油页岩中灰分含量高,通常超过40%[1],其中的硅酸盐对页岩油的生成有抑制作用[2].酸洗是常见的煤、油页岩脱灰方法,Al-Harahshe等[3]发现经酸洗脱灰处理后页岩的油产率提高. Siskin等[4]发现在油页岩热解过程中矿物质会固定部分有机质,降低油收率.薛向欣等[5]发现无机矿物质的去除使干酪根的生油潜力得到提高.所以干馏前对油页岩进行酸洗脱灰处理有利于提高页岩油的产率.在酸洗过程中,由于酯的水解和傅氏反应会改变煤的化学结构[6],在油页岩酸洗脱灰过程中也存在类似的影响.通过酸洗的方法研究脱灰对油页岩有机质化学官能团的影响,掌握脱灰后有机质官能团的变化规律,对改善页岩油提取工艺,提高页岩油产率,实现油页岩的高效利用意义重大.
傅里叶变换红外光谱(FT-IR)分析方法因具有方便快速、分辨率高、不破坏被测样品结构等优点,被很多学者用于分析煤和干酪根中官能团的含量和化学结构[7-8].梁虎珍等[9]采用FT-IR详细分析了酸洗前后褐煤中脂肪族、芳香族、含氧类3种官能团的变化情况.发现酸洗对脂氢和羟基氢键有机结构影响较小,对芳香结构和含氧官能团的影响较大,此过程中发生了水解反应.近年有学者采用FT-IR研究油页岩的化学结构.谢芳芳等[10]用FT-IR考察了桦甸油页岩的结构,结果表明油页岩中有机质所含官能团主要以脂肪烃为主. Nadia等[11]对几种不同成熟度的干酪根化学结构进行了FT-IR分析,得到了干酪根成熟度与官能团之间的关系. Aboulkas等[12]使用FT-IR发现摩洛哥油页岩经HCl和HF两级酸洗后有机质的化学结构没有改变.薛向欣等[5]使用FT-IR考察了油页岩酸洗前后干酪根化学结构的的变化,结果表明随着去矿物质程度加深,干酪根中脂肪链含量先增加后减小.
本文选取山东龙口油页岩作为实验样品,研究酸洗脱灰对油页岩化学结构的影响.样品中黄铁矿含量较高,采用HCl→HF→HNO3 三级酸洗脱灰方法去除其中的矿物质,应用FT-IR分析酸洗对油页岩化学结构的影响.
1 实验材料和方法 1.1 样品制备将油页岩原样(LK)粉碎后筛分得到直径≤0.2 mm的样品,在真空干燥箱中105 ℃环境下干燥24 h后密封保存备用.
1.2 样品脱灰处理按文献[13]中的方法酸洗脱灰得到有机质试样,记为LK-O,酸洗流程见图 1.油页岩样品与酸溶液的比例为1 g:10 mL,酸纯度均为分析纯.具体步骤为:首先称量50 g油页岩置于烧杯中,向其中加入浓度为5 mol/L的HCl 500 mL,室温下搅拌24 h后抽滤,并用去离子水洗涤至中性,在60 ℃下真空干燥12 h得到去碳酸盐的样品;在去碳酸盐样品中加入质量分数40% HF和5 mol/L HCl(1:1)的混合酸溶液,按照与HCl酸洗相同的条件搅拌、洗涤、抽滤、干燥得到去硅酸盐的样品;在去硅酸盐样品中加入质量分数为20%的HNO3溶液,室温下搅拌2 h,过滤、洗涤并干燥后得到去黄铁矿的油页岩样品(LK-O).由于HNO3的强氧化性会引入羧基等官能团而造成有机质中氮、氧含量增加,所以采用低浓度的硝酸和较短的酸洗时间,以此降低油页岩中干酪根的被氧化程度[14].

|
图 1 逐级酸洗实验流程示意 Figure 1 Acid-treatment process of oil shale |
油页岩酸洗前后的工业分析(基于空气干燥基)和元素分析(基于干燥无灰基)测定结果见表 1.结果表明,经过3步酸洗脱灰,油页岩样品的灰分从原来的57.86%下降到1%以下,矿物质脱除率满足要求,说明此酸洗方法可有效脱除矿物质,得到的有机质纯度较高.
| 表 1 油页岩和酸洗后油页岩的工业分析和元素分析(质量分数) Table 1 Proximate and ultimate analysis of LK and LK-O |
油页岩灰分含量高,组成复杂,采用XRD考察酸洗前后矿物质的变化.实验采用德国Bruker公司生产的D8Focus型X射线衍射仪,电压:40 kV;电流:40 mA;扫描步径:0.03 °;步扫时间0.5 s;扫描范围:2θ=4°~90°.
1.3.3 红外光谱分析实验采用美国Bio-rad公司生产的FTS-165红外光谱仪对酸洗前后样品中的官能团进行测试.将样品和KBr在干燥箱中105 ℃下干燥24 h,取1 mg样品与200 mg KBr在玛瑙研钵中充分混匀、研磨,然后压片,扫描.仪器的分辨率设定为4 cm-1,扫描范围为400~4 000 cm-1[15].
2 实验结果与讨论 2.1 XRD分析图 2是酸洗前后油页岩的X射线衍射强度图.由图 2可知,油页岩中含有的矿物质主要有碳酸盐和硅酸盐两类,碳酸盐主要由方解石组成,硅酸盐包含二氧化硅和黏土矿物,油页岩中还含有少量的黄铁矿. LK-O的XRD图谱中表示石英、方解石、黄铁矿的矿物质吸收峰消失,在2θ=20°处出现了一个较宽的峰包,代表其中的有机质[16],上述结果表明油页岩中的矿物质通过上述3步酸洗的方法基本都被脱除,用此方法制得的有机质纯度较高.
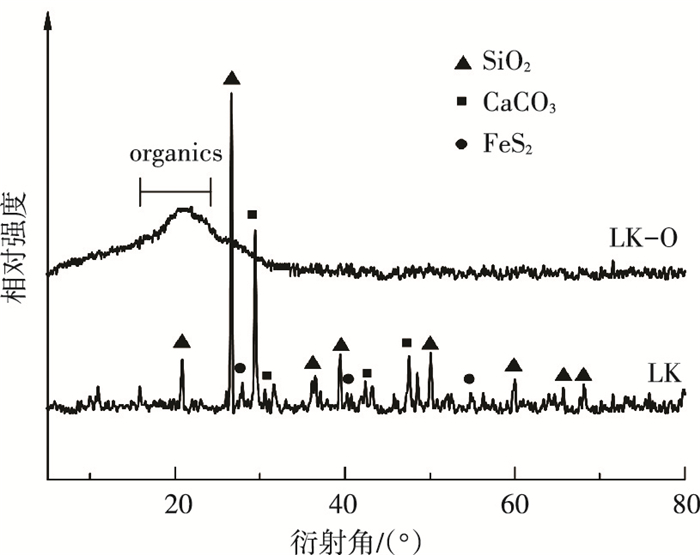
|
图 2 酸洗前后样品的XRD光谱 Figure 2 XRD patterns of LK and LK-O samples |
图 3是LK和LK-O的FT-IR测试图.图谱显示油页岩中矿物质对应的图谱很明显,说明其含量很高,甚至在一定程度上掩盖了相同峰位的有机质峰[10].其中,出现在1 170~1 060、804~780、520~480和3 620 cm-1 4个区间的吸收峰代表油页岩中的石英和黏土类矿物质(如蒙脱石、伊利石、高岭石),该类矿物质的吸收峰强度最大, 范围最广;方解石的特征吸收峰出现1 430和877 cm-1处,其峰强度和范围次之;540 cm-1代表Fe—O的振动.对比酸洗前后两种样品的红外光谱谱图发现,经酸洗处理后上述代表各类矿物质的峰均消失,与之前XRD分析结果一致,表明采用3级酸洗方法能有效地脱除油页岩中的矿物质.
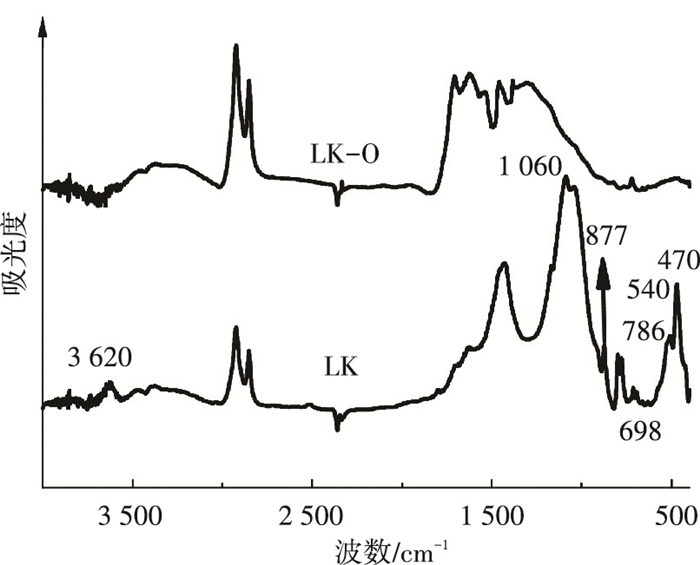
|
图 3 酸洗前后样品的红外光谱 Figure 3 FT-IR spectra of LK and LK-O samples |
参考文献[17-20]给出的红外光谱拟合过程中各类官能团代表性峰位值,使用PeakFit软件进行分段分峰拟合,拟合函数选用高斯和洛仑兹函数,并保证拟合系数在0.999以上[18, 21].
2.3.1 芳香结构的红外光谱拟合700~900 cm-1区域的谱图代表了苯环各种取代方式的官能团. 700 cm-1附近是苯环单取代, 720和750 cm-1附近是苯环二取代, 760 cm-1附近是苯环三取代,815 cm-1附近是苯环四取代,870 cm-1附近是苯环五取代[22-25]. 图 4给出了油页岩酸洗前后芳香结构的分峰拟合图,拟合参数的详细信息见表 2.
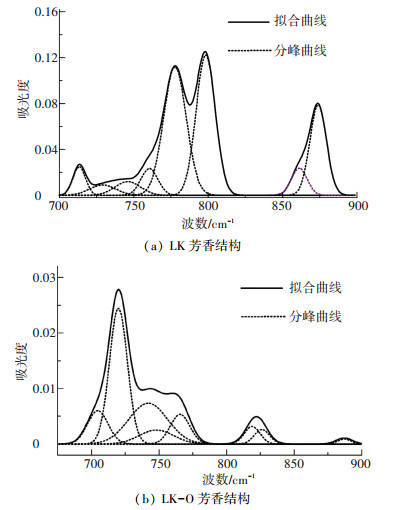
|
图 4 样品芳香结构的红外光谱 Figure 4 FTIR spectra of aromatic structure of samples |
| 表 2 LK和LK-O芳香结构红外光谱分峰拟合各吸收峰参数 Table 2 Parameters of FT-IR spectrum of aromatic structure for LK and LK-O by curving-fitting |
酸洗后700~710 cm-1附近吸收峰面积由3.99%上升到10.78%;720~750 cm-1区间吸收峰面积由6.95%上升到70.90%;750~810 cm-1区间吸收峰面积从67.49%降到9.09%;810~860 cm-1区间吸收峰面积由4.75%上升到7.77%;860~900 cm-1区间吸收峰面积由16.82%下降到1.46%.龙口油页岩酸洗过程中发生了取代反应.
2.3.2 含氧官能团的红外光谱拟合1 00~1 800 cm-1区域的谱图主要是各类含氧官能团的归属峰.峰值为1 036、1 094、1 168、1 222、1 274、1 350、1 377、1 401、1 437、1 458、1 500、1 586、1 610、1 650、1 703、1 772 cm-1处的峰位能代表典型含氧官能团的归属[22, 26]. 图 5给出了油页岩酸洗前后含氧官能团的分峰拟合图,拟合参数的详细信息见表 3.
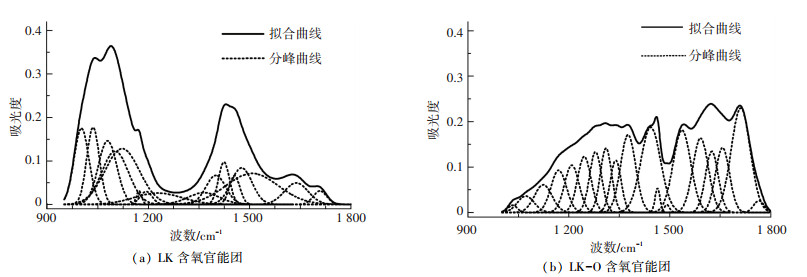
|
图 5 样品含氧官能团的红外光谱 Figure 5 FTIR spectra of oxygen-containing functional group of samples |
| 表 3 LK和LK-O含氧官能团分峰拟合各吸收峰参数 Table 3 Parameters of FT-IR spectrum of oxygen-containing functional group for LK and LK-O by curving-fitting |
油页岩中含氧官能团主要由醚氧、羧基、羟基和羰基4类组成.酸洗后1 002 cm-1附近的吸收峰消失,表明对应的无机矿物质被脱除; 1 035 cm-1附近吸收峰面积由7.96%下降到0.46%,可能是烷基醚在酸性条件下转换为酚;1 080 cm-1附近吸收峰面积由11.69%下降到1.90%,表明芳香醚含量减少;1 120 cm-1到1 340 cm-1区间吸收峰面积由22.03%上升到31.97%,可能是由于酸洗脱除了和含氧官能团结合的大量无机元素[6];1 380 cm-1、1 450 cm-1附近吸收峰面积变化不大;1 500~1 590 cm-1区间吸收峰面积由13.33%上升到18.47%;1 640 cm-1附近的吸收峰面积由4.21%上升到11.73%;1 710 cm-1附近吸收峰面积由1.50%上升到13.82%,主要是由羧酸盐向羧酸的转变造成的.油页岩中的羧酸盐通过羧基基团与无机元素(如碱和碱土金属)以离子交联的形式结合在一起,酸洗过程破坏了这种离子交联作用,使羧酸盐中的无机元素被氢取代转变为羧酸.
2.3.3 脂肪结构的红外光谱拟合2 700~3 000 cm-1区域的谱图主要归属于各类脂肪结构.代表脂肪结构的典型峰位有2 853、2 879、2 896、2 923、2 953 cm-1[27-29]. 图 6是酸洗前后脂肪结构的分峰拟合图,拟合参数的详细信息见表 4.酸洗后2 852 cm-1附近吸收峰面积由30.77%下降到25.57%,表明酸洗后对称的CH2伸缩振动减小;2 922 cm-1附近吸收峰面积由26.08%上升到52.92%,表明酸洗后不对称的CH2伸缩振动增加,约增加1倍;酸洗前2 939 cm-1附近吸收峰面积从33.05%下降到酸洗后2 959 cm-1附近吸收峰面积9.16%,表明酸洗后不对称的CH3伸缩振动吸减小;2 897 cm-1附近吸收峰面积从10.09%下降到6.92%,表明酸洗后CH伸缩振动减小;酸洗后在2 870 cm-1处出现了新的峰位,为对称的CH3伸缩振动.龙口油页岩酸洗后,CH伸缩振动和不对称的CH3伸缩振动会向不对称的CH2伸缩振动转变,脂肪链长度增加.
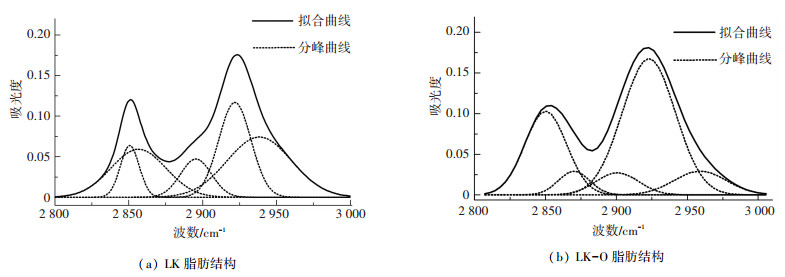
|
图 6 酸洗前后脂肪结构的红外光谱 Figure 6 FTIR spectra of aliphatic structure of samples |
| 表 4 LK和LK-O脂肪结构分峰拟合各吸收峰参数 Table 4 Parameters of FT-IR spectrum of aliphatic for LK and LK-O by curving-fitting |
3 000~3 600 cm-1区域的谱图主要归属于各类羟基结构.代表羟基结构的典型峰位有3 050、3 200、3 300、3 400、3 516、3 610 cm-1[20, 30-31].羟基在煤中属重要官能团,是形成氢键的主要官能团,氢键又是构成煤大分子结构的一种主要非共价键[10].由于油页岩中有机质与煤中有机质形成过程类似,有必要对羟基的变化作详细分析. 图 7是油页岩酸洗前后羟基的分峰拟合图,拟合参数的详细信息见表 5.酸洗后3 035和3 078 cm-1附近附近吸收峰面积变化不大且相对量很小,表明芳烃CH伸缩振动和OH—N含量很少;3 130~3 260 cm-1区间吸收峰面积从33.21%上升到38.07%;3 320~3 390 cm-1附近吸收峰面积从41.29%下降到33.21%;3 400~3 470 cm-1附近吸收峰面积从24.29%下降到24.02%.酸洗对环羟基、OH—O影响较大,对OH—OH影响很小.
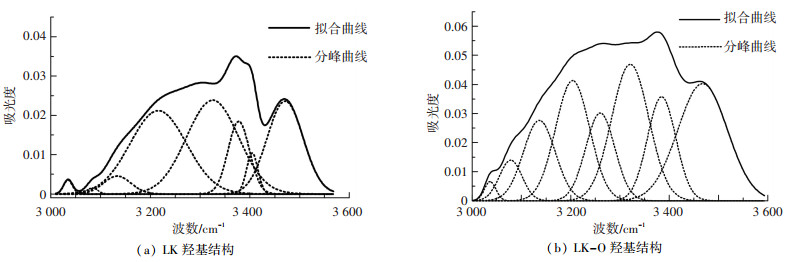
|
图 7 酸洗前后羟基结构的红外光谱 Figure 7 FTIR spectra of hydroxyl of samples |
| 表 5 LK和LK-O羟基分峰拟合各吸收峰参数 Table 5 Parameters of FT-IR spectrum of hydroxyl for LK and LK-O by curving-fitting |
1) 采用HCl→HF→HNO3三级酸洗可以脱除大部分的矿物质,得到纯度较高的有机质.
2) 龙口油页岩酸洗过程中发生了取代反应,苯环结构从以苯环三取代为主变为以苯环二取代为主;酸性条件下烷基醚可能转换为酚,羧酸盐与酸之间发生离子交换,芳香醚含量减少,羧酸含量增加.
3) 酸洗后CH伸缩振动和不对称的CH3伸缩振动会向不对称的CH2伸缩振动转变,脂肪链长度增加;酸洗对油页岩的环羟基、OH—O影响较大,对OH—OH影响很小.
| [1] |
薛晴, 宋静丽. 油页岩中有机质组成结构研究[J].
科技风, 2012(13): 235.
XUE Qing, SONG Jingli. Research of organic structure compounds in oil shale[J]. Technology Wind, 2012(13): 235. DOI: 10.3969/j.issn.1671-7341.2012.13.200 |
| [2] |
王擎, 张宏喜, 迟铭书, 等. 矿物质对桦甸油页岩热解产物影响特性[J].
燃料化学学报, 2016, 44(3): 328-334.
WANG Qing, ZHANG Hongxi, CHI Mingshu, et al. Effect of mineral matter on product evolution during pyrolysis of Huadian oil shale[J]. Journal of Fuel Chemistry and Technology, 2016, 44(3): 328-334. DOI: 10.3969/j.issn.0253-2409.2016.03.010 |
| [3] |
AL-HARAHSHEH A, AL-HARAHSHEH M, AL-OTOO M A, et al. Effect of demineralization of El-lajjun Jordanian oil shale on oil yield[J].
Fuel Processing Technology, 2009, 90(6): 818-824.
DOI: 10.1016/j.fuproc.2009.03.005 |
| [4] |
SISKIN M, BRONS G, PAYACK J F. Disruption of kerogen-mineral interactions in oil shales[J].
Energy Fuels, 1987, 1(3): 248-252.
DOI: 10.1021/ef00003a004 |
| [5] |
薛向欣, 刘艳辉, 李勇, 等. 红外光谱法研究油页岩及干酪根的生油能力[J].
东北大学学报(自然科学版), 2010, 31(9): 1292-1295.
XUE Xiangxin, LIU Yanhui, LI Yong, et al. Study on oil producibility from oil shale and kerogen by infrared spectra[J]. Journal of Northeastern University(Natural Science), 2010, 31(9): 1292-1295. DOI: 10.3969/j.issn.1005-3026.2010.09.019 |
| [6] |
LARSEN J W P C S. Effect of demineralization on the macromolecular structure of coals[J].
Energy Fuels, 1989, 3(5): 557-561.
DOI: 10.1021/ef00017a004 |
| [7] |
LIS G P, MASTALERZ M, SCHIMMELMANN A. FTIR absorption indices for thermal maturity in comparison with vitrinite reflectance R0 in type-Ⅱ kerogens from Devonian black shales[J].
Organic Geochemistry, 2005, 36(11): 1533-1552.
DOI: 10.1016/j.orggeochem.2005.07.001 |
| [8] |
WANG S, TANG Y, SCHOBERT H H, et al. FTIR and 13C NMR investigation of coal component of late permian coals from southern China[J].
Energy & Fuels, 2011, 25(12): 5672-5677.
|
| [9] |
梁虎珍, 王传格, 曾凡桂, 等. 应用红外光谱研究脱灰对伊敏褐煤结构的影响[J].
燃料化学学报, 2014, 42(2): 129-137.
LIANG Huzhen, WANG Chuange, ZENG Fangui, et al. Effect of demineralization on lignite structure from Yinmin coalfied by FT-IR investigation[J]. Journal of Fuel Chemistry and Technology, 2014, 42(2): 129-137. |
| [10] |
谢芳芳, 王泽, 宋文立, 等. 吉林桦甸油页岩及热解产物的红外光谱分析[J].
光谱学与光谱分析, 2011, 31(1): 91-94.
XIE Fangfang, WANG Ze, SONG Wenli, et al. FTIR analysis of oil shale from Huadian Jilin and their pyrolysates[J]. Spectroscopy and Spectral Analysis, 2011, 31(1): 91-94. DOI: 10.3964/j.issn.1000-0593(2011)01-0091-04 |
| [11] |
NADIA A, SCHWARZBAUER J, VOLK H, et al. Alteration of organic material during maturation: A pyrolytic and infrared spectroscopic study of isolated bisaccate pollen and total organic matter (Lower Jurassic, Hils Syncline, Germany)[J].
Organic Geochemistry, 2013, 59(59): 22-36.
DOI: 10.1016/j.orggeochem.2013.03.006 |
| [12] |
ABOULKAS A, EL HARFI K. Effects of acid treatments on Moroccan Tarfaya oil shale and pyrolysis of oil shale and their kerogen[J].
Journal of Fuel Chemistry and Technology, 2009, 37(6): 659-667.
DOI: 10.1016/S1872-5813(10)60013-8 |
| [13] |
GAI R, JIN L, ZHANG J, et al. Effect of inherent and additional pyrite on the pyrolysis behavior of oil shale[J].
Journal of Analytical and Applied Pyrolysis, 2014, 105(5): 342-347.
DOI: 10.1016/j.jaap.2013.11.022 |
| [14] |
SAXBY J D. Isolation of kerogen in sediments by chemical methods[J].
Chemical Geology, 1970, 6(3): 173-184.
|
| [15] |
李美芬. 低级煤热解模拟过程中主要气态产物的生成动力学及其机理的实验研究[D]. 太原: 太原理工大学, 2009.
LI Meifen. Experimental study on kinetics and mechanisms of the main gaseous products generation from low rank coal pyrolysis[D]. Taiyuan: Taiyuan University of Technology, 2009. http://doi.wanfangdata.com.cn/10.7666/d.d082479 |
| [16] |
YAN J, JIANG X, HAN X, et al. A TG-FTIR investigation to the catalytic effect of mineral matrix in oil shale on the pyrolysis and combustion of kerogen[J].
Fuel, 2013, 104(2): 307-317.
DOI: 10.1016/j.fuel.2012.10.024 |
| [17] |
MENG F, TAHMASEBI A, YU J, et al. Low-temperature oxidation characteristics of lignite chars from low-temperature pyrolysis[J].
Energy and Fuels, 2014, 28(9): 5612-5622.
DOI: 10.1021/ef501004t |
| [18] |
TAHMASEBI A, YU J, HAN Y, et al. Study of chemical structure changes of Chinese lignite upon drying in superheated steam, microwave, and hot air[J].
Energy & Fuels, 2012, 26(6): 405-408.
DOI: 10.1021/ef300559b |
| [19] |
TAHMASEBI A, YU J, HAN Y, et al. A study of chemical structure changes of Chinese lignite during fluidized-bed drying in nitrogen and air[J].
Fuel Processing Technology, 2012, 102(22): 85-93.
DOI: 10.1016/j.fuproc.2012.04.005 |
| [20] |
PAINTER P C, SOBKOWIAK M, YOUTCHEFF J. FT-IR. Study of hydrogen bonding in coal[J].
Fuel, 1987, 66(7): 973-978.
DOI: 10.1016/0016-2361(87)90338-3 |
| [21] |
PAINTER P C, SNYDER R W, STARSINIC M, et al. Concerning the application of FT-IR to the study of coal:a critical assessment of band assignments and the application of spectral analysis programs[J].
Applied Spectroscopy, 1981, 35(5): 475-485.
DOI: 10.1366/0003702814732256 |
| [22] |
IBARRA J, MUNOZ E, MOLINER R. FTIR study of the evolution of coal structure during the coalification process[J].
Organic Geochemistry, 1996, 24(6/7): 725-735.
DOI: 10.1016/0146-6380(96)00063-0 |
| [23] |
WANG S H, GRIFFITHS P R. Resolution enhancement of diffuse reflectance IR spectra of coals by Fourier self-deconvolution:1. C-H stretching and bending modes[J].
Fuel, 1985, 64(2): 229-236.
DOI: 10.1016/0016-2361(85)90223-6 |
| [24] |
YEN T F, WU W H, CHILINGAR G V. A study of the structure of petroleum asphaltenes and related substances by infrared spectroscopy[J].
Energy Sources, 1984, 7(3): 203-235.
DOI: 10.1080/00908318408908084 |
| [25] |
PAINTER P C, COLEMAN M M. Application of Fourier-transform infrared spectroscopy to the characterization of fractionated coal liquids[J].
Fuel, 1979, 58(4): 301-308.
DOI: 10.1016/0016-2361(79)90141-8 |
| [26] |
GEORGAKOPOULOS A. Study of low rank greek coals using FTIR spectroscopy[J].
Energy Sources, 2003, 25(10): 995-1005.
DOI: 10.1080/00908310390232442 |
| [27] |
IBARRA J, MOLINER R, BONET A J. FT-IR investigation on char formation during the early stages of coal pyrolysis[J].
Fuel, 1994, 73(6): 918-924.
DOI: 10.1016/0016-2361(94)90287-9 |
| [28] |
IBARRA J V, MUNOZ E, MOLINER R. FTIR study of the evolution of coal structure during the coalification process[J].
Organic Geochemistry, 1996, 24(6/7): 725-735.
DOI: 10.1016/0146-6380(96)00063-0 |
| [29] |
MICHAELIAN K H, FRIESEN W I. Photoacoustic FT-IR. spectra of separated western Canadian coal macerals: analysis of the CH stretching region by curve-fitting and deconvolution[J].
Fuel, 1990, 69(10): 1271-1275.
DOI: 10.1016/0016-2361(90)90288-2 |
| [30] |
MIURA K, MAE K, LI W, et al. Estimation of hydrogen bond distribution in coal through the analysis of OH stretching bands in diffuse reflectance infrared spectrum measured by in-situ technique[J].
Energy & Fuels, 2001, 15(3): 599-610.
DOI: 10.1021/ef0001787 |
| [31] |
MIURA K, MIURA K. A new method to estimate hydrogen bondings in coal by utilizing FTIR and DSC[J].
Fuel & Energy Abstracts, 1998, 39(3): 167-167.
|
 2018, Vol. 50
2018, Vol. 50


