2. 城市水资源与水环境国家重点实验室(哈尔滨工业大学), 哈尔滨 150090
2. State Key Laboratory of Urban Water Resource and Environment(Harbin Institute of Technology), Harbin 150090, China
随着中国城市建设由外延式扩张向内涵式发展的不断推进,城市生态环境保护得到了高度重视.在此过程中,污水处理设施在中国大量建设,城市污水处理率从10年前的不到50%提高至目前的90%以上,水环境得到了明显改善[1-2].在中国污水处理设施投资建设过程中,用于污泥处理处置费用仅占投资费用的20%~30%,远低于欧美的50%~60%,“重水轻泥”现象严重,致使大量污泥未得到有效处理.研究数据表明, 每处理1万t污水产生含水率80%污泥量6~9 t;据统计,目前中国每年产生的市政剩余污泥产量已超(含水率按80%计)4 000万t,预计到2020年污泥产量将达到6 000万t[3-5].
污泥中含有微生物、重金属和有机污染物等,处理不当极易造成二次污染,危及污泥最终出路(如图 1所示);同时,污泥中存在大量的N、P、K等营养元素,不加以合理利用会造成资源浪费[6-7].常见的污泥处置工艺包括焚烧、绿化、填埋和农业利用等,其中农业利用约占45%,土地填埋约占35%,还有10%未经任何处理[8].在上述处置过程中,污泥中的病原微生物、有机污染物可以通过堆肥、厌氧消化等技术得到有效降解,而重金属却随着污泥处理不断迁移和富集,给生态系统和人类健康造成极大威胁[9-10],严重制约了污泥最终的土地利用[11].

|
图 1 污水处理过程中污水中重金属在污泥中的迁移富集特征 Figure 1 Transformation of heavy metals during the biological wastewater treatment and their accumulation in sewage sludge |
研究发现,污泥胞外聚合物(EPS)是污泥中重金属最主要的吸附场所,其能够通过电中和、成键、络合等物理化学过程完成对常见重金属(锌、铜、镉、铬等)的吸附[12-14].污泥胞外聚合物是污泥有机质的主要成分,由微生物代谢产物、微生物衰亡絮体以及吸附物构成,EPS中蛋白质、多糖、腐殖酸等物质含有的羧基、羟基、磷酸基等是最主要的吸附位点.污泥EPS的吸附位点随污泥泥质和EPS提取方法的变化而呈现出显著差异,其不同重金属离子间的吸附特征亦差异明显;重金属在污泥EPS上吸附后一方面会降低水体中重金属离子的质量浓度,有利于待处理污水中金属离子质量浓度达到排放标准;但另一方面过量的重金属离子在污泥絮体上吸附后会影响污泥的活性和功能.近年来,随着污泥处理处置的日渐重视和重金属危害的广泛关注,典型重金属在污泥EPS上的吸附特征成为环境领域的研究热点之一.
1 EPS的化学组成与结构特征 1.1 EPS的化学组成污泥EPS主要由微生物絮体、附着在黏液层的有机物以及细胞衰亡产物等构成,总体上污泥EPS约占污泥总有机质的50%~90%、干物质的20%左右,是污泥中最主要有机物质[15-18].EPS主要是由以C和O为主要元素的蛋白质、腐殖酸、多糖、核酸、磷酸酯等物质构成;其中蛋白质(40%~60%)和多糖(30%)含量最高,约占EPS总量的70%~80%,是最容易被微生物有效利用的碳源和能源[19-20].
EPS的组成受污泥种类的影响,Liu et al.[19]采用不同提取方法对好氧污泥、产酸活性污泥和产甲烷污泥EPS进行了研究,发现EPS提取量基本呈现出产酸污泥>好氧污泥>产甲烷污泥,并且不同提取方法得到的EPS含量和组成存在差异,产酸污泥EPS中碳水化合物占主导地位(占62%),产甲烷污泥中蛋白质含量较高(占41%).另外,不同的废水基质对EPS各部分组成也有一定的影响,有研究表明,污泥分别在高蛋白质底物(HiP-ProS)和低蛋白质底物(LoP-ProS)条件下进行培养,EPS中蛋白质含量分别增加了13%(LoP-ProS)和28%(HiP- ProS),而多糖分别下降23%和64%[21].为了进一步揭示污泥中EPS的化学组成和分子质量分布特征,Wei et al.[13, 22]采用XAD-8/XAD-4分级技术对活性污泥、厌氧絮状污泥和厌氧颗粒污泥EPS进行了化学分级,得到亲水性有机物(HPI)、疏水性酸性有机物(HPO-A)、疏水中性有机物(HPO-N)、过渡亲水酸性有机物(TPI-A)和过渡亲水中性有机物(TPI-A)5种物质,结果显示,EPS中蛋白质、多糖均主要由疏水性酸性有机物(HPO-A)和亲水性有机物(HPI)构成,且疏水性较强的疏水性酸性有机物(HPO-A)分子质量高、呈聚合结构,而易于生物降解的亲水性有机物(HPI)组分分子质量最低.
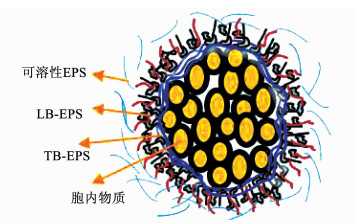
1.2 EPS的结构和功能EPS作为微生物抵御外界不良环境效应的保护层,具有网状孔隙结构,在一定程度上能够防止重金属离子等物质破坏细胞结构,起到了微生物群“细胞壁”的作用[23-25].活性污泥EPS空间结构呈现出三维凝胶状,许多学者根据EPS是否与微生物细胞结合将EPS划分为结合型EPS和溶解型EPS,前者呈现出双层结构[13];根据其与微生物细胞结合的紧密程度可将结合型EPS分为TB-EPS(紧密结合型EPS)和LB-EPS(松散结合型EPS)[26](如图 2所示).Yu et al.[27].提出以毛细管吸入时间(CST)作为EPS分层分析的一个重要指标,将污泥絮体分为上清液、黏液层、LB-EPS、TB-EPS和颗粒5部分.
|
图 2 EPS组成及结构示意 Figure 2 Schematic diagram of composition and chemical structure of sludge EPS |
当萃取污泥EPS中的LB-EPS后,污泥呈现出更加致密和坚固的网状结构,表明LB-EPS对EPS结构稳定贡献较小;而萃取TB-EPS后(可通过超声预处理进行有效提取),EPS的三维网状结构被破坏,表明TB-EPS是形成EPS致密结构的关键物质[28-30].总体上,TB-EPS性质稳定,具有很强的吸附能力和絮凝能力,能够对包括重金属在内的多种污染物进行良好的吸附[31-32].此外,组成EPS的蛋白质、腐殖酸、多糖等组分含有羟基、羧基等阴离子官能团,能够通过电中和、络合、离子交换、静电吸引等作用吸附重金属[33-34];此外,EPS具有的三维网状结构能够通过表面微絮凝、表面沉淀等作用实现对重金属的有效吸附[35](如图 3所示).

|
图 3 EPS与金属离子相互作用机制示意 Figure 3 Interaction mechanism of typical heavy metals and sludge EPS |
目前,根据EPS吸附重金属研究对象的不同可将研究方向大致归为3类,即未分级EPS吸附重金属的研究、TB-EPS型和LB-EPS型吸附重金属的研究.总体上,未分级EPS型吸附重金属的研究开展较早,研究相对深入,故将EPS型吸附重金属分为对单一重金属吸附和对多种金属共存条件下的吸附.上述研究成果探究和揭示了不同结构污泥EPS吸附重金属的特征及机制[36-37].基于国内外文献和研究成果的梳理,整理了近年来部分学者在不同反应条件下EPS吸附重金属的吸附效能及相关模型方面研究的成果,具体见表 1.
| 表 1 吸附反应条件、吸附效能及相关模型 Table 1 Reaction conditions, adsorption efficiency and related models |
Sun et al. [38]通过研究TB-EPS对Zn2+和Co2+吸附前后傅里叶红外(FT-IR)光谱谱图结构的变化,发现TB-EPS对这两种金属离子的吸附主要依靠官能团中羧基和氨基的络合作用.在此基础上,有学者进一步研究了TB-EPS中官能团结构特征对重金属吸附的影响.通过剩余污泥提取TB-EPS对Cu2+进行吸附,研究发现TB-EPS含量的增高能促进污泥对金属离子的吸附,并且在较高Cu2+质量浓度下,TB-EPS的结合位点将达到吸附伪饱和;扫描电子显微镜观察结果表明,TB-EPS粗糙的表面和长链结构能够更好地吸附Cu2+;TB-EPS中羟基(-OH)、羧基(-COOH)和酰胺(N-H)对重金属的去除起到重要作用,其中,羧基含量对重金属离子的吸附贡献较大[39].另外,EPS吸附纳米级CuO后,TB-EPS对应的三维荧光光谱图受金属离子吸附的影响较少,谱图基本未发生变化,表明大颗粒纳米颗粒吸附以物理吸附为主[40].
2.1.2 LB-EPS型重金属吸附由于LB-EPS的组成和性质不稳定,受外界条件影响较大,将污泥EPS组分中的LB-EPS分离出来进行重金属吸附的研究相对较少.目前,针对LB-EPS吸附重金属的研究可以分为吸附传统重金属以及吸附纳米级金属.Sun et al.[38]研究了好氧颗粒污泥中LB-EPS吸附对Zn2+和Co2+的吸附机制,发现LB-EPS不仅能够起到螯合剂作用,还能通过絮凝作用强化吸附过程.Miao et al.[40]通过研究MBR反应器中纳米级CuO对活性污泥EPS组成影响,发现LB-EPS是CuO与活性污泥接触和相互作用的主要场所,随着CuO浓度的不断升高,污泥EPS中的LB-EPS组分,特别是蛋白质和多糖不断增加.You et al.[41]通过研究CeO2对序批式膜生物反应器中活性污泥胞外聚合物的影响,发现加入CeO2会导致LB-EPS中蛋白质和多糖成分增加,但是LB-EPS的絮凝作用会受到抑制;三维荧光光谱显示吸附CeO2后增强的蛋白质类物质百分比,表明蛋白质对CeO2的吸附能力较弱,FT-IR光谱显示主要是EPS中羟基(-OH)和氨基(-NH2)与CeO2结合.
2.1.3 EPS型重金属吸附本部分内容将EPS型重金属吸附分为对单一金属离子的吸附和对多种金属共存条件下的吸附;其中对单一金属离子的吸附重点探究不同条件下的吸附机制和吸附模式,而对多种金属离子的吸附重点研究金属离子的性质对其吸附能力、吸附位点占有等的影响.
1) 对单一金属离子的吸附
① 对某一种金属离子的吸附研究.Li et al.[42]通过好氧颗粒污泥EPS和厌氧颗粒污泥EPS(AGS和AnGS)对Ni2+吸附的研究,发现AGS对Ni2+吸附能力小于AnGS,且AGS对Ni2+吸附符合Langmuir吸附模型,而AnGS更倾向于Freundlich型吸附;通过FT-IR光谱分析,两种EPS中的羟基和氨基是对Ni2+吸附的关键基团.Fang et al.[43]通过对EPS吸附Cu2+的研究,发现EPS基团中羧基和磷酸酯基团对Cu2+吸附贡献较大.通过活性污泥EPS吸附Cu2+的热力学特征研究,得出EPS中的蛋白质和腐殖酸都是Cu2+的强配体,Cu2+能够与其中羧基上的氧原子有效结合[44].好氧污泥EPS对Zn2+吸附后,与EPS中多糖和蛋白质相关的三维荧光光谱和FT-IR特征峰均出现下降,谱图变化显示Zn2+与羟基、氨基和羰基等官能团均发生作用;此外,蛋白质与腐殖酸会竞争吸附金属离子[45].结合离散配体模型进行拟合和拓展X射线吸收精细结构光谱(EXAFS)分析手段,可发现当EPS对Cr3+吸附时,羧基和磷酸基位点能够促进EPS对Cr3+的吸附,并形成EPS-Cr3+配体化合物[46].
② 对多种金属离子的吸附效能比较.Zhang et al.[47]通过对比有无EPS的枯草芽孢杆菌对Pb2+和Cd2+的吸附,发现EPS对Pb2+的亲和力高于对Cd2+,与蛋白质对Pb2+的结合能力高于Cd2+有关,上述两金属离子吸附后没有EPS的枯草芽孢杆菌细胞中蛋白质二级结构发生了变化,表明EPS能够在一定程度上保护细胞免受金属离子的毒性影响.通过对颗粒污泥EPS在不同pH下吸附Pb2+和Cd2+的研究,发现EPS对Pb2+的吸附能力大于Cd2+的吸附能力,并且对于两者的吸附效能差异明显,这种现象与离子交换、沉淀和表面络合等综合作用密切相关[48].Zhang et al.[49]通过对好氧污泥EPS吸附Hg2+和Sb2+的研究,发现上述两金属离子主要是与EPS中蛋白质和多糖发生吸附,其中EPS中蛋白质对Hg2+离子的吸附更为显著(发生明显荧光的淬灭),pH对该吸附过程影响显著;此外,EPS与Hg2+结合后在环境条件变化下短时间内会释放出Hg2+,发生解吸附[50].
Yin et al.利用EPS分别对Fe3+、Cu2+和Pb2+进行了吸附研究,结合紫外-可见吸收光谱和差分吸收法分析后发现金属诱导的EPS光学性质,即DAS强度随着金属质量浓度(Fe3+、Cu2+和Pb2+)发生显著变化,EPS对Cu2+和Pb2+的吸附能力大于Fe3+;EPS吸附不同重金属后将产生不同的高斯谱带,EPS的三维荧光强度在3种金属离子吸附后均出现下降,FT-IR光谱显示EPS中的官能团(如磷酸基团和羧酸基团)在吸附过程中起主要作用[51].Guibaud et al.[52]通过对EPS吸附Pb2+、Ni2+和Cd2+研究,结合SMDE极谱法分析,发现EPS对3种离子的吸附能力呈现出Pb2+>Ni2+>Cd2+;FT-IR谱图显示其吸附机制除静电吸附以外,磷酸和羧酸基团的络合作用对吸附贡献较大.基于Kurbatov模型分析,Guibaud et al. [53]发现3种不同的厌氧颗粒污泥EPS对Cd2+的吸附能力大于Pb2+,通过质子与金属离子的摩尔比分析,发现除了离子交换外,EPS络合作用参与了金属离子吸附过程.
2) 多种金属共存条件下的吸附
基于EPS吸附单个金属离子的研究,许多学者研究了不同金属离子共存下EPS对重金属吸附的特征及效能.Yang et al.[54]基于活性污泥克雷伯菌属提取EPS吸附Cu2+和Zn2+的研究,发现Cu2+的存在显著降低了EPS对Zn2+的吸附,竞争吸附显著;FT-IR结果显示,EPS中羧基和酰胺基团对上述两金属离子吸附贡献较大,且Cu2+的吸附位点与Zn2+的吸附位点重叠.采用平行因子法分析好氧和厌氧污泥EPS在Ca2+和Hg2+共存体系下的吸附作用,发现Ca2+吸附后对EPS的Zeta电位影响很大,而Hg2+对体系电位影响甚小,表明Ca2+以物理吸附为主;相对应的Hg2+以化学吸附为主(EEM谱图峰值变化显著);总体上,上述两种金属离子基本不存在竞争关系[55].
Yan et al.[56]采用牛血清蛋白、葡聚糖和腐殖酸钠3种物质配置成人工EPS,对Zn2+、Cd2+、Fe3+进行吸附,结果表明,静电作用是导致二价阳离子出现竞争吸附的主要原因,而三价阳离子比二价更容易与EPS结合,归因于三价离子与EPS结合位点会形成紧密的络合键.通过两个不同污水厂污泥EPS对Pb2+、Cu2+和Cd2+进行吸附,示差脉冲极谱法(DPP)分析结果显示EPS的pKa受pH影响较大,导致其吸附3种重金属的效能有所不同,在较低pH时,EPS的结合位点主要为羧基和磷酸基,吸附能力Pb2+> Cu2+> Cd2+;在较高pH时,EPS吸附位点是酚和氨基官能团,吸附能力Cu2+> Pb2+> Cd2+[57].
2.2 重金属在污泥中的存在形态污泥中重金属生物毒性、迁移能力以及富集能力不仅受重金属含量的影响,也受到重金属形态的影响[60].因此,研究污泥EPS中重金属吸附前后形态的变化,对减少其潜在的生物迁移与转化能力十分重要.污泥中重金属存在形态大致可以分为5种状态:可交换态、碳酸化合态、铁锰氧化物结合态、有机结合态和残渣态,其中可交换态最不稳定,迁移能力最强,而残渣态几乎不迁移[61].Zhang et al.[62]发现8种重金属离子经热水解和厌氧消化后其对应的稳定态组分出现了不同程度的增加,并且硫化物能够与重金属有效结合,增加重金属的吸附效能.通过对不同的城市污水厂中污泥各个阶段的重金属(Zn、Cu、Ni、Pb、Cd、Cr和Hg)进行研究,发现不同的污泥中金属元素含量存在差异,并且不同金属在相同污泥中迁移能力各有差别,但这几种金属元素都主要以铁锰氧化物结合态、有机结合态和残渣态存在[63].
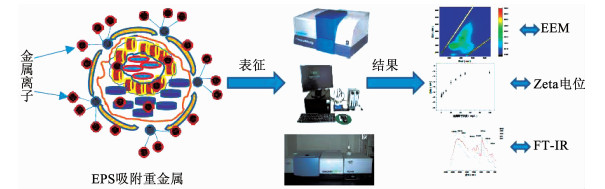
2.3 EPS吸附重金属后的表征方法重金属吸附前后EPS结构会出现显著的变化,由于EPS结构和组成均非常复杂,常需要一种或几种复杂的表征方法来对上述变化过程进行揭示.通常,EEM多用来表征EPS中蛋白质类物质、腐殖酸类物质以及可溶性微生物沥出物等的荧光物质变化,FT-IR可作为分析有机物化学基团变化的工具,扫描电子显微镜和透射电子显微镜可以得知EPS的表面结构,拉曼光谱多用于确定EPS的分子信息等(如图 4所示).随着对EPS吸附重金属研究的深入以及研究领域的不断扩展,越来越多的表征方法将用来研究ESP结构以及EPS吸附重金属的机制.近年来研究EPS及EPS吸附重金属的常用表征方法见表 2.
|
图 4 EPS吸附重金属表征方法 Figure 4 Structural characterization methods of heavy metals adsorption processes by sludge EPS |
| 表 2 常用表征方法 Table 2 Characterization methods commonly used |
不同EPS提取方法的提取效率和提取的潜在吸附位点存在差异,从而影响EPS吸附重金属效果.从污泥中最大效率地提取EPS是研究EPS吸附重金属的一个重要前提[38].目前,EPS的提取方法有很多,主要原理均为破坏细胞与EPS之间的结合来分离EPS[32, 66].EPS的定量和定性分析均依赖其提取方法,不同的提取方法可以得到不同的结论[67].通过对膜生物反应器污泥采取甲醛+氢氧化钠、离心、加热和EDTA分别提取EPS,其中膜生物反应器污泥EPS中蛋白质含量分别为:甲醛+氢氧化钠(76.74 mg/g VS)>加热(53.98 mg/g VS)>EDTA(10.49 mg/g VS)>离心(7.07 mg/g VS),而多糖含量则是甲醛+氢氧化钠(14.5 mg/g VS)>加热(7.14 mg/g VS)>离心(3.75 mg/g VS)> EDTA(1.82 mg/g VS),同时EPS中的腐殖酸含量为:甲醛+氢氧化钠(50.4 mg/g VS)> EDTA(31.06 mg/g VS)>离心(21.34 mg/g VS)>加热(9.41 mg/g VS)[68].对试验剩余污泥采用加热和离心相结合的方法分别提取TB-EPS与LB-EPS,其中TB-EPS中蛋白质含量((114.67±6.7) mg/g VS)>多糖含量((29.8±3.2) mg/g VS);但LB-EPS中蛋白质含量((17.6±3.2) mg/g VS) < 多糖含量((25.2±1.4) mg/g VS)[39].总之,EPS的提取方法可以分为两大类,即化学法、物理法,具体方法原理、优缺点如表 3所示.两类方法提取的一般步骤为:1)样品的预处理:包括样品的洗涤、均质等;2)EPS的提取;3)对提取到的EPS进行纯化和处理[13].
| 表 3 EPS的提取方法及潜在吸附位点 Table 3 Extraction method and potential adsorption site of EPS |
由于不同提取过程中采用的化学试剂和物理参数差异明显,不同提取方式获得的EPS不仅提取效率存在显著差别,潜在的吸附位点也存在一定的差异.由于EPS中几乎不含有核酸物质,提取后核酸的含量水平常作为提取过程中细胞破解的一个重要指标,基于此,有学者用紫外-可见光谱法对细胞的破解水平进行评判[69].研究发现,污泥的种类及提取时间都会对EPS的提取效率和细胞破解产生影响,一般化学法提取效率高于物理法[70].有学者在研究提取EPS时,研究离子交换树脂、硫化钠、氢氧化钠和三聚磷酸钠提取不同种类污泥,通过这4种方法按顺序提取更加高效,避免了污泥种类对提取效率的影响[71].Caudan et al.[72]结合超声和EDTA两种方法,通过超声阶段主要提取多糖物质,并在整个过程萃取蛋白质,结果表明,在提取EPS过程中采取多种方法结合的方式,EPS提取效率更高.综上,总结了不同EPS的提取方法、潜在吸附位点(见表 3)和EPS潜在吸附位点与金属离子的结合能力(见表 4).
| 表 4 EPS潜在吸附位点与金属离子的结合能力 Table 4 Capability of potential binding sites of EPS to metal ions |
进入市政污水的大量重金属不可避免对污水及污泥生物处理系统产生影响.污泥EPS作为微生物抵抗外界不良环境的保护层,通过吸附重金属能够有效降低生物毒性;但大量重金属离子在EPS上吸附后会造成EPS自身结构及功能的变化.通过对SBR反应器中投加不同质量浓度的铜离子溶液,发现当溶液中Cu2+质量浓度在2.5 mg/L及以下时,随着Cu2+质量浓度增高EPS(包括蛋白质和腐殖酸组分)含量增大,此时系统反硝化脱磷效率维持在95%左右,表明投加适当质量浓度(2.5 mg/L以下)的铜离子有助于生物系统生成EPS[79].通过研究Cu2+对SBR工艺脱氮的影响,发现投加一定含量的铜离子溶液后,硝化菌和异养菌通过增加EPS含量有效降低了Cu2+的生物毒性[80].此外,利用SBR工艺在好氧-厌氧条件下培养暴露在含有Ni2+(质量浓度为0.1~10 mg/L)废水的颗粒污泥,研究发现,随着金属离子质量浓度的增加,TB-EPS与LB-EPS的含量呈现出下降趋势,但两种EPS中的多糖组分基本没有变化,主要是蛋白质组分出现明显的降低;暴露在Ni2+中的两种EPS的三维荧光光谱谱图显示代表芳香族蛋白质和腐殖酸物质峰出现显著淬灭,尤其是LB-EPS结构变化更加明显,表明Ni2+对LB-EPS影响更大,傅里叶红外光谱从官能团结构上揭示芳香族蛋白质能够与Ni2+结合主要原因是羰基和羧基起作用[81].可见,高质量浓度的金属离子在EPS上吸附后会严重影响污泥的结构和功能.
5 结论与展望目前,EPS吸附重金属的研究已经取得一定进展,但是对于EPS的提取、分类以及EPS吸附重金属的机制研究还是不够明晰,所以,笔者认为EPS吸附重金属的研究可以从以下3点考虑:
1) 目前,EPS吸附重金属主要通过离子交换、络合以及表面沉淀3种作用机制完成,但是不同重金属吸附过程中3种机制作用的主次顺序尚未确定;所以,对于同一体系中不同重金属离子的吸附过程,可以尝试通过建立吸附模型,为后续应用EPS吸附重金属的实际应用创造理论基础.
2) EPS的成分很大程度影响着EPS吸附重金属的吸附效能、机制,所以,在研究EPS吸附重金属的过程中,EPS的提取、分类十分重要,但是目前对于EPS的提取效率仍缺乏统一标准,建立评价提取EPS方法的优劣体系可以作为一个研究重点;虽然EPS可以划分为TB-EPS与LB-EPS,但是可以在此基础上根据其组成进一步划分,为充分研究EPS吸附重金属的机制奠定基础.
3) EPS吸附重金属的研究一般采用是将污泥中EPS提取出来,对单一重金属的体系或者多种重金属共存体系的研究,缺乏实际反应器中污泥EPS对重金属吸附的研究.因此,可以结合反应器的培养过程中重金属对污泥EPS的影响,揭示实际运行条件下污泥吸附重金属的效能和反应机制.
| [1] |
郭伟杰, 刘燕. 我国城市污水处理现状及发展趋势[J]. 科技风, 2017(5): 146. DOI:10.19392/j.cnki.1671-7341.201705133 |
| [2] |
祝妍华. 我国城市污水处理事业的现状及发展趋势[J]. 江苏环境科技, 2008, 21(a01): 117. ZHU Yanhua. Present situation and future of our country' s wastewater treatment cause[J]. Jiangsu Environmental Science and Technology, 2008, 21(a01): 117. |
| [3] |
戴晓虎. 我国城镇污泥处理处置现状及思考[J]. 给水排水, 2012, 38(2): 1. DOI:10.13789/j.cnki.wwe1964.2012.02.006 |
| [4] |
杨金满, 贾瑞宝. 城市污泥资源化利用研究进展[J]. 工业用水与废水, 2011, 42(5): 1. YANG Jinman, JIA Ruibao. Research progress of resource utilization of urban sewage sludge[J]. Industrial Water & Wastewater, 2011, 42(5): 1. |
| [5] |
XUE S, ZHAO Q, WEI L, et al. Reduction of dissolved organic matter in secondary municipal effluents by enhanced coagulation[J]. Environmental Progress & Sustainable Energy, 2015, 34(3): 751. DOI:10.1002/ep.12060 |
| [6] |
王菲, 杨国录, 刘林双, 等. 城市污泥资源化利用现状及发展探讨[J]. 南水北调与水利科技, 2013(2): 99. WANG Fei, YANG Guolu, LIU Linshuang, et al. Research on development and utilization of municipal sewage sludge[J]. South-to-North Water Transfers and Water Science & Technology, 2013(2): 99. |
| [7] |
王厚成. 城市污泥生命周期内重金属变化及对土壤污染植物修复的影响研究[D]. 兰州: 兰州大学, 2017. WANG Houcheng. Changs of heavy metals during life cycle of municipal sludge and effects on phytoremediation of contaminated soil[D]. Lanzhou: Lanzhou University, 2017. |
| [8] |
张冬, 董岳, 黄瑛, 等. 国内外污泥处理处置技术研究与应用现状[J]. 环境工程, 2015(Sup1): 600. ZHANG Dong, DONG Yue, HUANG Ying, et al. Research and application of situation of sludge treatment and disposal technology at home and abroad[J]. Chinese Journal of Environmental Engineering, 2015(Sup1): 600. |
| [9] |
陈超, 袁建伟, 张晓, 等. 剩余污泥重金属污染去除研究进展[J]. 资源节约与环保, 2017(8): 8. DOI:10.16317/j.cnki.12-1377/x.2017.08.005 |
| [10] |
WEI L, QIN K, ZHAO Q, et al. Utilization of artificial recharged effluent for irrigation: pollutants' removal and risk assessment[J]. Journal of Water Reuse and Desalination, 2017, 7(1): 77. DOI:10.2166/wrd.2016.134 |
| [11] |
黄晨悦, 郭盾, 薛崇灏, 等. 污泥中重金属的稳定及去除技术研究[J]. 化工技术与开发, 2017, 46(8): 46. HUANG Chenyue, GUO Dun, XUE Chongqin, et al. Study on stabilization and removal of heavy metals from sewage sludge[J]. Technology & Development of Chemical Industry, 2017, 46(8): 46. |
| [12] |
WEI L, LI Y, NOGUERA D R, et al. Adsorption of Cu2+and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism[J]. Journal of Hazardous Materials, 2017, 321: 473. DOI:10.1016/j.jhazmat.2016.05.016 |
| [13] |
SHENG G, YU H, LI X. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review[J]. Biotechnology Advances, 2010, 28(6): 882. DOI:10.1016/j.biotechadv.2010.08.001 |
| [14] |
XUE S, ZHAO Q, WEI L, et al. Fluorescence spectroscopic studies of the effect of granular activated carbon adsorption on structural properties of dissolved organic matter fractions[J]. Frontiers of Environmental Science & Engineering, 2012, 6(6): 784. DOI:10.1007/s11783-012-0436-5 |
| [15] |
NI B, FANG F, XIE W, et al. Characterization of extracellular polymeric substances produced by mixed microorganisms in activated sludge with gel-permeating chromatography, excitation-emission matrix fluorescence spectroscopy measurement and kinetic modeling[J]. Water Research, 2009, 43(5): 1350. DOI:10.1016/j.watres.2008.12.004 |
| [16] |
SHENG G, XU J, LI W, et al. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge[J]. Chemosphere, 2013, 93(7): 1436. DOI:10.1016/j.chemosphere.2013.07.076 |
| [17] |
宋悦, 魏亮亮, 赵庆良, 等. 活性污泥胞外聚合物的组成与结构特点及环境行为[J]. 环境保护科学, 2017, 43(2): 35. SONG Yue, WEI Liangliang, ZHAO Qingliang, et al. Chemical structure and environmental behavior of extracellular polymeric substances in sludge: A review[J]. Environmental Protection Science, 2017, 43(2): 35. DOI:10.16803/j.cnki.issn.1004-6216.2017.02.007 |
| [18] |
NIELSEN P H, FROLUND B, KEIDING K. Changes in the composition of extracellular polymeric substances in activated sludge during anaerobic storage[J]. Applied Microbiology and Biotechnology, 1996, 44(6): 823. DOI:10.1007/BF00178625 |
| [19] |
LIU H, FANG H H P. Extraction of extracellular polymeric substances (EPS) of sludges[J]. Journal of Biotechnology, 2002, 95(3): 249. DOI:10.1016/S0168-1656(02)00025-1 |
| [20] |
WEI L, WANG K, KONG X, et al. Application of ultra-sonication, acid precipitation and membranefiltration for co-recovery of protein and humic acid from sewage sludge[J]. Frontiers of Environmental Science & Engineering, 2016, 10(2): 327. DOI:10.1007/s11783-014-0763-9 |
| [21] |
WANG B, LIU X, CHEN J, et al. Composition and functional group characterization of extracellular polymeric substances (EPS) in activated sludge: The impacts of polymerization degree of proteinaceous substrates[J]. Water Research, 2018, 129: 133. DOI:10.1016/j.watres.2017.11.008 |
| [22] |
WEI L, ZHAO Q, XUE S, et al. Removal and transformation of dissolved organic matter in secondary effluent during granular activated carbon treatment[J]. Journal of Zhejiang University-Science A, 2008, 9(7): 994. DOI:10.1631/jzus.A071508 |
| [23] |
郑蕾. 活性污泥胞外聚合物吸附重金属效能与机制研究[D]. 哈尔滨: 哈尔滨工业大学, 2006. ZHENG Lei. Research on efficiency and mechanisms of heavy metal adsorption by EPS from activated sludge[D]. Harbin: Harbin Institute of Technology, 2006. |
| [24] |
WEI L, WANG G, JIANG J, et al. Co-removal of phosphorus and nitrogen with commercial 201 x 7 anion exchange resin during tertiary treatment of WWTP effluent and phosphate recovery[J]. Desalination and Water Treatment, 2015, 56(6): 1633. DOI:10.1080/19443994.2014.951966 |
| [25] |
WEI L, AN X, WANG S, et al. Effect of hydraulic retention time on deterioration/restarting of sludge anaerobic digestion:Extracellular polymeric substances and microbial response[J]. Bioresource Technology, 2017, 244(1): 261. DOI:10.1016/j.biortech.2017.07.110 |
| [26] |
NIELSEN P J A. Extraction of EPS[J]. Microbial Extracellular Polymeric Substances, 1999, 49. |
| [27] |
YU G, HE P, SHAO L, et al. Stratification structure of sludge flocs with implications to dewaterability[J]. Environmental Science & Technology, 2008, 42(21): 7944. DOI:10.1021/es8016717 |
| [28] |
ERDINCLER A, VESILIND P A. Effect of sludge water distribution on the liquid-solid separation of a biological sludge[J]. J Environ Sci Health A Tox Hazard Subst Environ Eng, 2003, 38(10): 2391. DOI:10.1081/ESE-120023439 |
| [29] |
LI X Y, YANG S F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge[J]. Water Research, 2007, 5(41): 1022. DOI:10.1016/j.watres.2006.06.037 |
| [30] |
YUAN D, WANG Y, QIAN X. Variations of internal structure and moisture distribution in activated sludge with stratified extracellular polymeric substances extraction[J]. International Biodeterioration & Biodegradation, 2017, 116: 1. DOI:10.1016/j.ibiod.2016.09.012 |
| [31] |
GAO J, ZHANG Q, WANG J, et al. Contributions of functional groups and extracellular polymeric substances on the biosorption of dyes by aerobic granules[J]. Bioresource Technology, 2011, 102(2): 805. DOI:10.1016/j.biortech.2010.08.119 |
| [32] |
LIANG Z, LI W, YANG S, et al. Extraction and structural characteristics of extracellular polymeric substances (EPS), pellets in autotrophic nitrifying biofilm and activated sludge[J]. Chemosphere, 2010, 81(5): 626. DOI:10.1016/j.chemosphere.2010.03.043 |
| [33] |
LIU W, ZHANG J, JIN Y, et al. Adsorption of Pb(Ⅱ), Cd(Ⅱ) and Zn(Ⅱ) by extracellular polymeric substances extracted from aerobic granular sludge: Efficiency of protein[J]. Journal of Environmental Chemical Engineering, 2015, 3(2): 1223. DOI:10.1016/j.jece.2015.04.009 |
| [34] |
JIANG J, ZHAO Q, WEI L, et al. Extracellular biological organic matters in microbial fuel cell using sewage sludge as fuel[J]. Water Research, 2010, 44(7): 2163. DOI:10.1016/j.watres.2009.12.033 |
| [35] |
LI W, YU H. Insight into the roles of microbial extracellular polymer substances in metal biosorption[J]. Biotechnology & Bioengineering, 2014, 160(SupI): 15. DOI:10.1016/j.biortech.2013.11.074 |
| [36] |
LIU H, FANG H. Characterization of electrostatic binding sites of extracellular polymers by linear programming analysis of titration data[J]. Biotechnology & Bioengineering, 2002, 80(7): 806. DOI:10.1002/bit.10432 |
| [37] |
ISMAIL S B, DE LA P C J, TEMMINK H, et al. Extracellular polymeric substances (EPS) in upflow anaerobic sludge blanket (UASB) reactors operated under high salinity conditions[J]. Water Research, 2010, 44(6): 1909. DOI:10.1016/j.watres.2009.11.039 |
| [38] |
SUN X, WANG S, ZHANG X, et al. Spectroscopic study of Zn2+ and Co2+ binding to extracellular polymeric substances (EPS) from aerobic granules[J]. Journal of Colloid and Interface Science, 2009, 335(1): 11. DOI:10.1016/j.jcis.2009.03.088 |
| [39] |
MIAO L, WANG C, HOU J, et al. Contributions of different fractions of extracellular polymeric substances from waste-activated sludge to Cu(Ⅱ) biosorption[J]. Desalination and Water Treatment, 2016, 57(45): 21405. DOI:10.1080/19443994.2015.1120686 |
| [40] |
MIAO L, WANG C, HOU J, et al. Response of wastewater biofilm to CuO nanoparticle exposure in terms of extracellular polymeric substances and microbial community structure[J]. Science of the Total Environment, 2017, 579: 588. DOI:10.1016/j.scitotenv.2016.11.056 |
| [41] |
YOU G, HOU J, XU Y, et al. Effects of CeO2 nanoparticles on production and physicochemical characteristics of extracellular polymeric substances in biofilms in sequencing batch biofilm reactor[J]. Bioresource Technology, 2015, 194: 91. DOI:10.1016/j.biortech.2015.07.006 |
| [42] |
LI N, WEI D, WANG S, et al. Comparative study of the role of extracellular polymeric substances in biosorption of Ni(Ⅱ) onto aerobic/anaerobic granular sludge[J]. Journal of Colloid and Interface Science, 2017, 490: 754. DOI:10.1016/j.jcis.2016.12.006 |
| [43] |
FANG L, WEI X, CAI P, et al. Role of extracellular polymeric substances in Cu(Ⅱ) adsorption on Bacillus subtilis and Pseudomonas putida[J]. Bioresource Technology, 2011, 102(2): 1137. DOI:10.1016/j.biortech.2010.09.006 |
| [44] |
SHENG G, XU J, LUO H, et al. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge[J]. Water Research, 2013, 47(2): 607. DOI:10.1016/j.watres.2012.10.037 |
| [45] |
WEI D, LI M, WANG X, et al. Extracellular polymeric substances for Zn (Ⅱ) binding during its sorption process onto aerobic granular sludge[J]. Journal of Hazardous Materials, 2016, 301: 407. DOI:10.1016/j.jhazmat.2015.09.018 |
| [46] |
KANTAR C, DEMIRAY H, DOGAN N M, et al. Role of microbial exopolymeric substances (EPS) on chromium sorption and transport in heterogeneous subsurface soils: I. Cr(Ⅲ) complexation with EPS in aqueous solution[J]. Chemosphere, 2011, 82(10): 1489. DOI:10.1016/j.chemosphere.2011.01.009 |
| [47] |
ZHANG P, FANG F, CHEN Y, et al. Composition of EPS fractions from suspended sludge and biofilm and their roles in microbial cell aggregation[J]. Chemosphere, 2014, 117: 59. DOI:10.1016/j.chemosphere.2014.05.070 |
| [48] |
D'ABZAC P, BORDAS F, VAN Hullebusch E, et al. Effects of extraction procedures on metal binding properties of extracellular polymeric substances (EPS) from anaerobic granular sludges[J]. Colloids and Surfaces B-Biointerfaces, 2010, 80(2): 161. DOI:10.1016/j.colsurfb.2010.05.043 |
| [49] |
ZHANG D, LEE D, PAN X. Desorption of Hg(Ⅱ) and Sb(Ⅴ) on extracellular polymeric substances: Effects of pH, EDTA, Ca(Ⅱ) and temperature shocks[J]. Bioresource Technology, 2013, 128: 711. DOI:10.1016/j.biortech.2012.10.089 |
| [50] |
ZHANG D, PAN X, MOSTOFA K M G, et al. Complexation between Hg(Ⅱ) and biofilm extracellular polymeric substances: An application of fluorescence spectroscopy[J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 359. DOI:10.1016/j.jhazmat.2009.10.011 |
| [51] |
YIN C, MENG F, MENG Y, et al. Differential ultraviolet-visible absorbance spectra for characterizing metal ions binding onto extracellular polymeric substances in different mixed microbial cultures[J]. Chemosphere, 2016, 159: 267. DOI:10.1016/j.chemosphere.2016.05.089 |
| [52] |
GUIBAUD G, COMTE S, BORDAS F, et al. Comparison of the complexation potential of extracellular polymeric substances (EPS), extracted from activated sludges and produced by pure bacteria strains, for cadmium, lead and nickel[J]. Chemosphere, 2005, 59(5): 629. DOI:10.1016/j.chemosphere.2004.10.028 |
| [53] |
GUIBAUD G, BHATIA D, D'ABZAC P, et al. Cd(Ⅱ) and Pb(Ⅱ) sorption by extracellular polymeric substances (EPS) extracted from anaerobic granular biofilms: Evidence of a pH sorption-edge[J]. Journal of the Taiwan Institute of Chemical Engineering, 2012, 43(3): 444. DOI:10.1016/j.jtice.2011.12.007 |
| [54] |
YANG J, WEI W, PI S, et al. Competitive adsorption of heavy metals by extracellular polymeric substances extracted from Klebsiella sp J1[J]. Bioresource Technology, 2015, 196: 533. DOI:10.1016/j.biortech.2015.08.011 |
| [55] |
SHENG G, XU J, LI W, et al. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge[J]. Chemosphere, 2013, 93(7): 1436. DOI:10.1016/j.chemosphere.2013.07.076 |
| [56] |
YAN P, XIA J, CHEN Y, et al. Thermodynamics of binding interactions between extracellular polymeric substances and heavy metals by isothermal titration microcalorimetry[J]. Bioresource Technology, 2017, 232: 354. DOI:10.1016/j.biortech.2017.02.067 |
| [57] |
COMTE S, GUIBAUD G, BAUDU M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values[J]. Journal of Hazardous Materials, 2008, 151(1): 185. DOI:10.1016/j.jhazmat.2007.0.070 |
| [58] |
ZHANG Z, ZHOU Y, ZHANG J, et al. Copper (Ⅱ) adsorption by the extracellular polymeric substance extracted from waste activated sludge after short-time aerobic digestion[J]. Environmental Science and Pollution Research, 2014, 21(3): 2132D. DOI:10.1007/s11356-013-2078-y |
| [59] |
ZHANG Z, ZHANG J. Characteristics of cadmium(Ⅱ) adsorbed by the extracellular polymeric substance extracted from waste-activated sludge after short-time aerobic digestion[J]. Water Air and Soil Pollution, 2014, 225(18502). DOI:10.1007/s11270-013-1850-4 |
| [60] |
HE M, TIAN G, LIANG X. Phytotoxicity and speciation of copper, zinc and lead during the aerobic composting of sewage sludge[J]. Journal of Hazardous Materials, 2009, 163(2-3): 671. DOI:10.1016/j.jhazmat.2008.07.013 |
| [61] |
TESSIER A C P G C. Sequential extraction procedure for the speciation of particulate trace metals[J]. Analytical Chemistry, 1979, 51(7): 844. DOI:10.1021/ac50043a017 |
| [62] |
ZHANG Q, ZHANG L, SANG W, et al. Chemical speciation of heavy metals in excess sludge treatment by thermal hydrolysis and anaerobic digestion process[J]. Desalination and Water Treatment, 2016, 57(27): 12770. DOI:10.1080/19443994.2015.1055518 |
| [63] |
TYTLA M, WIDZIEWICZ K, ZIELEWICZ E. Heavy metals and its chemical speciation in sewage sludge at different stages of processing[J]. Environmental Technology, 2016, 37(7): 899. DOI:10.1080/09593330.2015.1090482 |
| [64] |
ZHANG P, FANG F, CHEN Y, et al. Composition of EPS fractions from suspended sludge and biofilm and their roles in microbial cell aggregation[J]. Chemosphere, 2014, 117: 59. DOI:10.1016/j.chemosphere.2014.05.070 |
| [65] |
ZHANG P, CHEN Y, PENG M, et al. Extracellular polymeric substances dependence of surface interactions of Bacillus subtilis with Cd2+ and Pb2+: An investigation combined with surface plasmon resonance and infrared spectra[J]. Colloids and Surfaces B-Biointerfaces, 2017, 154: 357. DOI:10.1016/j.colsurfb.2017.03.046 |
| [66] |
ABZAC P, BORDAS F, VAN HULLEBUSCH E, et al. Extraction of extracellular polymeric substances(EPS) from anaerobic granular sludges: Comparison of chemical and physical extraction protocols[J]. Applied Microbiology and Biotechnology, 2010, 85(5): 1589. DOI:10.1007/s00253-009-2288-x |
| [67] |
JIA F, YANG Q, HAN J, et al. Modeling optimization and evaluation of tightly bound extracellular polymeric substances extraction by sonication[J]. Applied Microbiology & Biotechnology, 2016, 100(19): 1. DOI:10.1007/s00253-016-7748-5 |
| [68] |
ZHANG L, REN H, DING L. Comparison of extracellular polymeric substances (EPS) extraction from two different activated sludges[J]. Water Science & Technology, 2012, 66(7): 1558. DOI:10.2166/wst.2012.295 |
| [69] |
SHENG G P, YU H Q, YU Z. Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila[J]. Applied Microbiology & Biotechnology, 2005, 67(1): h125. DOI:10.1007/s00253-004-1704-5 |
| [70] |
SUN M, LI W, YU H, et al. A novel integrated approach to quantitatively evaluate the efficiency of extracellular polymeric substances (EPS) extraction process[J]. Applied Microbiology and Biotechnology, 2012, 96(6): 1577. DOI:10.1007/s00253-012-4478-1 |
| [71] |
SUN L P, CHEN L, GUO W Z, et al. Extraction of extracellular polymeric substances in activated sludge using sequential extraction[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(8): 1448. DOI:10.1002/jctb.4449 |
| [72] |
CAUDAN C, FILALI A, LEFEBVRE D, et al. Extracellular polymeric substances (EPS) from aerobic granular sludges: Extraction, fractionation, and anionic properties[J]. Applied Biochemistry and Biotechnolohy, 2012, 166(7): 1685. DOI:10.1007/s12010-012-9569-z |
| [73] |
PARK C, NOVAK J T. Characterization of activated sludge exocellular polymers using several cation-associated extraction methods[J]. Water Research, 2007, 41(8): 1679. DOI:10.1016/j.watres.2007.01.031 |
| [74] |
PARK C, NOVAK J T, HELM R F, et al. Evaluation of the extracellular proteins in full-scale activated sludges[J]. Water Research, 2008, 42(14): 3879-3889. DOI:10.1016/j.watres.2008.05.014 |
| [75] |
LU Q, CHANG M, YU Z, et al. The effects of three commonly used extraction methods on the redox properties of extracellular polymeric substances from activated sludge[J]. Environmental Technology, 2015, 36(22): 2884. DOI:10.1080/09593330.2015.1051590 |
| [76] |
LU X, ZHEN G, ESTRADA A L, et al. Operation performance and granule characterization of upflow anaerobic sludge blanket (UASB) reactor treating wastewater with starch as the sole carbon source[J]. Bioresource Technology, 2015, 180: 264. DOI:10.1016/j.biortech.2015.01.010 |
| [77] |
ZHANG L, REN H, DING L. Comparison of extracellular polymeric substances (EPS) extraction from two different activated sludges[J]. Water Science and Technology, 2012, 66(7): 1558. DOI:10.2166/wst.2012.295 |
| [78] |
ZHANG Z, WANG P, ZHANG J, et al. Removal and mechanism of Cu (Ⅱ) and Cd (Ⅱ) from aqueous single-metal solutions by a novel biosorbent from waste-activated sludge[J]. Environmental Science and Pollution Research, 2014, 21(18): 10823. DOI:10.1007/s11356-014-3013-6 |
| [79] |
WANG Y, QIN J, ZHOU S, et al. Identification of the function of extracellular polymeric substances (EPS) in denitrifying phosphorus removal sludge in the presence of copper ion[J]. Water Research, 2015, 73: 252. DOI:10.1016/j.watres.2015.01.034 |
| [80] |
SONG J S, MAENG M, LEE K, et al. The role of extracellular polymeric substances in reducing copper inhibition to nitrification in activated sludge[J]. Biotechnology and Bioprocess Engineering, 2016, 21(5): 683. DOI:10.1007/s12257-016-0329-8 |
| [81] |
YANG Q, SUN J, WANG D, et al. Effect of nickel on the flocculability, settleability, and dewaterability of activated sludge[J]. Bioresource Technology, 2017, 224: 188. DOI:10.1016/j.biortech.2016.11.018 |
 2018, Vol. 50
2018, Vol. 50


