了TSCM的孔道特性和形貌,同时对其中的TiO2粒子起到包覆-支撑作用,而且对TiO2粒子的生长和相变有限制作用。TSCM的SiO2骨架可以在微孔、介孔、大孔的广泛范围内调变,为不同催化的反应提供了丰富的选择。合成过程中使用的结构导向剂、Ti/Si比等参数是控制TSCM的形貌、孔道结构的核心制备要素。如何在得到特定晶相和粒子尺寸的TiO2物种的同时,得到特定孔道特性和形貌特征的SiO2骨架仍是研究的难点和热点。
在TSCM的合成中,两步法无需同时控制TiO2和SiO2的制备参数,重现性好,较多用于特殊形貌(如核壳型材料)的TSCM制备,但其中TiO2的分散度不如一步法。一步法需要在同一合成体系中同时调控相互影响的TiO2和SiO2物种的形成过程及其制备参数,常需要加入螯合剂、改性剂、结构导向剂,用以调节pH值进而降低钛源和硅源的水解速率,达到调变SiO2骨架的孔道结构和TiO2粒子尺寸和分布的目的,且重现性不如两步法。但一步法制备流程简短,更容易得到多孔-大比表面积材料,有利于实现TiO2粒子的尺寸控制及其在SiO2骨架中的均匀分布,从而得到更丰富的Ti—O—Si界面活性物种。
| [1] |
FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. DOI:10.1038/238037a0 |
| [2] |
MO S D, CHING W Y. Electronic and optical properties of three phases of titanium dioxide: rutile, anatase, and brookite[J]. Physical Review B, Condensed Matter, 1995, 51: 13023-13032. DOI:10.1103/physrevb.51.13023 |
| [3] |
ROMERO-MORAN A, SANCHEZ-SALAS J L, MOLINA-REYES J. Influence of selected reactive oxygen species on photocatalytic activity of TiO2/SiO2 composite coatings processed at low temperature[J]. Applied Catalysis B: Environmental, 2021, 291: 119685. DOI:10.1016/j.apcatb.2020.119685 |
| [4] |
VYATSKIKH A, NG R C, EDWARDS B, et al. Additive manufacturing of high-refractive-index, nanoarchitected titanium dioxide for 3D dielectric photonic crystals[J]. Nano Letters, 2020, 20(5): 3513-3520. DOI:10.1021/acs.nanolett.0c00454 |
| [5] |
SMEETS V, BOISSIÈRE C, SANCHEZ C, et al. Aerosol route to TiO2-SiO2 catalysts with tailored pore architecture and high epoxidation activity[J]. Chemistry of Materials, 2019, 31(5): 1610-1619. DOI:10.1021/acs.chemmater.8b04843 |
| [6] |
LEE V Y, AOKI S, YOKOYAMA T, et al. Toward a silicon version of metathesis: From schrock-type titanium silylidenes to silatitanacyclobutenes[J]. Journal of the American Chemical Society, 2013, 135(8): 2987-2990. DOI:10.1021/ja401072j |
| [7] |
DIAMANTI M V, GADELRAB K R, PEDEFERRI M P, et al. Nanoscale investigation of photoinduced hydrophilicity variations in anatase and rutile nanopowders[J]. Langmuir, 2013, 29(47): 14512-14518. DOI:10.1021/la4034723 |
| [8] |
HU J, ZHOU Y, SHENG X. Preparation, characterization and application of soluble TiO2@SiO2 nanospheres by a simple modified sol-gel procedure[J]. Journal of Sol-Gel Science and Technology, 2015, 74(1): 181-186. DOI:10.1007/s10971-014-3594-z |
| [9] |
YANG H, LIU Z, GAO H, et al. Synthesis and characterization of hierarchical titania-silica monolith[J]. Catalysis Today, 2013, 216(6): 90-94. DOI:10.1016/j.cattod.2013.05.025 |
| [10] |
MAEDA M, YAMASAKI S. Effect of silica addition on crystallinity and photo-induced hydrophilicity of titania-silica mixed films prepared by sol-gel process[J]. Thin Solid Films, 2005, 483(1): 102-106. DOI:10.1016/j.tsf.2004.12.042 |
| [11] |
LIU Y Y, QIAN L Q, GUO C, et al. Natural superhydrophilic TiO2/SiO2 composite thin films deposited by radio frequency magnetron sputtering[J]. Journal of Alloys and Compounds, 2009, 479(1): 532-535. DOI:10.1016/j.jallcom.2008.12.125 |
| [12] |
CISNEROS S, CHEN S, DIEMANT T, et al. Effects of SiO2-doping on high-surface-area Ru/TiO2 catalysts for the selective CO methanation[J]. Applied Catalysis B: Environmental, 2021, 282: 119483. DOI:10.1016/j.apcatb.2020.119483 |
| [13] |
ZHANG P, SUN Y, LU M, et al. High-loading nickel phosphide catalysts supported on SiO2-TiO2 for hydrodeoxygenation of guaiacol[J]. Energy & Fuels, 2019, 33(8): 7696-7704. DOI:10.1021/acs.energyfuels.9b01538 |
| [14] |
LI Y, LIU J, HE J, et al. Silica/titania composite-supported NiCo catalysts with combined catalytic effects for phenol hydrogenation under fast and mild conditions[J]. Applied Catalysis A: General, 2020, 591: 117409. DOI:10.1016/j.apcata.2020.117409 |
| [15] |
GUO J, BENZ D, NGUYEN T T D, et al. Tuning the photocatalytic activity of TiO2 nanoparticles by ultrathin SiO2 films grown by low-temperature atmospheric pressure atomic layer deposition[J]. Applied Surface Science, 2020, 530: 147244. DOI:10.1016/j.apsusc.2020.147244 |
| [16] |
WEI Q, LI Y, ZHANG T, et al. TiO2-SiO2-Composite-supported catalysts for residue fluid catalytic cracking diesel hydrotreating[J]. Energy & Fuels, 2014, 28(12): 7343-7351. DOI:10.1021/ef500799t |
| [17] |
LONG T, XU Y, LV X J, et al. Fabrication of freestanding SiO2-TiO2 composite by a facile one pot method[J]. Materials and Manufacturing Processes, 2019, 34(6): 624-629. DOI:10.1080/10426914.2018.1532090 |
| [18] |
LIU J, ZHANG Y, WANG Z, et al. Amorphous TiO2-SiO2 composites as selective heterogeneous catalysts for the oxidation of styrene to 1, 2-epoxyethylbenzene[J]. Journal of the Iranian Chemical Society, 2019, 16(7): 1373-1381. DOI:10.1007/s13738-019-01611-8 |
| [19] |
SUN J, XU K, SHI C, et al. Influence of core/shell TiO2@SiO2 nanoparticles on cement hydration[J]. Construction and Building Materials, 2017, 156: 114-122. DOI:10.1016/j.conbuildmat.2017.08.124 |
| [20] |
SONG X, ZHANG Q, ZHANG G, et al. Intrinsic effect of crystalline phases in TiO2 on the fischer-tropsch synthesis over well-defined and uniform pore-structure Fe/TiO2/SiO2 catalysts[J]. Reaction Kinetics, Mechanisms and Catalysis, 2020, 129(2): 743-753. DOI:10.1007/s11144-020-01748-1 |
| [21] |
LEWKOWICZ A, BOJARSKI P, SYNAK A, et al. Concentration-dependent fluorescence properties of rhodamine 6G in titanium dioxide and silicon dioxide nanolayers[J]. The Journal of Physical Chemistry C, 2012, 116(22): 12304-12311. DOI:10.1021/jp3022562 |
| [22] |
ZHOU J, GAO Z, XIANG G, et al. Interfacial compatibility critically controls Ru/TiO2 metal-support interaction modes in CO2 hydrogenation[J]. Nature Communications, 2022, 13(1): 327. DOI:10.1038/s41467-021-27910-4 |
| [23] |
KITSOU I, PANAGOPOULOS P, MAGGOS T, et al. Development of SiO2@TiO2 core-shell nanospheres for catalytic applications[J]. Applied Surface Science, 2018, 441: 223-231. DOI:10.1016/j.apsusc.2018.02.008 |
| [24] |
CETINKAYA T, NEUWIRTHOVA L, KUTLAKOVA K M, et al. Synthesis of nanostructured TiO2/SiO2 as an effective photocatalyst for degradation of acid orange[J]. Applied Surface Science, 2013, 279: 384-390. DOI:10.1016/j.apsusc.2013.04.121 |
| [25] |
AL-QAYSI K, NAYEBZADEH H, SAGHATOLESLAMI N. Comprehensive study on the effect of preparation conditions on the activity of sulfated silica-titania for green biofuel production[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2020, 30(10): 3999-4013. DOI:10.1007/s10904-020-01545-2 |
| [26] |
YENER H B, HELVACI Ş Ş. Effect of synthesis temperature on the structural properties and photocatalytic activity of TiO2/SiO2 composites synthesized using rice husk ash as a SiO2 source[J]. Separation and Purification Technology, 2015, 140: 84-93. DOI:10.1016/j.seppur.2014.11.013 |
| [27] |
HOSSEINI-ZORI M. Co-doped TiO2 nanostructures as a strong antibacterial agent and self-cleaning cover: synthesis, characterization and investigation of photocatalytic activity under UV irradiation[J]. Journal of Photochemistry and Photobiology B: Biology, 2018, 178: 512-520. DOI:10.1016/j.jphotobiol.2017.12.008 |
| [28] |
LIU B, YAN L, WANG J. Liquid N2 quenching induced oxygen defects and surface distortion in TiO2 and the effect on the photocatalysis of methylene blue and acetone[J]. Applied Surface Science, 2019, 494: 266-274. DOI:10.1016/j.apsusc.2019.07.095 |
| [29] |
CHEN X, DONG W, YAO Y, et al. Preparation of mesoporous anatase titania with large secondary mesopores and extraordinarily high photocatalytic performances[J]. Applied Catalysis B: Environmental, 2020, 269: 118756. DOI:10.1016/j.apcatb.2020.118756 |
| [30] |
ALFIERI I, LORENZI A, RANZENIGO L, et al. Synthesis and characterization of photocatalytic hydrophobic hybrid TiO2-SiO2 coatings for building applications[J]. Building and Environment, 2017, 111: 72-79. DOI:10.1016/j.buildenv.2016.10.019 |
| [31] |
REN J, LI H, JIN Y, et al. Silica/titania composite-supported Ni catalysts for CO methanation: Effects of Ti species on the activity, anti-sintering, and anti-coking properties[J]. Applied Catalysis B: Environmental, 2017, 201: 561-572. DOI:10.1016/j.apcatb.2016.08.061 |
| [32] |
ZHANG J, LI M, FENG Z, et al. UV Raman spectroscopic study on TiO2. I. Phase transformation at the surface and in the bulk[J]. Journal of Physical Chemistry B, 2006, 119(2): 927-935. DOI:10.1021/jp0552473 |
| [33] |
ZHANG J, XU Q, LI M, et al. UV raman spectroscopic study on TiO2. Ⅱ. effect of nanoparticle size on the outer/inner phase transformations[J]. The Journal of Physical Chemistry C, 2009, 113(5): 1698-1704. DOI:10.1021/jp808013k |
| [34] |
BEDILO A F, SHUVARAKOVA E I, VOLODIN A M. Silica-coated nanocrystalline TiO2 with improved thermal stability[J]. Ceramics International, 2019, 45(3): 3547-3553. DOI:10.1016/s1003-6326(17)60256-5 |
| [35] |
LI Z, BO H, XU Y, et al. Comparative study of sol-gel-hydrothermal and sol-gel synthesis of titania-silica composite nanoparticles[J]. Journal of Solid State Chemistry, 2005, 178(5): 1395-1405. DOI:10.1016/j.jssc.2004.12.034 |
| [36] |
WU J M. Nanostructured TiO2 layers on Ti for bone bonding[J]. Bioceramics, 2021, 25-76. DOI:10.1016/B978-0-08-102999-2.00003-X |
| [37] |
BU Y, ZHANG L, MA D, et al. Low-temperature synthesis of micro-mesoporous TiO2-SiO2 composite film containing Fe-N co-doped anatase nanocrystals for photocatalytic NO removal[J]. Catalysis Letters, 2021, 151(8): 2396-2407. DOI:10.1007/s10562-020-03466-8 |
| [38] |
BAI Y, LI Z, CHENG B, et al. Higher UV-shielding ability and lower photocatalytic activity of TiO2@SiO2/APTES and its excellent performance in enhancing the photostability of poly(p-phenylene sulfide)[J]. Rsc Advances, 2017, 7(35): 21758-21767. DOI:10.1039/C6RA28098F |
| [39] |
张亚楠, 陈强, 肖鹏飞, 等. SiO2/TiO2复合纳米粒子的可控制备及表征[J]. 应用化工, 2020, 49(1): 1-4. ZHANG Yanan, CHEN Qiang, XIAO Pengfei, et al. Controllable synthesis and characterization of composite nanoparticles SiO2@TiO2[J]. Applied Chemical Industry, 2020, 49(1): 1-4. DOI:10.16581/j.cnki.issn1671-3206.2020.01.001 |
| [40] |
ZHANG Q, KANG J, WANG Y. Development of novel catalysts for fischer-tropsch synthesis: tuning the product selectivity[J]. Chem Cat Chem, 2010, 2(9): 1030-1058. DOI:10.1002/cctc.201000071 |
| [41] |
FANG L, HOU L, ZHANG Y, et al. Synthesis of highly hydrophobic rutile titania-silica nanocomposites by an improved hydrolysis co-precipitation method[J]. Ceramics International, 2017, 43(7): 5592-5598. DOI:10.1016/j.ceramint.2017.01.091 |
| [42] |
张海东, 李晓捷, 周玉凤. 一种TiO2-SiO2氧化物复合材料及其制备方法: ZL202010758357.1[P]. 2020-07-31.
|
| [43] |
PAOLA A D, BELLARDITA M, PALMISANO L. Brookite, the least known TiO2 photocatalyst[J]. Catalysts, 2013, 3(1): 36-73. DOI:10.3390/catal3010036 |
| [44] |
MORLANDO A, MCNAMARA J, REHMAN Y, et al. Hydrothermal synthesis of rutile TiO2 nanorods and their decoration with CeO2 nanoparticles as low-photocatalytic active ingredients in UV filtering applications[J]. Journal of Materials Science, 2020, 55(19): 8095-8108. DOI:10.1007/s10853-020-04598-3 |
| [45] |
ARIER V Ö A. Optical and structural properties of sol-gel derived brookite TiO2-SiO2 nano-composite films with different SiO2: TiO2 ratios[J]. Optik, 2016, 127(16): 6439-6445. DOI:10.1016/j.ijleo.2016.04.038 |
| [46] |
王鲁燕, 孙彦平, 许并社. 钛硅纳米复合氧化物粉体表面化学结构特征[J]. 科学通报, 2008(9): 1036-1044. WANG Luyan, SUN Yanpin, XU Bingshe. Structure Characteristics of TiO2-SiO2 Nanocomposite[J]. Chinese Science Bulletin, 2008(9): 1036-1044. DOI:10.1360/csb2008-53-9-1036 |
| [47] |
ZHANG J, XU Q, FENG Z, et al. Importance of the relationship between surface phases and photocatalytic activity of TiO2[J]. Angewandte Chemie International Edition, 2008, 47(9): 1766-1769. DOI:10.1002/anie.200704788 |
| [48] |
BAO Y, GUO R, GAO M, et al. Morphology control of 3D hierarchical urchin-like hollow SiO2@TiO2 spheres for photocatalytic degradation: influence of calcination temperature[J]. Journal of Alloys and Compounds, 2021, 853: 157202. DOI:10.1016/j.jallcom.2020.157202 |
| [49] |
张海东, 申渝, 陈佳, 等. 一种介孔氧化钛-氧化硅氧化物复合材料及其制备方法和应用: CN201811550925.8[P]. 2018-12-18.
|
| [50] |
WANG H N, YUAN P, ZHOU L, et al. Synthesis and characterization of TiO2-incorporated silica foams[J]. Journal of Materials Science, 2009, 44(24): 6484-6489. DOI:10.1007/s10853-009-3578-5 |
| [51] |
TANG R, CHEN T, CHEN Y, et al. Core-shell TiO2@ SiO2 catalyst for transesterification of dimethyl carbonate and phenol to diphenyl carbonate[J]. Chinese Journal of Catalysis, 2014, 35(4): 457-461. DOI:10.1016/S1872-2067(14)60059-0 |
| [52] |
EKKA B, SAHU M K, PATEL R K, et al. Titania coated silica nanocomposite prepared via encapsulation method for the degradation of Safranin-O dye from aqueous solution: Optimization using statistical design[J]. Water Resources and Industry, 2019, 22: 100071. DOI:10.1016/j.wri.2016.08.001 |
| [53] |
DELANNOY L, FAJERWERG K, LAKSHMANAN P, et al. Supported gold catalysts for the decomposition of VOC: Total oxidation of propene in low concentration as model reaction[J]. Applied Catalysis B: Environmental, 2010, 94(1): 117-124. DOI:10.1016/j.apcatb.2009.10.028 |
| [54] |
CUI F, HAN W, SI Y, et al. In situ synthesis of MnO2@SiO2-TiO2 nanofibrous membranes for room temperature degradation of formaldehyde[J]. Composites Communications, 2019, 16: 61-66. DOI:10.1016/j.coco.2019.08.002 |
| [55] |
PAN J H, ZHAO X S, LEE W I. Block copolymer-templated synthesis of highly organized mesoporous TiO2-based films and their photoelectrochemical applications[J]. Chemical Engineering Journal, 2011, 170(2): 363-380. DOI:10.1016/j.cej.2010.11.040 |
| [56] |
WANG X, XUE J, WANG X, et al. Heterogeneous Ag-TiO2-SiO2 composite materials as novel catalytic systems for selective epoxidation of cyclohexene by H2O2[J]. PLOS One, 2017, 12(5): e0176332. DOI:10.1371/journal.pone.0176332 |
| [57] |
郑亚超, 王亮, 朱雯倩, 等. 不同模板剂对介孔SiO2-TiO2复合材料光催化性能的影响[J]. 广州化工, 2020, 48(10): 48-52. ZHENG Yachao, WANG Liang, ZHU Wenqian, et al. Effect of different templates on photocatalytic properties of mesoporous SiO2-TiO2 composite materials[J]. Guangzhou Chemical Industry, 2020, 48(10): 48-52. DOI: CNKI:SUN:GZHA.0.2020-10-019 |
| [58] |
BAZYARI A, KHODADADI A A, MAMAGHANI A H, et al. Microporous titania-silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization[J]. Applied Catalysis B: Environmental, 2016, 180: 65-77. DOI:10.1016/j.apcatb.2015.06.011 |
| [59] |
ZHANG H, SUN S, DING H, et al. Effect of calcination temperature on the structure and properties of SiO2 microspheres/nano-TiO2 composites[J]. Materials Science in Semiconductor Processing, 2020, 115: 105099. DOI:10.1016/j.mssp.2020.105099 |
| [60] |
RASALINGAM S, KIBOMBO H S, WU C M, et al. Influence of Ti—O—Si hetero-linkages in the photocatalytic degradation of rhodamine B[J]. Catalysis Communications, 2013, 31: 66-70. DOI:10.1016/j.catcom.2012.11.016 |
| [61] |
MAHESH K P O, KUO D H, HUANG B R. Facile synthesis of heterostructured Ag-deposited SiO2@TiO2 composite spheres with enhanced catalytic activity towards the photodegradation of AB 1 dye[J]. Journal of Molecular Catalysis A: Chemical, 2015, 396: 290-296. DOI:10.1016/j.molcata.2014.10.017 |
| [62] |
SADEGH-SAMIEI S, ABDOLMOHAMMADI S. TiO2-SiO2 nanocomposite-promoted efficient cyclocondensation reaction of arylmethylidenepyruvic acids with dimedone in aqueous media[J]. Journal of the Chinese Chemical Society, 2018, 65(10): 1155-1159. DOI:10.1002/jccs.201800057 |
| [63] |
MACINO M, BARNES A J, ALTHAHBAN S M, et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene[J]. Nature Catalysis, 2019, 2(10): 873-881. DOI:10.1038/s41929-019-0334-3 |
| [64] |
HUANG Y, ZHANG W, YUE Z, et al. Performance of SiO2-TiO2 binary oxides supported Cu-ZnO catalyst in ethyl acetate hydrogenation to ethanol[J]. Catalysis Letters, 2017, 147(11): 2817-2825. DOI:10.1007/s10562-017-2165-7 |
| [65] |
MOHAMED R M, MKHALID I A. Characterization and catalytic properties of nano-sized Ag metal catalyst on TiO2-SiO2 synthesized by photo-assisted deposition and impregnation methods[J]. Journal of Alloys and Compounds, 2010, 501(2): 301-306. DOI:10.1016/j.jallcom.2010.04.092 |
| [66] |
PARK E J, LEE J H, KIM K D, et al. Toluene oxidation catalyzed by NiO/SiO2 and NiO/TiO2/SiO2: towards development of humidity-resistant catalysts[J]. Catalysis Today, 2016, 260: 100-106. DOI:10.1016/j.cattod.2015.03.038 |
 2023, Vol. 31
2023, Vol. 31



