2. 苏州科技大学 水处理技术与材料协同创新中心,江苏 苏州 215009;
3. 常州工程职业技术学院 检验检测认证学院,江苏 常州 213164;
4. 苏州科特环保股份有限公司,江苏 苏州 215164
2. Collaborative Innovation Center of Water Treatment Technology and Material, Suzhou University of Science and Technology, Suzhou 215009, China;
3. Changzhou Vocational Institute of Engineering, School of Inspection, Testing and Certification, Changzhou 213164, China;
4. Suzhou Kete Environmental Protection Co., Ltd., Suzhou 215164, China
环境污染是全世界面临的重要问题。每年全球发展中国家因工业生产而直接排放的污染物达(300~400)×106 t[1-2]。我国排放的非甲烷总烃由1990年的9.76×106 t骤增192%至2017年的2.85×107 t[3]。随着“碳中和、碳达峰”目标的提出,催生了我国对于含碳有机物转化治理的极大需求[4]。然而,当前以生化处理为代表的传统环境治理技术在应对污染问题时,不仅微生物的活性极易受到其中所含新兴毒害有机物的冲击而剧烈波动[5],而且最终将不可避免地造成CO2的大量排放。因此,研发新型高效低耗的环境治理技术、开发相应的功能材料,将其用于环境污染治理中就显得尤为迫切。
以纳米材料为代表的新型环境功能材料,因其具有独特的尺寸效应、较大的比表面积和可修饰性,而具有活性高、见效快的特点,近年来在水体修复、废气治理中颇受关注[6-7]。但研究中发现,由于该类材料具有较高的表面能,极易自发团聚而导致活性位点减少,最终使得处理效果显著下降[8-9],极大地阻碍了其在污染治理中的应用。因此,如何维持其高效的性能是当前纳米材料需要解决的重要问题。
将纳米材料的活性中心“封闭”在纳米尺度的空间内,可有效限制其迁移,从而维持其活性[10-12]。同时,在水环境中,纳米尺度不仅会影响水的氢键网络结构,而且容易致使反应底物富集。此外,在纳米空间内,晶体的生长和成核过程会被影响,形成亚稳态晶型,从而加快特定晶型的结晶速率。最后,纳米空间还会影响反应物、催化剂、生成物和过渡态之间的相互作用,最终影响到反应中中间活性粒子的演化和反应产物生成[13-16]。狭小的限域空间孔道入口也会阻挡某些杂质与活性中心的结合,减少副反应发生。这些特性最终会显著影响目标反应发生的速率[17-20]。Bao等[21-22]将这一现象命名为“纳米限域效应”,对应的材料即为“纳米限域材料”。这一发现为纳米材料的研发及其在环境领域的应用打开了新的思路。
本文系统梳理了纳米限域材料的制备方式并对比了不同方式在实际应用过程中的优缺点,归纳总结了近年纳米限域材料在环境污染物治理领域的应用研究进展,展望了今后纳米限域材料在环境污染物治理领域的研发及应用前景,以期有助于同行们围绕“纳米限域效应”有针对性地研发新型环境治理技术。
1 纳米限域材料的制备依照纳米材料和载体框架在纳米限域材料制备过程中的合成顺序不同,可将纳米限域材料的制备策略归纳为“瓶中造船”、“船外造瓶”和“一锅制备”。
1.1 “瓶中造船”的制备策略“瓶中造船”是一种被较广泛应用的合成策略。其合成步骤为先将纳米活性中心的前驱体引入基体材料的孔道内,再将其转化为目标活性中心[23]。
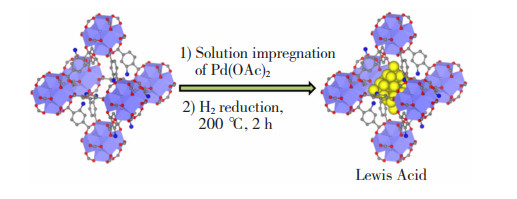
1.1.1 浸渍还原法将纳米颗粒(Nanoparticles,NPs)的前驱体以浸渍的方式引入基体材料的孔道内,而后以还原的方式将其转化为目标活性中心。不同的还原方式会影响材料制备的可操作性。研究证明,NPs粒径越小,分散性越好,激发出的活性位点就越多,所制备的纳米限域材料具有的催化活性越强[14, 18]。Li等[24]使用Pd(OAc)2为前体物,将Pd2+成功引入UiO-66-NH2孔道内,然后在200 ℃下用H2将其还原,制备得到Pd NPs平均粒径小于1.2 nm的纳米限域材料Pd@UiO-66-NH2,其合成步骤如图 1所示[24]。相较于UiO-66-NH2,该材料对苯丙醇的选择性转化率高出近98.4%。但使用H2有潜在的安全风险,使用其他较为温和和安全的还原剂也可达到类似的还原效果。
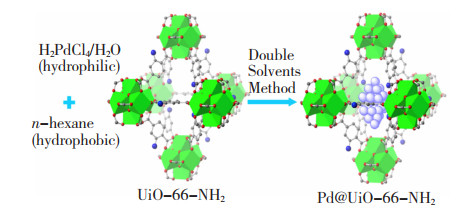
Feng等[25]将高温活化后的UiO-66-NH2框架均匀溶解于无水正己烷中,利用溶剂与NPs及其前驱体间亲和力和溶解度的不同,引入H2PdCl4水溶液形成双溶剂体系,经NaBH4还原后,得到Pd NPs粒径在0.8~2.6 nm的纳米限域材料Pd@UiO-66-NH2,其合成步骤示意图如图 2所示[25]。普通浸渍法制备得到的催化剂Pd/UiO-66-NH2中,Pd NPs平均粒径高达5.8 nm,远大于框架基体的尺寸,故其分散性较差。因此,减小NPs的尺寸可以减少其在载体表面的聚集,有效提高前驱体进入孔道内的效率。但NaBH4在使用中也有一定的生物毒性风险,需要尝试更为安全、环境友好的还原剂。Shi等[26]将纯化后的MIL-101(Cr)和正己烷混合,滴加不同摩尔质量的CuCl2水溶液,超声分散后引入环境友好的维生素C作为还原剂,过滤干燥后得到CuCl NPs的负载量分别为4和6 mmol/g的纳米限域材料4CuCl@MIL-101(Cr)和6CuCl@MIL-101(Cr),结果表明, 4CuCl@MIL-101(Cr)对噻吩的饱和吸附量是MIL-101(Cr)的近3倍。然而,6CuCl@MIL-101(Cr)对噻吩的吸附量却比4CuCl@MIL-101(Cr)低,这是因为引入过量的Cu+会堵塞框架孔隙,造成扩散阻力,影响吸附效果。Li等[27]将Pd2+引入MIL-101-NH2孔道中,经氨硼烷还原后得到Pd@MIL-101-NH2。在三乙基硅烷与水脱氢转化为三乙基硅醇的反应中,Pd@MIL-101-NH2的转化率分别高出Pd/Al2O3和Pd/Fe2O3的20%和23%。Wang等[28]将活化后的UiO-66-NH2分散于Cu(NO3)2 ·3H2O和C2H5OH溶液中,在氙弧灯的光催化还原下,得到负载量为0.39wt. %的Cu单原子(Single-Atom,SAs)纳米限域材料Cu SAs/UiO-66-NH2。在太阳能驱动下,Cu SAs/UiO-66-NH2催化CO2转化为CH3OH和C2H5OH的产率分别为5.33和4.22 μmol/h,远高于纯UiO-66-NH2的效果。
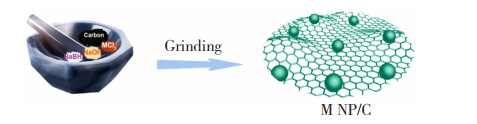
1.1.2 固体研磨法浸渍还原法制备而成的催化剂往往金属颗粒粒径较大且分散性较差。固体研磨法可以很好地解决这一问题。利用一些前体金属化合物、金属盐可挥发的特性,将金属NPs的前驱体和制备干燥后的载体框架直接混合研磨,依靠摩擦产生的热量使其挥发进入框架孔道内,最后将其还原获得负载型催化剂。宁欣[29]在室温条件下,将含Ni的前驱体硝酸镍和3种含有模板剂的介孔二氧化硅载体HMS、SBA-15和MCM-41进行混合研磨,使得Ni2+与模板剂十二胺产生较强的配位作用,而后在550 ℃空气中煅烧6 h,通过氧化镍与载体间所产生的强相互作用,制备得到Ni平均粒径分别为2.7、3.4和9.6 nm的纳米限域材料Ni@HMS、Ni@SBA-15和Ni@MCM-41。在催化1, 2-二氯乙烷气相加氢脱氯反应中,与采用浸渍还原法所制得的相同Ni负载量的Ni/SiO2催化剂相比,Ni@HMS、Ni@SBA-15和Ni@MCM-41的转化率分别提高了33.8%、21.8%和3.9%。此外,相较于Ni@SBA-15和Ni@MCM-41,Ni@HMS催化剂中Ni分布均匀且没有大的团聚颗粒,因此具有最高的催化反应活性。Wang等[30]在室温下,通过在玛瑙研钵中直接研磨混合前驱体,制备得到在各种碳载体上高度分散的Rh、Ru和Ir NPs,其合成步骤如图 3所示[30]。电化学实验表明,Rh NP/C在电流密度为10 mA/cm2时的过点位为7 mV,比商用Pt/C催化剂的28 mV小得多,其表现出优秀的析氢反应活性。这种合成方法不需要进行碳材料预处理,且不需要额外添加有机溶剂和封盖剂,不仅大大缩减了工作量,而且有利于大规模生产。
|
图 3 固体研磨法制备金属NPs/C的合成步骤示意图[30] Fig.3 Schematic diagram of synthesis steps of metal NPs/C by solid grinding method[30] |
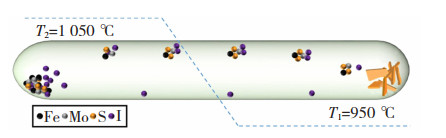
化学气相沉积(Chemical Vapor Deposition,CVD)可将金属类颗粒高温蒸发,经框架的孔道结构将金属蒸汽捕捉,最后结合光、电化学、热解等手段将其还原成NPs,从而构建出前述两种制备方法难以企及的高纯度纳米限域材料。文炎坤[31]以碳纳米纤维(Carbon Nanofibers,CNFs)为载体框架,利用静电纺丝工艺先将纺丝前驱体溶液制成Au-Mo纳米纤维膜,然后利用高温碳化和硫蒸气辅助的CVD于管式炉中构建出Au-MoS2/CNFs,由于Au和MoS2形成纳米限域晶体后,Au表面产生等离子体共振,其表现出比单一组分Au/CNFs和MoS2/CNFs更快的析氢速率和质子传输能力。Sun等[32]通过化学气相输运方法等将研磨后的含Mo、S、Fe的混合粉末通过高温蒸发使其限制在Mo晶格中,随后置于真空中得到Fe@MoS2,其合成步骤如图 4所示[32]。
1.2 “船外造瓶”的制备策略“瓶中造船”在使用中普遍存在纳米粒子不稳定且易团聚的问题。而通过将预先制备好的有稳定剂包裹的纳米活性中心直接负载到基体材料的孔道内,从而构建出目标活性中心的“船外造瓶”策略可以克服这一缺点[23]。
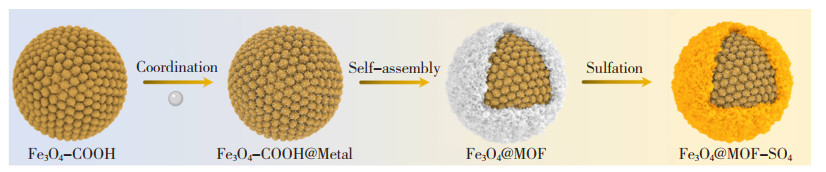
1.2.1 直接包覆法将可控活性中心与表面稳定剂混合后引入基体材料内,可构建出目标活性中心。Ahmadipouya等[33]将磁性Fe3O4 NPs引入3-(三甲氧基硅基)丙基甲基丙烯酸酯中,并与基体材料UiO-66的前体物共混溶解于DMF中,得到均匀分散且粒径减小的纳米限域材料Fe3O4@UiO-66,其合成步骤如图 5所示[33]。Alivand等[34]将Fe3O4-COOH纳米团簇分散于金属有机框架(Metal-Organic Framework,MOFs)的前体物溶液,加入含甲醇的2-MeIm溶液得到Fe3O4@MOF。将Fe3O4@MOF置于H2SO4水溶液中,利用硫酸根与金属团簇的配位可以得到Fe3O4粒径在2 ~3 nm的酸性纳米限域材料Fe3O4@MOF-SO4。研究发现,仅需0.1wt. %的Fe3O4@MOF-SO4即可使捕获CO2的能耗降低44.7%,且捕获效率比商用非均相催化剂提高了近10倍。
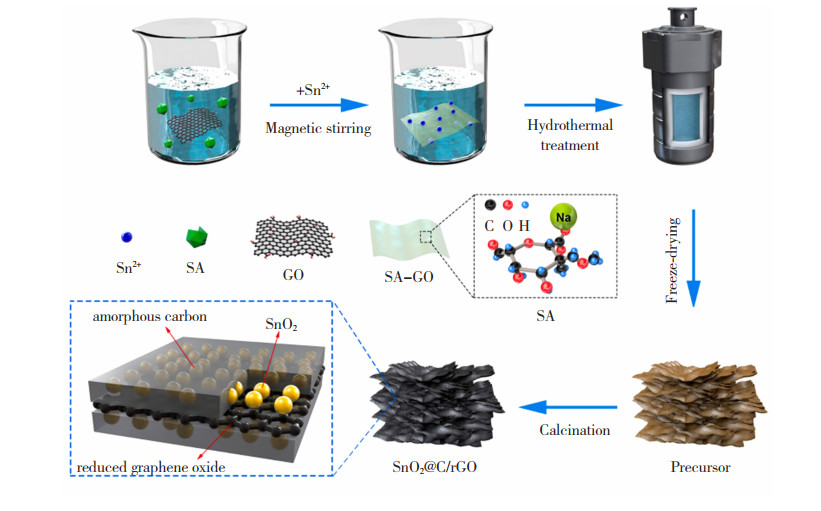
1.2.2 溶剂热法直接包覆法不易控制NPs的大小、晶型,故反应稳定性有待提高。以溶剂热法制备的纳米限域材料,其NPs的尺寸、形状都可稳定控制。Yang等[35]将含Ni(NO3)2 ·6H2O、Co(NO3)2 ·6H2O和尿素的过渡金属层状双羟基滑石(Layered Double Hydroxides,LDHs)的前驱体溶液NiCo-LDH进行超声处理,随后与含KCu7S4纳米线的C2H6O混合,得到KCu7S4 @NiCo-LDH。阻抗分析表明,电解液到KCu7S4 @NiCo-LDH电极上的质量扩散阻力相较于到KCu7S4上的扩散阻力更小,电荷转移更高效。Li等[36]将还原氧化石墨烯(reduced Graphene Oxide,rGO)和海藻酸钠溶液均匀分散在去离子水中,加入SnO2形成均相溶液,180 ℃下水热反应12 h。将所得的黑色固体在管式炉中500 ℃氩气流下煅烧1 h,得到纳米限域材料SnO2@C/rGO,其合成步骤如图 6所示[36]。由于双碳催化剂SnO2@C/rGO复合材料的ID/IG峰强度比SnO2@C和SnO2/rGO低了0.25和0.13,其表现出更强的导电性能。
1.3 “一锅制备”的制备策略“船外造瓶”虽然可均匀分散NPs,但其仍存在基体材料和纳米活性中心间晶格不匹配的问题,导致NPs无法生长,影响材料的性能和寿命。
“一锅制备”是指将纳米活性中心的前驱体和基体材料的前体物直接混合,通过控制反应的时间和温度、各种溶液的浓度以及稳定剂的类型等调控基体材料的自成核、纳米活性中心在基体材料内的封装及目标活性中心的转化三者之间的关系[23]。其可直接通过从源头上实现纳米粒子生长的精准调控,从而更有效地调控纳米限域材料的自组装过程。
Lu等[37]将含乙酰丙酮镍的油酸溶液超声处理得到乳状溶液,向其中添加正硅酸乙酯和3-氨基丙基三乙氧基硅烷混合物,在800 ℃空气中煅烧得到Ni NPs负载量为2.8wt. %且平均粒径为4.47 nm的纳米限域催化剂Ni@HSS。然而常规浸渍法合成的Ni/HSS和Ni/SiO2催化剂中平均粒径达到13.13和24.9 nm。60 h内Ni@HSS对CH4的转化率可稳定在93%左右,Ni/HSS和Ni/ SiO2则分别降至59%和56%。由于800 ℃的煅烧温度较为苛刻,Hoyos等[38]将含Ni(NO3)2 ·6H2O的C2H5OH溶液、硅酸钠和表面活性剂溶液混合后,通过调节pH值再结合微波加热,在马弗炉中823 K下煅烧6 h后得到Ni NPs含量为8.8wt. %的纳米限域材料Ni-MCM。相较于普通浸渍法得到的催化剂Ni/MCM,前者中含有的Ni NPs尺寸更小,还原性更强,与载体的相互作用更高。在甲烷干法重整反应中,Ni-MCM对CO2的转化率比
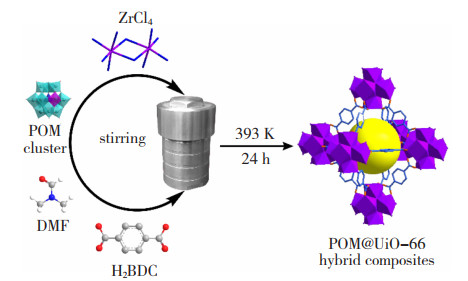
Ni/MCM提高了5%,且在937 K下工作100 h后仍能保持稳定。由于高温存在一定的易燃易爆风险,Hu等[39]将ZrCl4、HCl、H2BDC和PMo12或Co取代的PMo11Co溶于DMF中,在393 K下加热24 h得到多金属氧酸盐(Polyoxometalate,POM)的负载量分别为7.3wt. %和6.7wt. %的纳米限域材料PMo12@UiO-66和PMo11Co@UiO-66。POM@UiO-66的合成步骤如图 7所示[39]。由于PMo11Co@UiO-66的转换频率为19 h-1,高于PMo12@UiO-66的11 h-1,其表现出比PMo12@UiO-66更强的催化活性。在对氧化环辛烯环(C8H14)的催化实验中,由于POM和UiO-66间的协同作用,99%的C8H14可被PMo11Co@UiO-66选择性氧化,转化率可达52%,而UiO-66的转化率仅为3%。
根据前述的介绍,本文总结了当前纳米限域材料3种制备策略中不同制备方法的原理,具体见表 1。
| 表 1 3种制备策略中不同制备方法的原理 Table 1 Principles of different preparation methods of three preparation strategies |
纳米限域材料具有活性高、稳定性强的特点,并与流体环境间存在协同关系[40],这为研发新型环境治理和能源开发技术拓宽了新的思路。
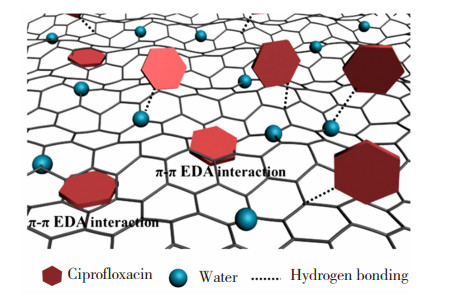
2.1 吸附水受限于纳米尺度的空间时,水的氢键网络结构被打散破坏,导致平均密度和平均氢键数减小,从而与其他分子的氢键相互作用显著增强,故此时的受限水,整体介电常数会发生变化,呈现出强化吸附物质的特性[41-43]。Liu等[44]采用“瓶中造船”策略将Pd NPs限域在具有高稳定三维阴离子拓扑结构的(Ln-OFs)[Ho2(BPTC)2][(CH3)2NH2]2的孔道内,构建出Pd NPs的负载量为2.81wt. %,且平均尺寸为1 nm的Pd@[Ho2(BPTC)2][(CH3)2NH2]2。对比发现,其对甲基蓝的吸附量可达1 413.7 mg/g,远高于GO的397 mg/g。Sun等[45]利用石墨烯水凝胶的自组装构建出纳米限域空间和受限水,通过调节氧化石墨烯(Graphene Oxide,GO)分散pH值来调节限域空间的孔径和含氧量。结果表明,疏水纳米限域条件下,随着GO分散pH值的增加,环丙沙星在石墨烯水凝胶上的吸附量从243.04 mg/g增加到442.91 mg/g,这主要是由于纳米受限水的平均氢键数较小,导致与污染物分子的氢键相互作用增强,从而暴露出更多的吸附位点[46-48],其机理如图 8所示[46]。
以沸石分子筛为代表的传统吸附剂常存在脱附和再生问题,纳米限域效应可通过加热去除吸附剂中的受限水,从而降低材料的吸附能力,促进解吸,减少化学洗脱剂的使用,实现吸附材料的绿色再生。
纳米限域材料可以有效抑制纳米颗粒的生长团聚,且其具有更大的比表面积和更丰富的活性位点,极大增加了吸附质和吸附剂活性位点的接触几率,有利于提高整体的吸附性能[49-51]。Au NPs对H2S具有吸附惰性,而在高温下易团聚,Wang等[52]采用“瓶中造船”策略,以三甲基氯硅烷为外表面钝化剂,3-氨基丙基三乙氧基硅烷为内激活剂,将直径为1.4 nm的超细Au NPs限域在MCM-48分子筛的三维介孔孔道中,合成出一种在含H2S和H2O的天然气中可完全捕获Hg0的纳米材料Au/MCM-48,其中Au负载量为0.98wt. %。相较于MCM-48对Hg0的去除效率,同样投加量条件下该材料对Hg0的去除效率提高了近70%,且能够保持10 h的高稳定性。这是由于经过三甲基氯硅烷改性的MCM-48分子筛具有良好的疏水性,纳米限域效应有效抑制了热处理过程中Au NPs的生长和团聚,从而获得优异的再生性能。Zhang等[53]采用“船外造瓶”策略将粒径范围为4.4~5.0 nm的Cu-Zn NPs限域在SBA-15分子筛的孔道内,合成出1Cu1Zn/SBA-15,其中Cu和Zn的负载量分别为14.03wt. %和14.64wt. %,且SBA的孔径随着Cu-Zn混合氧化物负载后从6~8 nm减小到3~7 nm。对比发现,低温下1Cu1Zn/SBA-15相较于SBA-15对Hg0和H2S的吸附率分别提高了90%和85%,并表现出优异的稳定和再生性能。其同时脱附Hg0和H2S的机理如图 9所示[53]。
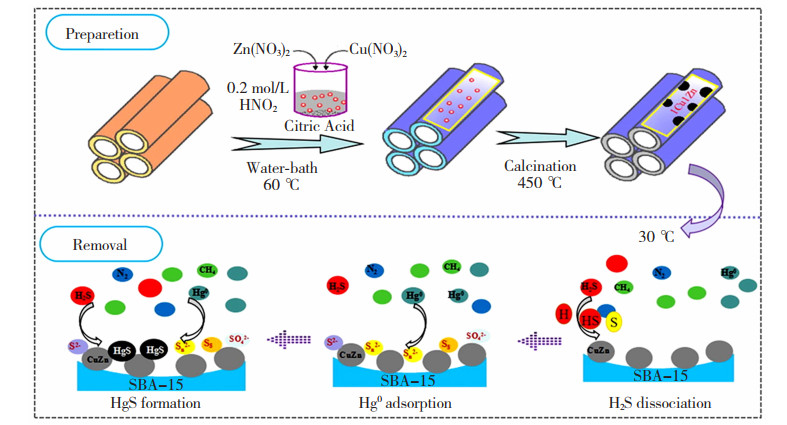
|
图 9 1Cu1Zn/SBA-15吸附剂同时脱除H2S和Hg0的机理图[53] Fig.9 Mechanism of simultaneous removal H2S and Hg0 over the 1Cu1Zn/SBA-15 absorbent[53] |
纳米限域空间会影响身处其中的晶体的晶型结构,这为研发新型吸附剂提供了新思路。控制自然水体中内源性底泥磷的释放是水体富营养化修复的关键之一。投加锁磷剂是当前公认的控制手段之一。目前, 澳大利亚CISRO组织开发的镧改性膨润土(Lanthanum Modified Bentonite, LMB)是一种颇受好评的商业锁磷剂,但使用中由于腐殖质与碳酸氢盐竞争性络合活性镧物种,导致难以稳定地发挥除磷效果[54-56]。通过采用纳米限域材料,利用含磷沉淀晶体在限域空间内热力学和动力学方面特异的晶体成核和生长方式,达到形成特定晶型的目的,从而提高与天然有机质之间的竞争速率,提高整体的除磷效果。Zhang等[57]采用“瓶中造船”策略将La氧化物NPs负载在Mg/Fe LDHs载体的纳米限域层状空间内,构建出纳米限域锁磷剂L-CMF-1.0。L-CMF-1.0的平均孔径仅为7.0 nm,比LMB的平均孔径减小了4.5 nm,其吸附PO43-的能力是LMB的5倍,对总磷(Total Phosphorus,TP)和可溶性活性磷(Soluble Active Phosphorus,SRP)的去除率分别达95.0%和98.3%,且可经38 d运行后除磷效率波动不大;而LMB对TP和SRP的去除效率仅分别为91.8%和94.7%,且经30 d运行后均告失效。研究表明,改变纳米限域材料的尺寸可以改变不同离子的亲水性、电荷密度等,从而影响其吉布斯自由能或显著调节其脱水自由能垒,形成更稳定的晶型[58-62]。Zhang等[63]采用“瓶中造船”策略在聚苯乙烯结构中的介孔孔道内限域生长出平均粒径为3.5 nm的亚稳态磷酸锆(γ-ZrP),其吸附分配系数(Kd)是正常α-ZrP的10~90倍,表现出超强的磷吸附能力,γ-ZrP及α-ZrP对Pb2+的吸附机理如图 10所示[63]。
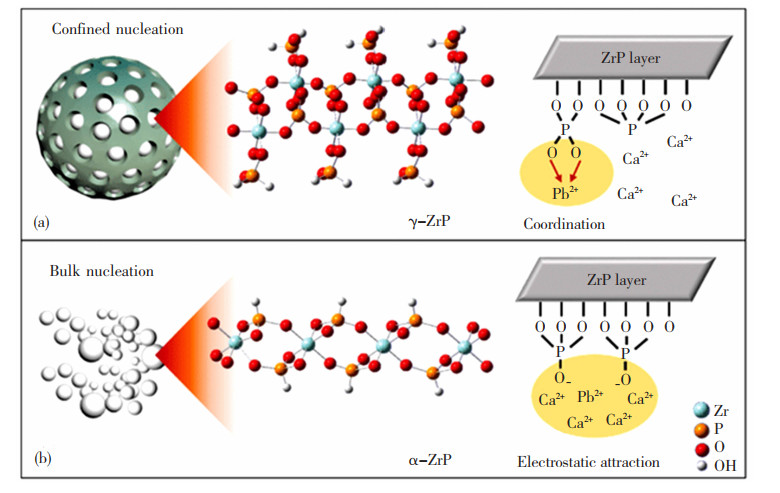
|
图 10 (a)γ-ZrP和(b) α-ZrP吸附铅离子示意图[63] Fig.10 Schematic diagram of (a)γ-ZrPand (b) α-ZrP adsorption of lead ions[63] |
这是因为正常α-ZrP的合成主要是靠非特异性静电吸引,而纳米限域使得层间空间从1.22 nm减小到0.98 nm,晶体的成核机制由非均相成核转变为均相成核,增加了自由能势垒,从而更易形成亚稳态晶型γ相[64-66]。Zhang等[67]将纳米水合氧化铁(Nano-hydrated Ferric Oxide,HFO)分散限域于交联聚苯乙烯球的纳米孔道内,合成了HFO@201。对比发现,HFO@201-CaCl2吸附体系对PO43-的吸附量比非纳米限域体系D-201-CaCl2提高了5 mg/g。由于HFO@201-CaCl2体系内除磷过程可分为单独的HFO表面吸附除磷和限域材料内部形成磷酸钙或羟基磷灰石两个步骤,所形成的羟基磷灰石可在限域材料内部独立结晶,不影响HFO外表面的磷酸盐吸附再生循环[68-69]。
虽然上述研究已经表明纳米限域效应会影响结晶,提高水处理效果,但限于实验中所适用的水质较为单一,而天然水体中的有机质常较为复杂多变,研究中单一的目标污染物往往不能涵盖实际水质,故纳米限域效应、纳米颗粒的结晶过程与污染物吸附性能之间的关系还有待深入研究。同时,对于纳米限域材料的连续性运行效果、材料使用寿命方面的研究也需进一步深入,以期可以更科学地评估其今后在工程化应用中的潜力。
2.2 高级氧化当前,高级氧化工艺降解污染物时主要凭藉的是以羟基自由基·OH或硫酸根自由基为代表的非选择性自由基。它们的产生需要投入过量氧化剂或能量输入,产生效率不高,这将造成很大的浪费。通过将目标污染物尺寸放入排斥效应或将其限制在某个特定的纳米空间内,可以有效调节氧化剂的可及性,影响活性粒子的演化过程,最终可提高选择性自由基对污染降解的贡献率[70-73]。Zhang等[74]采用“瓶中造船”策略通过简单的吸附和热转化方法将α-Fe2O3纳米团簇限域进UiO-66的孔道内,构建出限域材料α-Fe2O3@UiO-66,其在可见光的照射下对有机污染物的光催化降解效率是α-Fe2O3的23.6倍。Zhang等[75]采用“船外造瓶”策略, 利用石墨烯气凝胶(Graphene Aerogel,GA)的三维纳米空间生长出MIL-101-Fe NPs,构建出纳米限域材料GA/MIL-101-Fe。GA/MIL-101-Fe中的MIL-101-Fe NPs尺寸小于未添加基底的MIL-101-Fe NPs,因此其表现出的催化活性比MIL-101-Fe的高72.4%。这主要是因为纳米限域效应可以排除干扰,增强目标污染物的吸附密集,从而提高降解速率[76-77]。
类Fenton反应是最常用的高级氧化技术,主要通过Fe2+提供电子活化H2O2中的过氧键,生成以羟基自由基(Hydroxyl Radicals,·OH)为代表的活性粒子,用于降解难生物降解有机物[78-80]。研究发现,减小纳米限域材料的尺寸使得纳米限域与反应物、生成物、催化剂以及过渡态之间产生良好的相互作用,降低活化能势垒,从而加速降解速率,甚至可能改变反应途径[81-84]。Yang等[85]采用“瓶中造船”策略在内径为7 nm的碳纳米管(Carbon Nanotube,CNT) 孔道内均匀分散平均粒径为1.9 nm的Fe2O3 NPs,形成Fe2O3@FCNT-H;另外, 将平均粒径为2.4 nm的Fe2O3 NPs负载在CNT外表面, 构建出催化剂Fe2O3/FCNT-L。此两者被分别用于类Fenton反应中,Fe2O3@FCNT-H降解亚甲基蓝的速率比Fe2O3/FCNT-L快了22.5倍,且反应中的ROS并非以经典的·OH为主,而是以单线态氧(Singlet Oxygen,1O2)为主。Fe2O3@FCNT-H/H2O2体系的降解机理如图 11所示[85]。
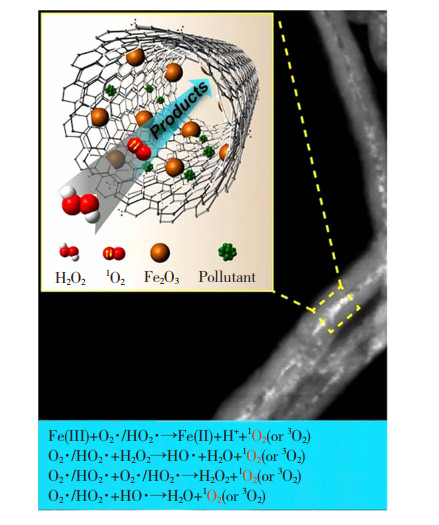
|
图 11 Fe2O3@FCNT-H/H2O2体系的污染物降解机理图[85] Fig.11 Pollutant degradation mechanism of Fe2O3@FCNT-H/H2O2 system[85] |
纳米限域效应使得碳纳米管缺电子,从而与Fe2O3 NPs产生强电子相互作用,选择性地产生1O2,在密闭空间内形成自由基的富集和有限的迁移而加速污染物的降解。Liu等[86]采用“瓶中造船”策略通过化学气相沉积法将Fe3O4 NPs均匀分散在CNT的孔道内,制备得到磁性纳米限域材料Fe3O4@CNT。催化降解四环素(Tetracycline,TL)实验中,单独Fe3O4 NPs和CNT均不能有效激活过二硫酸酯(Peroxydisulfate, PDS)降解TL,而Fe3O4@CNT可以且在40 min内TL去除率达到89.1%,比Fe3O4 NPs负载在外表面的材料Fe3O4/CNT高出49.6%。Fe3O4@CNT的表观速率常数是Fe3O4/CNT的6.3倍。猝灭实验证明,Fe3O4@CNT/PDS体系中存在除·OH之外的活性,还存在非自由基1O2。这是由于纳米限域可以提高Fe3O4 NPs的分散性和稳定性,加速Fe3O4 NPs和CNT之间的电子传递,从而增强催化剂的反应动力学,使其获得较强的电荷转移能力和氧化能力[87-89]。但由于化学环境的复杂性,目前对于纳米限域空间影响活性粒子种类演化的机制,仍限于以密度泛函为代表的量子力学计算推演并结合活性粒子捕获检测,缺乏直接有力的原位观测依据,故该研究还有待深入进行。
此外,纳米限域效应会从动力学和热力学两方面改变类Fenton反应途径,降低反应能垒[90-92]。Guo等[93]采用“瓶中造船”策略利用毛细力将Fe2O3 NPs限制在CNT侧壁内,构建出纳米复合膜电极Fe2O3-in-CNT,并制备了Fe2O3包覆在CNT外表面的Fe2O3-out-CNT。对比发现,Fe2O3-out-CNT中的优势自由基为·OH,而Fe2O3-in-CNT中优势自由基则以1O2为主, 其反应机理如图 12所示[93]。电Fenton反应体系中,Na2SiO3或Na2SO4作为电解质时,使用Fe2O3-in-CNT降解TL的效率比仅使用CNT的效率分别高出2.4和2.0倍,这是由于仅使用CNT时没有Fe2O3的参与,H2O2的氧化能力降低。此外,Fe2O3-in-CNT降解TL的伪一阶速率常数为1.02 h-1,是Fe2O3-out-CNT的1.65倍。在中性pH条件下,Fe2O3-in-CNT对TL的矿化效率比Fe2O3-out-CNT的高出8.1%。这是因为纳米限域缩短了自由基的扩散距离,使得TL在碳纳米管内大量富集,显著提高了电Fenton降解污染物的动力学过程[94-97]。在此基础上,Guo等[98]进一步利用湿化学法在CNT阴极管内封装MnOxNPs,构建出MnOx-in-CNT纳米限域复合膜电极,并在CNT的外表面包覆MnOxNPs,形成MnOx-out-CNT。对比发现,电Fenton反应体系中,MnOx-in-CNT降解双酚A的伪一阶速率常数为0.050 min-1,是MnOx-out-CNT的2.9倍。MnOx-in-CNT中占据主导地位的ROS是高价金属氧自由基Mn(IV),而MnOx-out-CNT中则是·OH和O2- ·占据主导地位。这是由于电场与纳米限域效应之间产生协同作用,使得纳米限域材料产生丰富的活性位点,增强对流的质量迁移并提高催化剂稳定性,产生更低的能量消耗。尽管在电化学领域已有一些研究证明纳米限域效应会影响离子运输和分子扩散[99-100],但纳米限域如何调控电活性分子的物理特性的方式仍有待深入探索。
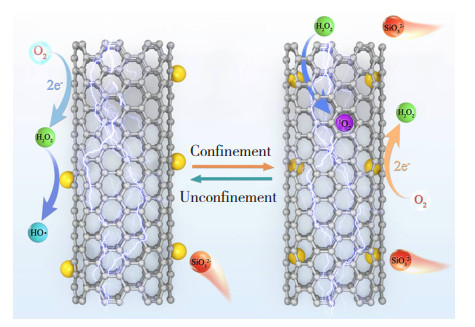
|
图 12 四环素在Fe2O3-in-CNT和Fe2O3-out-CNT体系中的降解机理图[93] Fig.12 Degradation mechanism of tetracycline in Fe2O3-in-CNT and Fe2O3-out-CNT systems[93] |
根据前述的介绍,本文总结了当前纳米限域材料在环境治理领域的应用及其具有的优势,具体见表 2。
| 表 2 纳米限域材料在环境治理中的应用 Table 2 Application of nano-confined materials in environmental governance |
本文系统梳理了当前纳米限域材料的制备方法,介绍了该材料在当前环境治理中的最新应用研究进展,展现了纳米限域效应在今后的污染治理中的广阔应用前景,但其中也反映出了一些亟需解决的问题。
纳米限域可以充分利用纳米材料比表面积大且活性位点丰富的优点,且能有效解决直接使用纳米颗粒材料所造成的易聚集失活,以及对生态系统带来的潜在生物毒性问题。然而,现有的纳米限域材料制备方法仍需要进一步优化。纳米限域材料依赖于纳米限域空间,然而,现有的制备策略大都受到操作温度和时间、外部压力以及前驱体溶液浓度的影响,因此,精确控制纳米限域空间对构建高性能的纳米限域材料具有重要意义。此外,金属NPs的晶型不同,其表现出的性能也大不相同,如何精准调控受限空间中金属NPs的晶型值得深入探索。部分纳米材料、贵重金属等经济成本较高,且会对生态环境和人体健康产生危害,如何通过更经济简便的方法得到更高效稳定的纳米限域材料值得深入研究。
虽然目前已有研究证明纳米限域效应可以提高纳米材料吸附和氧化降解污染物的性能[44-45],但仍然非常有限,因此,需要更多的研究来探索纳米限域效应,深入了解其作用机制。由于孔道的扩散限制,纳米限域吸附剂的脱附和再生方式与传统的吸附剂相比存在很大差异,其造成的使用寿命和更换周期问题仍值得进一步深入考察。而目前受限于经济成本,这方面内容还没有被探索。在纳米限域条件下,水分子会产生独特的结构,从动力学和热力学两方面,其晶体的成核和生长过程还会受到影响,最终影响目标污染物的吸附效果。然而,纳米限域效应、纳米颗粒的结晶过程与污染物吸附性能之间的关系还有待深入研究。化学反应的过程中,溶液pH值、浓度和电荷等可能会被改变,从而影响纳米活性中心的性能。此外,光、电、超声波等外部影响因素也可能参与到反应过程中,影响污染物的去除过程。但由于化学环境的复杂性,难以量化和评估纳米限域效应的贡献。纳米限域效应也在电化学领域影响着离子运输和分子扩散,使得反应物高度富集,从而影响催化反应速率。但纳米限域效应如何通过调控物理特征影响反应过程中活性粒子种类的演化还有待深入研究。此外,关于纳米限域效应的研究目前尚处于机理研究阶段,纳米限域材料的制备成本昂贵,距离实际应用还非常遥远。并且,实验室的简化环境和工程应用环境有着极大的差异,其中以各种无机离子和天然有机物为代表的杂质的影响不可忽略。因此,很有必要了解相关因素,如pH、温度等对纳米限域效应的影响。
综上所述,未来在研究具有高稳定性、高回收率、低二次污染的纳米限域材料时,还需要兼顾材料制备方法的经济性和环保性。此外,还需要进行更多深入的研究,才能深刻理解纳米限域效应下的结晶和其他化学反应机理。
| [1] |
BORETTI A, ROSA L. Reassessing the projections of the world water development report[J]. NPJ Clean Water, 2019, 2(1): 15. DOI:10.1038/s41545-019-0039-9 |
| [2] |
WANG Yubao, WEI Haoran, WANG Yuanzhu, et al. Chinese industrial water pollution and the prevention trends: An assessment based on environmental complaint reporting system (ECRS)[J]. Alexandria Engineering Journal, 2021, 60(6): 5803-5812. DOI:10.1016/j.aej.2021.04.015 |
| [3] |
LIU Biyuan, JI Jian, ZANG Boge, et al. Catalytic ozonation of VOCs at low temperature: A comprehensive review[J]. Journal of Hazardous Materials, 2022, 422: 126847. DOI:10.1016/j.jhazmat.2021.126847 |
| [4] |
邹才能, 熊波, 薛华庆, 等. 新能源在碳中和中的地位与作用[J]. 石油勘探与开发, 2021, 48(2): 480-491. ZOU Caineng, XIONG Bo, XUE Huaqing, et al. The status and role of new energy in carbon neutrality[J]. Petroleum Exploration and Development, 2021, 48(2): 480-491. DOI:10.11698/PED.2021.02.18 |
| [5] |
ZHANG Zhuowei, YU Yin, XI Hongbo, et al. Review of micro-aeration hydrolysis acidification for the pretreatment of toxic and refractory organic wastewater[J]. Journal of Cleaner Production, 2021, 317: 128343. DOI:10.1016/j.jclepro.2021.128343 |
| [6] |
AHMADI M, GHOORCHIAN A, DASHTIAN K, et al. Application of magnetic nanomaterials in electroanalytical methods: A review[J]. Talanta, 2021, 225: 121974. DOI:10.1016/j.talanta.2020.121974 |
| [7] |
ALVAREZ P J J, CHAN C K, ELIMELECH M, et al. Emerging opportunities for nanotechnology to enhance water security[J]. Nature Nanotechnology, 2018, 13(8): 634-641. DOI:10.1038/s41565-018-0203-2 |
| [8] |
ZHANG Weixian. Nanoscale iron particles for environmental remediation: An overview[J]. Journal of Nanoparticle Research, 2003, 5: 323-332. DOI:10.1023/A:1025520116015 |
| [9] |
DAS R, VECITIS C D, SCHULZE A, et al. Recent advances in nanomaterials for water protection and monitoring[J]. Chemical Society Reviews, 2017, 46(22): 6946-7020. DOI:10.1039/C6CS00921B |
| [10] |
LING Lan, ZHANG Weixian. Visualizing arsenate reactions and encapsulation in a single zero-valent iron nanoparticle[J]. Environmental Science & Technology, 2017, 51(4): 2288-2294. DOI:10.1021/acs.est.6b04315 |
| [11] |
GROMMET A B, FELLER M, KLAJN R. Chemical reactivity under nanoconfinement[J]. Nature Nanotechnology, 2020, 15(4): 256-271. DOI:10.1038/s41565-020-0652-2 |
| [12] |
QIAN Jieshu, GAO Xiang, PAN Bingcai. Nanoconfinement-mediated water treatment: From fundamental to application[J]. Environmental Science & Technology, 2020, 54(14): 8509-8526. DOI:10.1021/acs.est.0c01065 |
| [13] |
WANG Yong, MAO Jun, MENG Xianguang, et al. Catalysis with two-dimensional materials confining single atoms: Concept, design, and applications[J]. Chemical Reviews, 2018, 119(3): 1806-1854. DOI:10.1021/acs.chemrev.8b00501 |
| [14] |
FUMAGALLI L, ESFANDIAR A, FABREGAS R, et al. Anomalously low dielectric constant of confined water[J]. Science, 2018, 360(6395): 1339-1342. DOI:10.1126/science.aat4191 |
| [15] |
YE Jian, WANG Yi, LI Zhanguo, et al. 2D confinement freestanding graphene oxide composite membranes with enriched oxygen vacancies for enhanced organic contaminants removal via peroxy-monosulfate activation[J]. Journal of Hazardous Materials, 2021, 417: 126028. DOI:10.1016/j.jhazmat.2021.126028 |
| [16] |
YIN Yu, SHI Lei, LI Wenlang, et al. Boosting Fenton-like reactions via single atom Fe catalysis[J]. Environmental Science & Technology, 2019, 53(19): 11391-11400. DOI:10.1021/acs.est.9b03342 |
| [17] |
ZHANG Cuiqing, MA Yaya, LI Chengyu, et al. Spatially confined growth of Bi2O4 into hierarchical TiO2 spheres for improved visible light photocatalytic activity[J]. Journal of Materials Science, 2020, 55(8): 3181-3194. DOI:10.1007/s10853-019-04143-x |
| [18] |
ZHANG Shuo, HEDTKE T, ZHU Qianhong, et al. Membrane-confined iron oxychloride nanocatalysts for highly efficient heterogeneous Fenton water treatment[J]. Environmental Science & Technology, 2021, 55(13): 9266-9275. DOI:10.1021/acs.est.1c01391 |
| [19] |
LI Huarui, TIAN Jiayu, XIAO Feng, et al. Structure-dependent catalysis of cuprous oxides in peroxymonosulfate activation via nonradical pathway with a high oxidation capacity[J]. Journal of Hazardous Materials, 2020, 385: 121518. DOI:10.1016/j.jhazmat.2019.121518 |
| [20] |
HAN Yuhang, JIANG Bin, ZHANG Congcong, et al. Co@N-C nanocatalysts anchored in confined membrane pores for instantaneous pollutants degradation and antifouling via peroxymonosulfate activation[J]. Journal of Water Process Engineering, 2022, 47: 102639. DOI:10.1016/j.jwpe.2022.102639 |
| [21] |
包信和. 纳米限域体系的催化特性[J]. 中国科学(B辑: 化学), 2009, 39(10): 1125-1133. BAO Xinhe. Catalytic properties of nano-confined domain systems[J]. Chinese Science B: Chemistry, 2009, 39(10): 1125-1133. |
| [22] |
包信和. 催化基础理论研究发展浅析——兼述催化中的限域效应(代序)[J]. 中国科学: 化学, 2012, 42(4): 355-362. BAO Xinhe. An analysis of the development of basic theory of catalysis with reference to the domain-limiting effect in catalysis[J]. Chinese Science: Chemistry, 2012, 42(4): 355-362. DOI:10.1360/032012-130 |
| [23] |
陈小明, 张杰鹏. 金属-有机框架材料[M]. 北京: 化学工业出版社, 2017. CHEN Xiaoming, ZHANG Jiepeng. Metal-organic framework materials[M]. Beijing: Chemical Industry Press, 2017. |
| [24] |
LI Xinhe, GUO Zhiyong, XIAO Chaoxian, et al. Tandem catalysis by palladium nanoclusters encapsulated in metal-organic frameworks[J]. ACS Catalysis, 2014, 4(10): 3490-3497. DOI:10.1021/cs5006635 |
| [25] |
FENG Jian, LI Min, ZHONG Yanhui, et al. Hydrogenation of levulinic acid to γ-valerolactone over Pd@UiO-66-NH2 with high metal dispersion and excellent reusability[J]. Microporous and Mesoporous Materials, 2020, 294: 109858. DOI:10.1016/j.micromeso.2019.109858 |
| [26] |
SHI Shu, LI Yuxia, LI Shuaishuai, et al. Fabrication of Cu+ sites in confined spaces for adsorptive desulfurization by series connection double-solvent strategy[J]. Green Energy & Environment, 2022, 7(2): 345-351. DOI:10.1016/j.gee.2020.10.009 |
| [27] |
LI Luyan, LI Zhixin, YANG Weijie, et al. Integration of Pd nanoparticles with engineered pore walls in MOFs for enhanced catalysis[J]. Chem, 2021, 7(3): 686-698. DOI:10.1016/j.chempr.2020.11.023 |
| [28] |
WANG Gang, HE Chunting, HUANG Rong, et al. Photoinduction of Cu aingle atoms decorated on UiO-66-NH2 for enhanced photocatalytic reduction of CO2 to liquid fuels[J]. Journal of the American Chemical Society, 2020, 142(45): 19339-19345. DOI:10.1021/jacs.0c09599 |
| [29] |
宁欣. 限域型Ni基催化剂的制备及其在气相催化加氢脱氯中的行为研究[D]. 南京: 南京大学, 2019. NING Xin. Preparation of domain-limited Ni-based catalysts and their behavior in gas-phase catalytic hydrodechlorination[D]. Nanjing: Nanjing University, 2019. |
| [30] |
WANG Qi, MING Mei, NIU Shuai, et al. Scalable solid-state synthesis of highly dispersed uncapped metal (Rh, Ru, Ir) nanoparticles for efficient hydrogen evolution[J]. Advanced Energy Materials, 2018, 8(31): 1801698. DOI:10.1002/aenm.201801698 |
| [31] |
文炎坤. 超细碳纳米纤维限域调控贵金属@MoS2核壳纳米结构及其电催化行为[D]. 杭州: 浙江理工大学, 2018. WEN Yankun. Limit-domain modulation of noble metal@MoS2 core-shell nanostructures by ultrafine carbon nanofibers and their electrocatalytic behavior[D]. Hangzhou: Zhejiang University of Technology, 2018. |
| [32] |
SUN Yibing, LI Ruiping, SONG Chunli, et al. Origin of the improved reactivity of MoS2 single crystal by confining lattice Fe atom in peroxymonosulfate-based Fenton-like reaction[J]. Applied Catalysis B: Environmental, 2021, 298: 120537. DOI:10.1016/j.apcatb.2021.120537 |
| [33] |
AHMADIPOUYA S, HARIS M H, AHMADIJOKANI F, et al. Fe3O4@UiO-66 nanocomposite for rapid adsorption of organic dyes from aqueous solution[J]. Journal of Molecular Liquids, 2020, 322: 114910. DOI:10.1016/j.molliq.2020.114910 |
| [34] |
ALIVAND M S, MAZAHERI O, WU Yue, et al. Engineered assembly of water-dispersible nanocatalysts enables low-cost and green CO2 capture[J]. Nature Communications, 2022, 13(1): 1249. DOI:10.1038/s41467-022-28869-6 |
| [35] |
YANG Pan, JING Chuan, LIU Jingcheng, et al. Controllable crystal growth of a NiCo-LDH nanostructure anchored onto KCu7S4 nanowires via a facile solvothermal method for supercapacitor application[J]. Cryst Eng Comm, 2019, 22(9): 1602-1609. DOI:10.1039/C9CE01261C |
| [36] |
LI Hui, ZHANG Bao, ZHOU Qijie, et al. Dual-carbon confined SnO2 as ultralong-life anode for Li-ion batteries[J]. Ceramics International, 2019, 45(6): 7830-7838. DOI:10.1016/j.ceramint.2019.01.090 |
| [37] |
LU Yao, GUO Dan, RUAN Yongzhe, et al. Facile one-pot synthesis of Ni@HSS as a novel yolk-shell structure catalyst for dry reforming of methane[J]. Journal of CO2 Utilization, 2018, 24: 190-199. DOI:10.1016/j.jcou.2018.01.003 |
| [38] |
HOYOS L S, FAROLDI B M, CORNAGLIA L M. A coke-resistant catalyst for the dry reforming of methane based on Ni nanoparticles confined within rice husk-derived mesoporous materials[J]. Catalysis Communications, 2020, 135: 105898. DOI:10.1016/j.catcom.2019.105898 |
| [39] |
HU Dianwen, SONG Xiaojing, WU Shujie, et al. Solvothermal synthesis of Co-substituted phosphomolybdate acid encapsulated in the UiO-66 framework for catalytic application in olefin epoxidation[J]. Chinese Journal of Catalysis, 2021, 42(2): 356-366. DOI:10.1016/S1872-2067(20)63665-8 |
| [40] |
WANG Xuening, LI Hongchao, SHAN Chao, et al. Construction of model platforms to probe the confinement effect of nanocomposite-enabled water treatment[J]. Chemical Engineering Journal Advances, 2022, 9: 100229. DOI:10.1016/j.ceja.2021.100229 |
| [41] |
CHAKRABORTY S, KUMAR H, DASGUPTA C, et al. Confined water: Structure, dynamics, and thermodynamics[J]. Accounts of Chemical Research, 2017, 50(9): 2139-2146. DOI:10.1021/acs.accounts.6b00617 |
| [42] |
SCHLAICH A, KNAPP E W, NETZ R R. Water dielectric effects in planar confinement[J]. Physical Review Letters, 2016, 117(4): 048001. DOI:10.1103/PhysRevLett.117.048001 |
| [43] |
VARGHESE S, KANNAM S K, HANSEN J S, et al. Effect of hydrogen bonds on the dielectric properties of interfacial water[J]. Langmuir, 2019, 35(24): 8159-8166. DOI:10.1021/acs.langmuir.9b00543 |
| [44] |
LIU Yang, WANG Yuchen, YAN Hui, et al. Series of stable anionic lanthanide metal-organic frameworks as a platform for pollutant separation and efficient nanoparticle catalysis[J]. Inorganic Chemistry, 2022, 61(8): 3472-3483. DOI:10.1021/acs.inorgchem.1c03400 |
| [45] |
SUN Yiran, YU Fei, LI Cong, et al. Nano-/micro-confined water in graphene hydrogel as superadsorbents for water purification[J]. Nano-micro Letters, 2020, 12: 1-14. DOI:10.1007/s40820-019-0336-3 |
| [46] |
MA Jie, YANG Mingxuan, YU Fei, et al. Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel[J]. Scientific Reports, 2015, 5(1): 1-10. DOI:10.1038/srep13578 |
| [47] |
MA Jie, SUN Yiran, ZHANG Mingzhen, et al. Comparative study of graphene hydrogels and aerogels reveals the important role of buried water in pollutant adsorption[J]. Environmental Science & Technology, 2017, 51(21): 12283-12292. DOI:10.1021/acs.est.7b02227 |
| [48] |
LI Kun, TAO Yi, LI Zhongwu, et al. Selective ion-permeation through strained and charged graphene membranes[J]. Nanotechnology, 2017, 29(3): 035402. DOI:10.1088/1361-6528/aa9b0c |
| [49] |
WANG Liang, XU Shaodan, HE Shenxian, et al. Rational construction of metal nanoparticles fixed in zeolite crystals as highly efficient heterogeneous catalysts[J]. Nano Today, 2018, 20: 74-83. DOI:10.1016/j.nantod.2018.04.004 |
| [50] |
MALIK R, RANA P S, TOMER V K, et al. Nano gold supported on ordered mesoporous WO3/SBA-15 hybrid nanocomposite for oxidative decolorization of azo dye[J]. Microporous and Mesoporous Materials, 2016, 225: 245-254. DOI:10.1016/j.micromeso.2015.12.013 |
| [51] |
HOU Jifei, LIN Jingling, FU Heyun, et al. Vitamin B12 derived CoCNx composite confined in SBA-15 as highly effective catalyst to activate peroxymonosulfate for naproxen degradation[J]. Chemical Engineering Journal, 2020, 389: 124344. DOI:10.1016/j.cej.2020.124344 |
| [52] |
WANG Juan, ZHANG Mingzhu, LI Ge, et al. Ultrafine Au nanoparticles confined in three-dimensional mesopores of MCM-48 for efficient and regenerable Hg0 removal sorbent in H2S and H2O containing natural gas[J]. Fuel, 2021, 286: 119479. DOI:10.1016/j.fuel.2020.119479 |
| [53] |
ZHANG Huawei, WANG Juan, LIU Ting, et al. Cu-Zn oxides nanoparticles supported on SBA-15 zeolite as a novel adsorbent for simultaneous removal of H2S and Hg0 in natural gas[J]. Chemical Engineering Journal, 2021, 426: 131286. DOI:10.1016/j.cej.2021.131286 |
| [54] |
COPETTI D, FINSTERLE K, MARZIALI L, et al. Eutrophication management in surface waters using lanthanum modified bentonite: A review[J]. Water Research, 2016, 97: 162-174. DOI:10.1016/j.watres.2015.11.056 |
| [55] |
SPEARSA B M, MACKAYB E B, YASSERIC S, et al. A meta-analysis of water quality and aquatic macrophyte responses in 18 lakes treated with lanthanum modified bentonite (Phoslock®)[J]. Water Research, 2016, 97: 111-121. DOI:10.1016/j.watres.2015.08.020 |
| [56] |
ZHI Yue, CALL D F, GRIEGER K D, et al. Influence of natural organic matter and pH on phosphate removal by and filterable lanthanum release from lanthanum-modified bentonite[J]. Water Research, 2021, 202: 117399. DOI:10.1016/j.watres.2021.117399 |
| [57] |
ZHANG Yanyang, KONG Bo, SHEN Zhaoyang, et al. Phosphorus binding by lanthanum modified pyroaurite-like clay: Performance and mechanisms[J]. ACS ES&T Engineering, 2021, 1(11): 1565-1575. DOI:10.1021/acsestengg.1c00218 |
| [58] |
ZHANG Xiaolin, CHENG Cheng, QIAN Jieshu, et al. Highly efficient water decontamination by using sub-10 nm FeOOH confined within millimeter-sized mesoporous polystyrene beads[J]. Environmental Science & Technology, 2017, 51(16): 9210-9218. DOI:10.1021/acs.est.7b01608 |
| [59] |
PARIJA A, WAETZIG G R, ANDREWS J L, et al. Traversing energy landscapes away from equilibrium: Strategies for accessing and utilizing metastable phase space[J]. The Journal of Physical Chemistry C, 2018, 122(45): 25709-25728. DOI:10.1021/acs.jpcc.8b04622 |
| [60] |
QI Jiang, WARD M D. Crystallization under nanoscale confinement[J]. Chemical Society Reviews, 2014, 43(7): 2066-2079. DOI:10.1039/C3CS60234F |
| [61] |
NIE Fan, XU Wenyue, ZHANG Di, et al. 3D hierarchical local heterojunction as ultra-highly efficient Fenton-like catalyst: Mechanism of coupling the proton-coupled electron transfer under nanoconfinement effect[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107604. DOI:10.1016/j.jece.2022.107604 |
| [62] |
QU Wei, CHEN Cheng, TANG Zhuoyun, et al. Electron-rich/poor reaction sites enable ultrafast confining Fenton-like processes in facet-engineered BiOI membranes for water purification[J]. Applied Catalysis B: Environmental, 2022, 304: 120970. DOI:10.1016/j.apcatb.2021.120970 |
| [63] |
ZHANG Xiaolin, SHEN Jialin, PAN Siyuan, et al. Metastable zirconium phosphate under nanoconfinement with superior adsorption capability for water treatment[J]. Advanced Functional Materials, 2020, 30(12): 1909014. DOI:10.1002/adfm.201909014 |
| [64] |
NAVROTSKY A, MAZEINA L, MAJZLAN J. Size-driven structural and thermodynamic complexity in iron oxides[J]. Science, 2008, 319(5870): 1635-1638. DOI:10.1126/science.1148614 |
| [65] |
WANG Yunwei, ZENG Muling, MELDRUM F C, et al. Using confinement to study the crystallization pathway of calcium carbonate[J]. Crystal Growth & Design, 2017, 17(12): 6787-6792. DOI:10.1021/acs.cgd.7b01359 |
| [66] |
CHEN M A, KOCAR B D. Radium sorption to iron (hydr)oxides, pyrite, and montmorillonite: Implications for mobility[J]. Environmental Science & Technology, 2018, 52(7): 4023-4030. DOI:10.1021/acs.est.7b05443 |
| [67] |
ZHANG Yanyang, SHE Xinwei, GAO Xiang, et al. Unexpected favorable role of Ca2+ in phosphate removal by using nanosized ferric oxides confined in porous polystyrene beads[J]. Environmental Science & Technology, 2018, 53(1): 365-372. DOI:10.1021/acs.est.8b05177 |
| [68] |
CHEN Lei, LIU Fei, WU Yao, et al. In situ formation of La(OH)3-poly(vinylidene fluoride) composite filtration membrane with superior phosphate removal properties[J]. Chemical Engineering Journal, 2018, 347: 695-702. DOI:10.1016/j.cej.2018.04.086 |
| [69] |
CHEN Lei, LI Yuanzi, SUN Yibing, et al. La(OH)3 loaded magnetic mesoporous nanospheres with highly efficient phosphate removal properties and superior pH stability[J]. Chemical Engineering Journal, 2019, 360: 342-348. DOI:10.1016/j.cej.2018.11.234 |
| [70] |
STANBURY D M. The principle of detailed balancing, the iron-catalyzed disproportionation of hydrogen peroxide, and the Fenton reaction[J]. Dalton Transactions, 2022, 51(6): 2135-2157. DOI:10.1039/D1DT03645A |
| [71] |
WU Liying, SUN Zhiqiang, ZHEN Yufei, et al. Oxygen vacancy-induced nonradical degradation of organics: Critical trigger of oxygen (O2) in the Fe-Co LDH/peroxymonosulfate system[J]. Environmental Science & Technology, 2021, 55(22): 15400-15411. DOI:10.1021/acs.est.1c04600 |
| [72] |
YAO Yiyuan, WANG Chaohai, NA J, et al. Macroscopic MOF architectures: Effective strategies for practical application in water treatment[J]. Small, 2022, 18(8): 2104387. DOI:10.1002/smll.202104387 |
| [73] |
SHAHZAD A, ALI J, IFTHIKAR J, et al. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides[J]. Journal of Hazardous Materials, 2020, 392: 122316. DOI:10.1016/j.jhazmat.2020.122316 |
| [74] |
ZHANG Rongbin, DU Biao, LI Qiuchan, et al. α-Fe2O3 nanoclusters confined into UiO-66 for efficient visible-light photodegradation performance[J]. Applied Surface Science, 2019, 466: 956-963. DOI:10.1016/j.apsusc.2018.10.048 |
| [75] |
ZHANG Yuwen, LIU Fei, YANG Zhichao, et al. Weakly hydrophobic nanoconfinement by graphene aerogels greatly enhances the reactivity and ambient stability of reactivity of MIL-101-Fe in Fenton-like reaction[J]. Nano Research, 2021, 14: 2383-2389. DOI:10.1007/s12274-020-3239-1 |
| [76] |
YANG Zhichao, QIAN Jieshu, SHAN Chao, et al. Toward selective oxidation of contaminants in aqueous systems[J]. Environmental Science & Technology, 2021, 55(21): 14494-14514. DOI:10.1021/acs.est.1c05862 |
| [77] |
SHI Heng, HE Yi, LI Yubin, et al. Confined ultrasmall MOF nanoparticles anchored on a 3D-graphene network as efficient and broad pH-adaptive photo Fenton-like catalysts[J]. Environmental Science: Nano, 2022, 9(3): 1091-1105. DOI:10.1039/D1EN00944C |
| [78] |
SALIMI M, ESRAFILI A, GHOLAMI M, et al. Contaminants of emerging concern: A review of new approach in AOP technologies[J]. Environmental Monitoring and Assessment, 2017, 189(8): 414. DOI:10.1007/s10661-017-6097-x |
| [79] |
HODGES B C, CATES E L, KIM J H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials[J]. Nature Nanotechnology, 2018, 13(8): 642-650. DOI:10.1038/s41565-018-0216-x |
| [80] |
JIANG Zhenying, WANG Lingzhi, LEI Juying, et al. Photo-Fenton degradation of phenol by CdS/rGO/Fe2+ at natural pH with in situ-generated H2O2[J]. Applied Catalysis B: Environmental, 2019, 241: 367-374. DOI:10.1016/j.apcatb.2018.09.049 |
| [81] |
YANG Zhichao, YU Anqing, SHAN Chao, et al. Enhanced Fe(Ⅲ)-mediated Fenton oxidation of atrazine in the presence of functionalized multi-walled carbon nanotubes[J]. Water Research, 2018, 137: 37-46. DOI:10.1016/j.watres.2018.03.006 |
| [82] |
BRILLAS E, GARCIA-SEGURA S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule[J]. Separation and Purification Technology, 2020, 237: 116337. DOI:10.1016/j.seppur.2019.116337 |
| [83] |
XU Yanyan, WANG Yan, WAN Jinquan, et al. Reduced graphene oxide-supported metal organic framework as a synergistic catalyst for enhanced performance on persulfate induced degradation of trichlorophenol[J]. Chemosphere, 2020, 240: 124849. DOI:10.1016/j.chemosphere.2019.124849 |
| [84] |
ZHANG Shuo, SUN Meng, HEDTKE T, et al. Mechanism of heterogeneous Fenton reaction kinetics enhancement under nanoscale spatial confinement[J]. Environmental Science & Technology, 2020, 54(17): 10868-10875. DOI:10.1021/acs.est.0c02192 |
| [85] |
YANG Zhichao, QIAN Jieshu, PAN Bingcai, et al. Singlet oxygen mediate diron-based Fenton-like catalysis under nanoconfinement[J]. Proceedings of the National Academy of Sciences, 2019, 116: 6659-6664. DOI:10.1073/pnas.1819382116 |
| [86] |
LIU Biming, SONG Wenbin, ZHANG Wenwen, et al. Fe3O4@CNT as a high-effective and steady chainmail catalyst for tetracycline degradation with peroxydisulfate activation: Performance and mechanism[J]. Separation and Purification Technology, 2021, 273: 118705. DOI:10.1016/j.seppur.2021.118705 |
| [87] |
LUO Rui, LI Miaoqing, WANG Chaohai, et al. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition[J]. Water Research, 2019, 148: 416-424. DOI:10.1016/j.watres.2018.10.087 |
| [88] |
ZHANG Ming, WANG Chaohai, LIU Chao, et al. Metal-organic frame-work derived Co3O4/C@SiO2 yolk-shell nanoreactors with enhanced catalytic performance[J]. Journal of Materials Chemistry A, 2018, 6(24): 11226-11235. DOI:10.1039/C8TA03565B |
| [89] |
ZHU Shishu, JIN Chao, DUAN Xiaoguang, et al. Nonradical oxidation in persulfate activation by graphene-like nanosheets (GNS): Differentiating the contributions of singlet oxygen (1O2) and sorption-dependent electron transfer[J]. Chemical Engineering Journal, 2020, 393: 124725. DOI:10.1016/j.cej.2020.124725 |
| [90] |
ZHANG Botao, YAN Zihan, LIU Yuchun, et al. Nanoconfinement in advanced oxidation processes[J]. Critical Reviews in Environmental Science and Technology, 2023, 53(12): 1197-1228. DOI:10.1080/10643389.2022.2146981 |
| [91] |
SHI Wen, DU Dan, SHEN Bin, et al. Synthesis of Yolk-Shell structured Fe3O4@void@CdS nanoparticles: A general and effective structure design for Photo-Fenton reaction[J]. ACS Applied Materials & Interfaces, 2016, 8(32): 20831-20838. DOI:10.1021/acsami.6b07644 |
| [92] |
WANG Jing, LIU Chao, QI Junwen, et al. Enhanced heterogeneous Fenton-like systems based on highly dispersed FeO-Fe2O3 nanoparticles embedded ordered mesoporous carbon composite catalyst[J]. Environmental Pollution, 2018, 243: 1068-1077. DOI:10.1016/j.envpol.2018.09.057 |
| [93] |
GUO Dongli, LIU Yanbiao, JI Haodong, et al. Silicate-enhanced heterogeneous flow-through electro-Fenton system using iron oxides under nanoconfinement[J]. Environmental Science & Technology, 2021, 55(6): 4045-4053. DOI:10.1021/acs.est.1c00349 |
| [94] |
WU Peiwen, ZHU Wenshuai, DAI Bilian, et al. Copper nanoparticles advance electron mobility of graphene-like boron nitride for enhanced aerobic oxidative desulfurization[J]. Chemical Engineering Journal, 2016, 301: 123-131. DOI:10.1016/j.cej.2016.04.103 |
| [95] |
YANG Juan, HE Xiaoqian, DAI Jun, et al. Electron-transfer-dominated non-radical activation of peroxydisulfate for efficient removal of chlorophenol contaminants by one-pot synthesized nitrogen and sulfur codoped mesoporous carbon[J]. Environmental Research, 2021, 194: 110496. DOI:10.1016/j.envres.2020.110496 |
| [96] |
YAO Yunjin, YIN Hongyu, GAO Mengxue, et al. Electronic structure modulation of covalent organic frameworks by single-atom Fe doping for enhanced oxidation of aqueous contaminants[J]. Chemical Engineering Science, 2019, 209: 115211. DOI:10.1016/j.ces.2019.115211 |
| [97] |
DU Dan, SHI Wen, WANG Lingzhi, et al. Yolk-shell structured Fe3O4@void@TiO2 as a photo-Fenton-like catalyst for the extremely efficient elimination of tetracycline[J]. Applied Catalysis B: Environmental, 2017, 200: 484-492. DOI:10.1016/j.apcatb.2016.07.043 |
| [98] |
GUO Dongli, JIANG Shengtao, JIN Limin, et al. CNT encapsulated MnOx for enhanced flow-through electro-Fenton: The involvement of Mn(IV)[J]. Journal of Materials Chemistry A, 2022, 10(30): 15981-15989. DOI:10.1039/D2TA03445J |
| [99] |
GUO Dongli, YAO Yuan, YOU Shijie, et al. Ultrafast degradation of micropollutants in water via electro-periodate activation catalyzed by nanoconfined Fe2O3[J]. Applied Catalysis B: Environmental, 2022, 309: 121289. DOI:10.1016/j.apcatb.2022.121289 |
| [100] |
HE Hui, WANG Yuexin, LI Juan, et al. Confined conductive and light-adsorbed network in metal organic frameworks (MIL-88B(Fe)) with enhanced photo-Fenton catalytic activity for sulfamethoxazole degradation[J]. Chemical Engineering Journal, 2022, 427: 131962. DOI:10.1016/j.cej.2021.131962 |
 2024, Vol. 32
2024, Vol. 32